Abstract
In Huntington's disease (HD), mutation of huntingtin causes selective neurodegeneration of dopaminoceptive striatal medium spiny neurons. Transgenic HD model mice that express a portion of the disease-causing form of human huntingtin develop a behavioral phenotype that suggests dysfunction of dopaminergic neurotransmission. Here we show that presymtomatic mice have severe deficiencies in dopamine signaling in the striatum. These include selective reductions in total levels of dopamine- and cAMP-regulated phosphoprotein, Mr 32 kDA (DARPP-32) and other dopamine-regulated phosphoprotein markers of medium spiny neurons. HD mice also show defects in dopamine-regulated ion channels and in the D1 dopamine/DARPP-32 signaling cascade. These presymptomatic defects may contribute to HD pathology.
Huntington's disease (HD) is an inherited neurodegenerative disorder characterized by progressive motor, psychiatric, and cognitive disturbances. The motor disturbance begins subtly, as minor adventitious movements, and gradually progresses until the entire body is consumed in flagrant chorea and uncontrolled writhing, leading to death 10–20 years after onset (1). HD pathology is characterized by specific neurodegeneration of the caudate nucleus and putamen (2, 3). Genetic analysis has established a causative link between HD and abnormal expansion of a polyglutamine repeat in the protein huntingtin (4). The transgenic mouse line R6/2 (HD mice) is a well-characterized animal model of HD in which a fragment of a disease-causing variant of human huntingtin is ectopically expressed. These mice exhibit HD-like behavioral changes beginning after 8 weeks of life, progressively deteriorate over the succeeding 10 weeks, and die prematurely (5, 6). Although the HD mice are essentially asymptomatic until week 8, behavioral deficits are preceded by the appearance of intranuclear inclusion bodies in the central nervous system (7, 8). These inclusions are similar to those detected in HD and other diseases associated with polyglutamine expansions (7–9).
Both normal and mutant huntingtin are expressed throughout the body. The dramatic and selective loss of striatal tissue seen in HD, and the resultant pronounced behavioral and pathological effects, have remained unexplained (1, 10, 11). Given that the striatum is the predominant target of midbrain dopamine neurons, we sought to determine whether dopamine signaling might be altered in presymptomatic HD mice. We found that while many parameters of neuronal function remained unchanged, there were significant deficits including severely impaired electrophysiological and biochemical responses to activation of D1-class dopamine receptors, which precede the emergence of histopathological and behavioral changes.
Materials and Methods
Forskolin was purchased from LC Laboratories. 8-Bromo-cAMP and protease type XIV were purchased from Sigma. SKF81297, D1 receptor antibodies, kainic acid, dopamine, and γ-aminobutyric acid (GABA) were purchased from Research Biochemicals. Actin antibodies were purchased from BRL. DARPP-32 (12), ARPP-21 (13), ARPP-16/19 (14), Glu-R1 (15), and synapsin (16) antibodies have previously been characterized. Antibody specific for the detection of the α isoform of the catalytic subunit of protein kinase A (PKA) was purchased from Santa Cruz Biotechnology. HD, (C57BL/6 × CBA)F1 (B6CBA)-TgN(HDexon1)62 (R6/2), and control [wild-type (WT)] B6CBAF1/J mice used for initial slice experiments and in situ hybridizations were obtained from The Jackson Laboratory. For time-course experiments and immunohistochemistry, transgenic TgN(HDexon1)62Gpb mice were obtained from The Jackson Laboratory and maintained on a B6/CBA hybrid background at the Wadsworth Center (Albany, NY). Genotypes were verified by PCR, as described (17). The Institutional Animal Care and Use Committee approved all procedures.
Electrophysiology Studies.
Acutely dissociated striatal neurons were prepared as described (18, 19). Whole-cell recordings of voltage-gated calcium current, γ-aminobutyric acid type A (GABAA) receptor-mediated chloride conductance, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate-activated current were measured by using standard whole-cell voltage-clamp techniques (20). Where applicable, 100 μM dopamine, 100 μM GABA, 10 μM SKF81297, or 100 μM kainate was used. Summary data were expressed as SEM and P values were derived by using the paired t test.
Pharmacology and Quantitative Immunoblotting.
Striatal slices were prepared as described (21). Striatal slices were treated with either 1 μM SKF81297 for 5 min, 50 μM forskolin for 5 min, or 1 mM 8-bromo-cAMP for 10 min. Slices were homogenized in 1% SDS and 50 mM NaF. Equal amounts of protein from homogenates of striatal slices or rapidly dissected striata were subjected to SDS/PAGE followed by electrophoretic transfer to polyvinylidene fluoride membranes (Millipore). Immunoreactive proteins were detected either by chemiluminescence or by 125I-protein A and quantified by laser densitometry or PhosphorImager analysis (Molecular Dynamics), respectively. Data were analyzed statistically by a nonparametric Mann–Whitney U test.
