Abstract
The mammalian kidney develops in three successive steps from the initial pronephros via the mesonephros to the adult metanephros. Although the nephric lineage is specified during pronephros induction, no single regulator, including the transcription factor Pax2 or Pax8, has yet been identified to control this initial phase of kidney development. In this paper, we demonstrate that mouse embryos lacking both Pax2 and Pax8 are unable to form the pronephros or any later nephric structures. In these double-mutant embryos, the intermediate mesoderm does not undergo the mesenchymal-epithelial transitions required for nephric duct formation, fails to initiate the kidney-specific expression of Lim1 and c-Ret, and is lost by apoptosis 1 d after failed pronephric induction. Conversely, retroviral misexpression of Pax2 was sufficient to induce ectopic nephric structures in the intermediate mesoderm and genital ridge of chick embryos. Together, these data identify Pax2 and Pax8 as critical regulators that specify the nephric lineage.
Keywords: Pax2, Pax8, genetic redundancy, kidney development, mesenchymal-epithelial transition, lineage specification
Kidney development in mammals and birds proceeds in three successive steps that are all characterized by the mesenchymal-to-epithelial transformation of intermediate mesoderm cells. The development of the first kidney, the transient pronephros, is initiated by signals from the somite and surface ectoderm that induce cells in the intermediate mesoderm to undergo the transition to epithelial cells forming the nephric duct (Obara-Ishihara et al. 1999; Mauch et al. 2000). The caudal migration of the nephric duct subsequently induces the adjacent nephrogenic mesoderm to aggregate and form the tubules of the mesonephros, the second embryonic kidney. On further extension, the nephric duct reaches the metanephrogenic mesenchyme at the level of the developing hindlimb, where the ureteric bud evaginates from the nephric duct and invades the surrounding mesenchyme. Both the ureter and mesenchyme subsequently undergo reciprocal inductive interactions to form the nephrons and collecting ducts of the metanephros, the third and adult kidney. Ultimately, the development of the metanephros therefore depends on the proper formation of the nephric duct during pronephros induction (for review, see Saxén 1987; Vainio and Müller 1997).
Targeted mutagenesis in the mouse has identified a multitude of genes that are important for normal kidney development (for review, see Kuure et al. 2000; Davies and Brändli 2002). The majority of these genes are essential for proper morphogenesis of the metanephros with the most severe phenotypes being caused by the inactivation of the transcription factor genes Lim1 (Shawlot and Behringer 1995), WT1 (Kreidberg et al. 1993), and Pax2 (Torres et al. 1995; Favor et al. 1996). Lim1 mutant embryos fail to develop a metanephros and gonads (Shawlot and Behringer 1995), although a nephric duct is initially formed, but then degenerates in the posterior part of the mesonephros (Tsang et al. 2000). The Wilms' tumor suppressor gene WT1 is also necessary for metanephros and gonad development, as the metanephric mesenchyme is unresponsive to inductive signals and undergoes apoptosis in the absence of WT1 function (Kreidberg et al. 1993). The mesonephros, however, still develops in WT1-deficient embryos, although its most caudal tubules fail to form (Sainio et al. 1997). Interestingly, the Pax2 gene is still expressed in the mesonephros of both Lim1 and WT1 mutant embryos (Donovan et al. 1999; Tsang et al. 2000), suggesting that Pax2 acts upstream of these two transcription factors in kidney development. Pax2 is the first known kidney-specific gene to be expressed in the pronephros of the mouse embryo (Bouchard et al. 2000). Despite this early expression, the mesonephric duct is still formed in Pax2-deficient embryos, but then fails to extend to the metanephrogenic mesenchyme because of its rapid degeneration (Torres et al. 1995). As a consequence, the metanephros and genital tracts never develop in Pax2 mutant mice (Torres et al. 1995; Favor et al. 1996). Importantly, however, none of the known gene mutations—including Pax2—interferes with the earliest phase of kidney development, that is, the initial formation of the pro- and mesonephros.
Pax8, another member of the Pax2/5/8 family, is also expressed during pro-, meso-, and metanephros development (Plachov et al. 1990; Pfeffer et al. 1998). Surprisingly, kidney organogenesis is normal in Pax8 mutant mice that die postnatally of a defect in thyroid gland development (Mansouri et al. 1998). By gene replacement in the mouse, we have shown recently that the proteins of the Pax2/5/8 family can substitute for each other in development because of their equivalent biochemical function (Bouchard et al. 2000). By analyzing Pax2,Pax8 double-mutant embryos, we now demonstrate that these two transcription factors have redundant functions in kidney development. Pax2 and Pax8 together are required for the formation of the pro- and mesonephros, as the intermediate mesoderm of Pax2−/−Pax8−/− embryos was unable to undergo the initial mesenchymal-epithelial transitions and to express the early kidney-specific genes c-ret and Lim1. In complementary experiments, the retroviral misexpression of Pax2 was sufficient to induce ectopic nephric structures in the intermediate mesoderm and genital ridge of chick embryos. Together, these data demonstrate that Pax2 and Pax8 are both necessary and sufficient for specifying the nephric lineage.
Results
Inactivation of the Pax8 gene
Exon 3 of the Pax8 gene codes for the N-terminal part of the paired domain that is indispensable for DNA binding of the Pax2/5/8 transcription factors (Czerny et al. 1993). Using homologous recombination in embryonic stem (ES) cells, we therefore inactivated the Pax8 gene by replacing exon 3 with an in-frame insertion of a cre recombinase gene together with a neomycin (neo) resistance gene (Fig. 1A). Heterozygous Pax8neo/+ mice were obtained by blastocyst injection of targeted ES cells and were shown to express Cre activity in all Pax8