Confocal Microscopy.
Tissue was fixed by transcardiac perfusion according to standard methodology (22). Whole dissected brains were cryoprotected by incubation overnight at 4°C in PBS with 15% sucrose. Brain hemispheres were separated at the corpus callosum and mounted so that each section contained one hemisphere each from a WT and HD mouse. Coronal cryostat sections (14 μm) were melted onto coated slides (Fisher ProbeOn Plus) and incubated overnight with primary monoclonal antibodies to DARPP-32 or glutamic acid decarboxylase (GAD-6) in PBS containing 1% normal donkey serum and 1% Triton X-100. Sections were then incubated for 90 min in secondary donkey anti-mouse antibody conjugated to tetramethylrhodamine B isothiocyanate (Jackson ImmunoResearch). Sections were coverslipped with glycerol/PBS (5:1) containing 0.1% paraphenylenediamine and stored in the dark at −20°C. Fluorescent images were captured from a Zeiss LSM 510 laser scanning confocal microscope with ×10 and ×60 objectives using an argon laser with an excitation wavelength of 547 Å. Images of control and HD mice were captured from paired tissue processed on the same slide. Identical microscope settings including gain, off set, pinhole, and laser intensity were used. The immunocytochemical specificities of the DARPP-32 and GAD antibodies have previously been demonstrated (23, 24).
In Situ Hybridization Studies.
α-35S-UTP-labeled riboprobes were radiolabeled by in vitro transcription from cDNA clones corresponding to a 5′ fragment of the mouse ARPP-16 (14), and to full-length clones of rat DARPP-32 (25), rat GAD-67 and GAD-65 (26), rat preproenkephalin A (27), rat preprotachykinin A (28), and synapsin II (16) genes. Cryostat sections were hybridized as described (29). After hybridization, the sections were exposed to Biomax MR film (Kodak) for 2–6 days. All autoradiograms were analyzed with a Microcomputer Imaging system (M4; Imaging Research, St. Catherine's, ON, Canada) as described (30). Statistical analyses of the data were performed using a two-tailed unpaired Student's t test.
Results
Attenuation of Dopaminergic Modulation of Ion Channels in HD Mice.
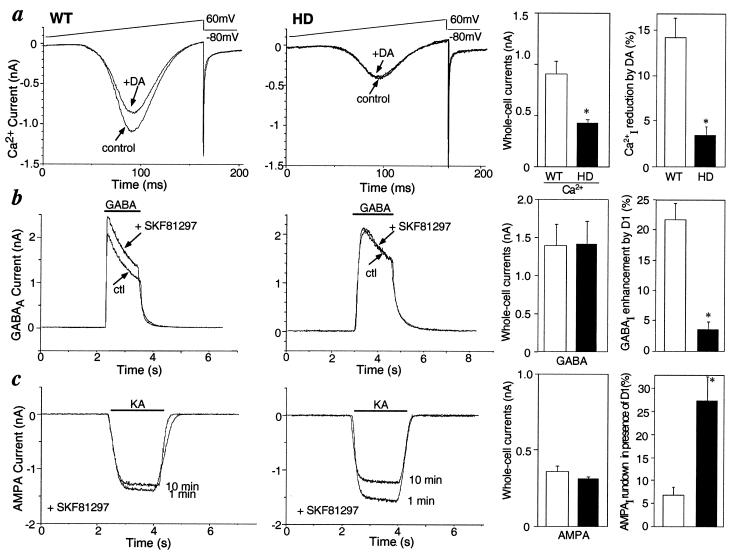
Various ion channels play a key role in regulating the excitability of striatal medium spiny neurons. D1-class dopamine receptors regulate voltage-gated Ca2+ currents, GABAA currents, and AMPA-type glutamate currents in these cells (18, 31, 32). The properties of these channels and the response of each to activation of D1-class dopamine receptors were compared in 6-week-old HD and control mice (Fig. 1). Application of dopamine reduced peak Ca2+ current in striatal neurons for control mice, in agreement with previous reports (18, 19). However, dopamine only slightly altered Ca2+ current in striatal neurons from HD mice (Fig. 1a: WT, 14.2 ± 2.1% reduction; HD, 3.5 ± 1% reduction, P < 0.05, n = 4). The amplitude of peak Ca2+ current induced by a voltage ramp (−80 to 60 mV) was significantly reduced in striatal neurons from HD mice (Fig. 1a: WT, 0.91 ± 0.35 nA; HD, 0.42 ± 0.11 nA, n = 8, P < 0.01). Activation and inactivation kinetics of Ca2+ currents in HD and control mice were indistinguishable. The addition of the D1-class dopamine receptor agonist SKF81297 potentiated GABAA current, as previously reported (33), but had no detectable effect in the striatal cells from HD mice (Fig. 1b: WT, 21.8 ± 3.0% increase; HD, 3.8 ± 3.0% increase, P < 0.005, n = 6). Addition of SKF81297 attenuated the run down of the AMPA current over a 10-min time period as reported previously (32), but had no such effect in striatal neurons from HD mice (Fig. 1c: WT, 6.9 ± 1.8% decrease; HD, 27.7 ± 5.0% decrease, P < 0.01, n = 4). No significant differences were detected in the peak amplitude and desensitization rate of GABAA- and AMPA-evoked currents in HD and control mice (Fig. 1 b and c). These results suggested a major attenuation in the ability of dopamine to modulate the ion channels that control the physiological state and excitability of medium spiny neurons in HD mice at 6 weeks of age.
Figure 1.
Comparison of electrophysiological properties of WT and HD mice. Dopamine effects on voltage-gated Ca2+ current (a), GABAA current (b), and AMPA current (c) . To the right of each are summary histograms of the peak amplitudes of whole-cell currents (Left) and of the percent change induced by dopamine (DA) or the D1-class receptor agonist SKF81297 (Right) in WT (□) and HD mice (■). Data represent means ± SEM for n = 4–9; ∗, P < 0.05 compared with WT.
Attenuation of Dopaminergic Signaling Cascade in HD Mice.
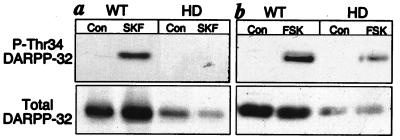
In an effort to define the underlying mechanism(s) responsible for loss of dopamine regulation of ion channels, analyses of the dopamine-signaling pathway were performed. D1-class dopamine receptors are positively coupled to the activation of PKA which phosphorylates a number of downstream protein targets. One such striatal-specific target is DARPP-32. Phosphorylation of DARPP-32 at Thr34 by PKA converts it into a potent inhibitor of the multifunctional serine/threonine phosphatase, protein phosphatase 1 (34). A phosphorylation state-specific antibody was used to measure phospho-Thr34 DARPP-32 levels in lysates from 6-week-old control and HD mice (35). A large increase in PKA-dependent phosphorylation of DARPP-32 was observed in response to the D1-class receptor activation by SKF81297 (Fig. 2a) or increases in intracellular levels of cAMP as a result of the activation of adenylyl cyclase by forskolin (Fig. 2b) in striatal slices from control mice. The response to SKF81297 was undetectable and the response to forskolin was greatly attenuated in HD mice of the same age. These results indicate that one or more severe defects exist in the D1/cAMP/PKA/DARPP-32 signaling cascade of presymptomatic HD mice. We sought to determine whether reduced levels of D1-class receptors and/or DARPP-32 contributed to the observed deficiencies in D1 dopamine signaling. Immunoprobing of the same blots that had been used to measure phospho-Thr34 DARPP-32 indicated that the level of D1 dopamine receptors was reduced to 74.4 ± 3% of the level detected in WT mice (n = 6, P < 0.05). The same polyvinylidene difluoride membranes were also immunoblotted with a monoclonal antibody that detected total (phosphorylated and unphosphorylated) DARPP-32 (Fig. 2, Lower). DARPP-32 levels in striatal slice homogenates from the HD mice were reduced to 40 ± 0.5% of controls (n = 6 slices, P < 0.05).
Figure 2.
Comparison of DARPP-32 phosphorylation in response to a D1 agonist and forskolin in WT and HD mice. Striatal slices from 6-week-old mice were treated with either a dopamine D1 receptor agonist (a), SKF81297 (SKF), or an activator of adenylyl cyclase (b), forskolin (FSK). Homogenates of the slices were subjected to SDS/PAGE, proteins were transferred to polyvinylidene difluoride membranes, and phospho-Thr34 DARPP-32 was analyzed by immunoblotting using a phosphorylation state-specific antibody. Blots were reprobed to determine total levels of DARPP-32 (Lower).
Selective Reduction in Dopamine Target Proteins in HD Mice.
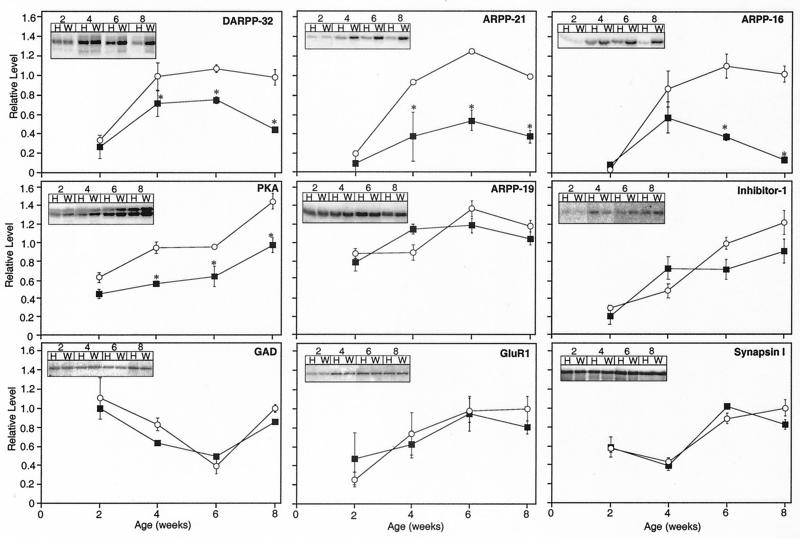
To determine whether protein deficiencies were limited to dopamine signaling targets, a more rigorous analysis was conducted with littermate-matched control mice at various ages before the onset of neurological symptoms (Fig. 3). DARPP-32 levels were reduced in HD mice relative to controls at 4, 6, and 8 weeks of age, as assessed by immunoblot analysis of striatal homogenates. Therefore, reduction of the adult striatal neuronal marker DARPP-32 precedes behavioral changes in HD mice. Moreover, ARPP-21 and ARPP-16, two other markers of end-differentiated striatal medium spiny neurons that serve as downstream targets of dopamine (14, 36), were dramatically reduced in the HD mice at all time points after 2 weeks. In addition, the levels of PKA were reduced significantly at 4, 6, and 8 weeks of age.
Figure 3.
Comparison of the levels of striatal proteins in WT and HD mice. Striatal homogenates of WT control (○) and HD (■) mice were subjected to SDS/PAGE and examined by immunoblotting with an antibody specific for the protein indicated. Mice were analyzed at 2, 4, 6, and 8 weeks of age. (Insets) Radiographic images showing representative protein bands detected in homogenates of HD (H) and WT (W) mice for each time point. Data represent means ± SEM for n = 6; ∗, P < 0.05 compared with WT.
To determine whether the decreases in the dopamine-regulated substrates DARPP-32, ARPP-21, and ARPP-16 were selective or representative of a general neurodegeneration of medium spiny neurons, the levels of various other neuronal proteins, not specifically localized to striatal medium spiny neurons, were examined by immunoblotting of the same homogenates. One such protein, the PKA-regulated phosphoprotein inhibitor-1 is a structural and functional homologue of DARPP-32 and is widely distributed throughout the brain and peripheral tissue (37). In contrast to DARPP-32, no difference was observed between the levels of inhibitor-1 in WT and HD mice (Fig. 3). Similarly, no difference was detected in the levels of ARPP-19, a homologue of ARPP-16 that exhibits a broader tissue distribution (14). The levels of the presynaptic GABAergic marker GAD, the GluR1 subunit of the AMPA-type glutamate receptor, a neuronal-specific presynaptic modulator of Ca2+-dependent neurotransmitter release, synapsin I, and actin (data not shown) were not significantly different in HD vs. control mice at any of the time points measured. These biochemical results reveal a dramatic and selective loss of dopaminergic signaling molecules during the period before behavioral changes in HD mice, while other more generally expressed markers remain unchanged.
Reduction in Striatal DARPP-32 Immunostaining in HD Mice.
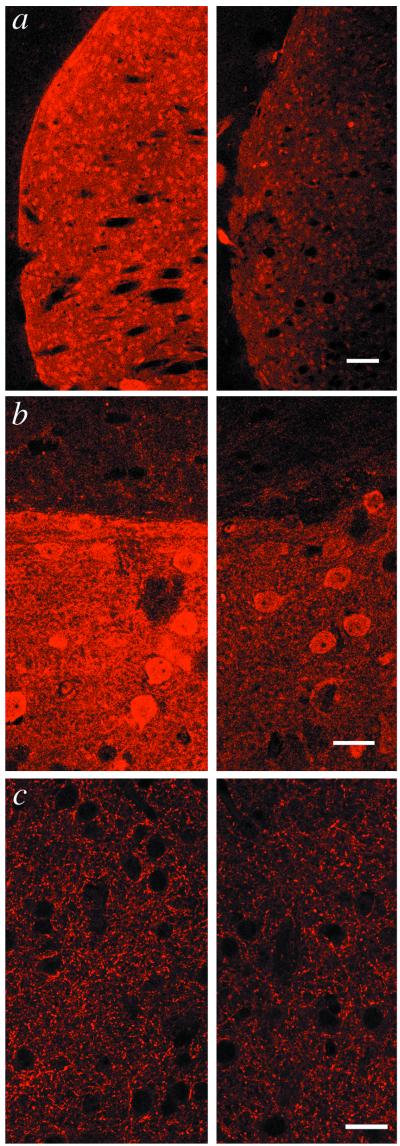
To examine the reduction of DARPP-32 at the cellular level, immunocytochemical studies were undertaken (Fig. 4). Coronal sections of whole brains from 6-week-old HD and littermate control mice were immunostained for DARPP-32 and GAD and analyzed by confocal microscopy under identical conditions (Fig. 4). There were no obvious differences between HD and control mice in morphology or tissue cytoarchitectonics. There was a dramatic reduction in the intensity of DARPP-32 staining of cell bodies and processes but no obvious decrease in the number of DARPP-32-positive neurons in the striatum of HD mice. In contrast, staining for GAD was indistinguishable in HD mice compared with control mice. These data suggest that the reduction in dopamine-signaling proteins is not due to any obvious changes in adult medium spiny neuron morphology or degeneration of striatal tissue.
Figure 4.
Comparison of immunocytochemical staining for DARPP-32 in WT and HD mice. Striatal tissue of WT (Left) and HD mice (Right) was immunostained for DARPP-32 and GAD, and fluorescent images were collected by confocal laser scanning microscopy. (a) Image of coronal sections showing immunoreactivity for DARPP-32 in somata and dendrites of caudatoputamen. (b) Morphology of DARPP-32-positive medium spiny neurons and neuropil architectonics appear to be unaffected in the HD mouse. Intensely staining caudatoputamen (lower portion) contrasts with weakly staining cortex (upper portion). Note reduction in signal intensity in HD tissue. (c) Staining of striatal tissue by GAD-6 antibodies at ×630 magnification shows no differences between WT and HD model mice. (Scale bars in a, b, and c represent 250, 25, and 25 μm, respectively.)
Selective Reduction in Striatal Gene Expression in HD Mice.
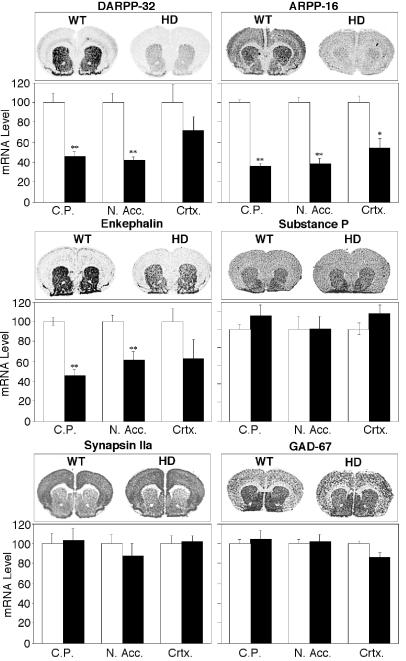
Reduced protein levels could result from altered rates of gene expression, protein translation, or protein degradation. To determine whether the observed changes in levels of dopamine-signaling proteins reflected alterations in gene transcription and mRNA levels, in situ hybridization studies were undertaken (Fig. 5). DARPP-32 and ARPP-16 mRNA levels were substantially reduced in the caudatoputamen and nucleus accumbens of HD mice at 6 weeks of age. The expression levels of the striatal neuropeptides enkephalin and substance P were assessed. These neuropeptides are expressed in different subpopulations of striatal projection neurons. Enkephalin is enriched in neurons that terminate in the globus pallidus, whereas substance P is mostly found in neurons that terminate in the substantia nigra or in the entepeduncular nucleus (38, 39). Enkephalin, but not substance P, mRNA levels were reduced in the striatum and nucleus accumbens of HD mice. mRNA levels of two glutamate decarboxylase isoforms, GAD-67 (Fig. 5) and GAD-65 (data not shown), were unaffected in HD mice compared with controls (40). Synapsin IIa mRNA levels were also unaffected. Of the transcripts examined, only ARPP-16 mRNA levels were significantly reduced in the cortex of HD mice compared with WT controls. These results indicate that the reductions in dopamine-signaling proteins reflect decreased mRNA levels, rather than altered protein degradation.
Figure 5.
Comparison of the levels of striatal gene expression in WT and HD mice. In situ hybridizations with probes specific for the detection of DARPP-32, ARPP-16, enkephalin, substance P, GAD-67, and synapsin IIa are shown. Representative labelings of coronal sections are shown for WT and HD mice. Histograms summarize quantification of signals in the caudatoputaman (C.P.), nucleus accumbens (N. Acc.) and cortex (Crtx) for WT (□) and HD mice (■). Data represent means ± SEM for n = 6; ∗∗, P < 0.001 and ∗, P < 0.01 compared with WT.
Discussion
The results presented here describe selective impairment in several aspects of dopaminergic signaling in a murine model for HD. These defects precede the hallmark HD-like behavioral changes that characterize these mice. The results indicate that HD mice are severely impaired in their ability to regulate the physiological state of striatal neurons via dopamine. DARPP-32 is a fundamental component of the dopamine-signaling cascade, and its expression is essential to the ability of dopamine to regulate the physiology of striatal neurons (34, 41). Striatal slice experiments indicated attenuation of the ability to invoke the D1/cAMP/PKA/DARPP-32 signaling cascade. Consistent with previous reports, we observed a reduction in D1 receptors in HD mice (42). We observed a reduction in the striatal-specific dopamine targets, PKA, DARPP-32, ARPP-16, and ARPP-21 in HD mice. The immunohistochemistry studies indicated that the reduction in dopamine-signaling proteins was not due to striatal neurodegeneration in the HD mice. Consistent with the results presented here, the “number” of DARPP-32 expressing neurons has been reported to be unaffected in HD mice (43). Although none of the observed reductions alone could account for a loss in dopamine signaling, the cumulative reduction of all components of the cascade could account for the observed loss in D1 receptor-dependent regulation of several critical downstream targets.
Recently, elevated intracellular Ca2+ levels in hippocampal neurons have been reported in a similar murine HD model (44). A reduced driving force for Ca2+ is in agreement with our observations of reduced voltage-gated Ca2+ current in striatal neurons of HD mice. Increased intracellular Ca2+ could also contribute to loss of dopamine signaling by activating the Ca2+/calmodulin-dependent protein phosphatase 2B (calcineurin) to dephosphorylate phospho-Thr34 DARPP-32 (45). Furthermore, the predicted loss in N-methyl-d-aspartate-mediated Ca2+ current could explain the resistance of HD model mice to quinolinic acid-induced striatal excitotoxicity (43).
HD mice at 6 weeks of age resemble DARPP-32−/− mice, which also exhibit a loss of dopamine regulation of medium spiny neuron physiology (34, 41, 46). Dopamine signaling is known to regulate gene transcription and mice lacking DARPP-32 exhibit altered gene transcriptional regulation (30, 41, 47). The in situ hybridization studies described here reveal a selective reduction in the expression of genes involved in dopamine signaling in HD mice. A selective reduction in the expression of enkephalin but not substance P was observed in HD mice. Preferential loss of enkephalin in the striatal tissue of HD patients has been reported previously (48, 49).
Expression of the disease-causing form of human huntingtin appears to have broad effects on mice as well as humans (i.e., decreased brain size, body weight loss) (5). The selective dysfunction in dopaminergic signaling is coincident with the appearance of polyglutamine-containing aggregates or inclusion bodies, which occurs throughout the central nervous system (7). Inclusion body formation and, perhaps, alteration in normal huntingtin function contribute to HD and may be associated with the loss of dopamine signaling. It is possible that inclusion body formation, although occurring throughout the central nervous system, specifically impairs striatum-specific gene expression (5, 50). The relationship between inclusion bodies and the disruption of various components of the dopaminergic signaling cascade is an important topic for future investigation. It is noteworthy that the expression of DARPP-32 in neuronal cultures correlates with the efficacy of intrastriatal tissue replacement therapy in HD model mice (51). It will be interesting to see what effect crossing HD mice with DARPP-32−/− mice will have on the period of onset of the neurological phenotype.
Acknowledgments
We thank Richard Huganir for the Glu-R1 receptor antibody. We thank Alan Towbin, Steven Sabol, and Hung-Teh Kao for the plasmids encoding GAD, preproenkephalin A, and synapsin, respectively. The GAD-selective antibody, GAD-6 was obtained from the Developmental Studies Hybridoma Bank at the University of Iowa. We are grateful to Beatriz Rendon and Carmina Valle for expert technical assistance. We thank Madaline Kuhn who inspired this work and provided financial support. This work was made possible by the financial support of the Huntington's Disease Society of America (J.A.B.), the Hereditary Disease Foundation (A.M.), and U.S. Public Health Service Grant MH-40899 (P.G.). Z.Y. is a recipient of the National Alliance for Research on Schizophrenia and Depression Young Investigator Award. P.S. is a recipient of a postdoctoral fellowship from Stiftelsen för Internationalisering av högree utbildning och forskning.
Abbreviations
- HD
Huntington's disease
- GABA
γ-aminobutyric acid
- PKA
protein kinase A
- GABAA
γ-aminobutyric acid type A
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GAD
glutamic acid decarboxylase
- WT
wild type
- DARPP-32
dopamine and cAMP-regulated phosphoprotein, Mr 32 kDa
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120166397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120166397
References
- 1.Gusella J F, MacDonald M E. Semin Cell Biol. 1995;6:21–28. doi: 10.1016/1043-4682(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 2.Vonsattel J P, Myers R H, Stevens T J J R, Ferrante E D B, Richardson E P. J Neurol Exp Neuropathol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Roos R A C. In: Handbook of Clinical Neurology. Vinke P J, Bruyn G W, Klawanm H L, editors. Vol. 49. Amsterdam: Elsevier; 1986. pp. 315–327. [Google Scholar]
- 4.Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 5.Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies S, Bates G P. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- 6.Carter R J, Lione L A, Humby T, Mangiarini L, Mahal A, Bates G P, Dunnett S B, Morton A J. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies S W, Turmaine M, Cozens B A, DiFiglia M, Sharp A H, Ross C A, Scherzinger E, Wanker E E, Mangiarini L, Bates G P. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 8.DiFiglia M, Sapp E, Chase K O, Davis S W, Bates G P, Vonsattel J P, Aronin N. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 9.Davies S W, Beardsall K, Turmaine M, DiFiglia M, Aronin N, Bates G P. Lancet. 1998;351:131–135. doi: 10.1016/S0140-6736(97)08360-8. [DOI] [PubMed] [Google Scholar]
- 10.Strong T, Tagle D, Valdes J, Elmer L, Boehm K, Swaroop M, Kaatz K, Collins F, Albin R. Nat Genet. 1993;5:259–263. doi: 10.1038/ng1193-259. [DOI] [PubMed] [Google Scholar]
- 11.Li A-H, Schilling G, Young S W, Li X-J, Margolis R L, Stine O C, Wagster M V, Abbott M H, Franz M L, et al. Neuron. 1993;11:985–993. doi: 10.1016/0896-6273(93)90127-d. [DOI] [PubMed] [Google Scholar]
- 12.Ouimet C C, Miller P E, Hemmings J, H C, Walaas S I, Greengard P. J Neurosci. 1984;4:114–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmings H C, Jr, Girault J-A, Williams K R, LoPresti M B, Greengard P. J Biol Chem. 1989;264:7726–7733. [PubMed] [Google Scholar]
- 14.Horiuchi A, Williams K R, Kurihara T, Nairn A C, Greengard P. J Biol Chem. 1990;265:9476–9484. [PubMed] [Google Scholar]
- 15.Blackstone C, Murphy T H, Moss S J, Baraban J M, Huganir R L. J Neurosci. 1994;14:7585–7593. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudhof T C, Czernik A J, Kao H T, Takei K, Johnston P A, Horiuchi A, Kanazir S D, Wagner M A, Perin M S, De Camilli P, Greengard P. Science. 1989;1989:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- 17.Manley K, Pugh J, Messer A. Mol Brain Res. 1999;835:74–79. doi: 10.1016/s0006-8993(99)01451-1. [DOI] [PubMed] [Google Scholar]
- 18.Surmeier D J, Bargas J, Hemmings H C, Jr, Nairn A C, Greengard P. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 19.Yan Z, Surmeier D J. J Neurosci. 1996;16:2592–2604. doi: 10.1523/JNEUROSCI.16-08-02592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 21.Nishi A, Snyder G L, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hockfield S, Carlson S, Evans C, Levitt P, Pintar J, Silberstein L. Selected Methods for Antibody and Nucleic Acid Probes. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. [Google Scholar]
- 23.Ouimet C C, Langly-Gullion K C, Greengard P. Brain Res. 1998;808:8–12. doi: 10.1016/s0006-8993(98)00724-0. [DOI] [PubMed] [Google Scholar]
- 24.Essrich C, Lorez M, Benson J A, Fritschy J-M, Lüscher B. Nat Neurosci. 1998;1:561–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich M E, Kurihara T, Greengard P. J Mol Neurosci. 1990;2:1–10. doi: 10.1007/BF02896920. [DOI] [PubMed] [Google Scholar]
- 26.Erlander M G, Tillakaratne N J, Feldblum S, Patel N, Tobin A J. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 27.Yoshikawa K, Williams C, Sabol S L. J Biol Chem. 1984;259:14301–14308. [PubMed] [Google Scholar]
- 28.Krause J E, Chirgwin J M, Carter M S, Xu S, Hershey A D. Proc Natl Acad Sci, USA. 1987;84:881–885. doi: 10.1073/pnas.84.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Moine C, Bloch B. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 30.Svenningsson, P., Fienberg, A. A., Allen, P. B., Le Moine, C., Lindskog, M., Fisone, G., Greengard, P. & Fredholm, B. B. (2000) J. Neurochem., in press. [DOI] [PubMed]
- 31.Flores-Hernandez, J., Hernandez, S., Snyder, G. L., Yan, Z., Fienberg, A. A., Moss, S. J., Greengard, P. & Surmeier, D. J. (2000) J. Neurophysiol., in press. [DOI] [PubMed]
- 32.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen P B, Fienberg A A, Nairn A C, Greengard P. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 33.Yan Z, Surmeier D J. Neuron. 1997;19:1115–1126. doi: 10.1016/s0896-6273(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 34.Greengard P, Allen P B, Nairn A C. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 35.Snyder G L, Girault J-A, Chen J Y C, Czernik A J, Kebabian J W, Nathanson J A, Greengard P. J Neurosci. 1992;12:3071–3083. doi: 10.1523/JNEUROSCI.12-08-03071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caporaso, G., Bibb, J. A., Snyder, G. L., Nairn, A. C., Hemmings, H. C., Jr. & Greengard, P. (2000) Neuropharmacology, in press. [DOI] [PubMed]
- 37.Hemmings H C, Jr, Girault J-A, Nairn A C, Bertuzzi G, Greengard P. J Neurochem. 1992;59:1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- 38.Gerfen C R, Wilson C J. In: Handbook of Chemical Neuroanatomy: Integrated Systems of the CNS. Swanson L W, Björklund A, Hökfelt T, editors. Amsterdam: Elsevier; 1996. pp. 371–467. [Google Scholar]
- 39.Graybiel A M. Trends Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- 40.LaPrade N, Soghomonian J-J. Synapse. 1999;33:36–48. doi: 10.1002/(SICI)1098-2396(199907)33:1<36::AID-SYN4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Fienberg A A, Hiroi N, Mermelstein P G, Song W-J, Snyder G L, Nishi A, Cheramy A, O'Callaghan J P, Miller D B, Cole, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 42.Cha J-H J, Kosinski C M, Kerner J A, Alsdorf A, Mangiarini L, Davies S W, Penney J B, Bates G P, Young A B. Proc Natl Acad Sci USA. 1998;95:6480–6485. doi: 10.1073/pnas.95.11.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansson O, Petersen A, Leist M, Nicotera P, Castilho R F, Brundin P. Proc Natl Acad Sci USA. 1999;96:8727–8732. doi: 10.1073/pnas.96.15.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodgson J G, Agopyan N, Gutekunst C A, Leavitt B R, LePiane F, Singaraja R, Smith D J, Bissada N, McCutcheon K, Nasir J, et al. Neuron. 1999;23:181–192. doi: 10.1016/s0896-6273(00)80764-3. [DOI] [PubMed] [Google Scholar]
- 45.Halpain S, Girault J-A, Greengard P. Nature (London) 1990;343:369–372. doi: 10.1038/343369a0. [DOI] [PubMed] [Google Scholar]
- 46.Fienberg A A, Greengard P. Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 47.Berke J D, Hyman S E. Neuron. 2000;25:513–537. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 48.Sapp E, Ge P, Bird A E, Penney J, Young A B, Vonsattel J-P, DiFiglia M. Neuroscience. 1995;64:397–404. doi: 10.1016/0306-4522(94)00427-7. [DOI] [PubMed] [Google Scholar]
- 49.Richfield E K, Magure-Zeiss K A, Vonkeman H E, Voorn P. Ann Neurol. 1995;38:852–861. doi: 10.1002/ana.410380605. [DOI] [PubMed] [Google Scholar]
- 50.Li S-H, Cheng A L, Li H, Li X-J. J Neurosci. 1999;19:5159–5172. doi: 10.1523/JNEUROSCI.19-13-05159.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakao N, Grasbon-Frodl E M, Widner H, Brundin P. Neuroscience. 1996;74:959–970. doi: 10.1016/0306-4522(96)00238-2. [DOI] [PubMed] [Google Scholar]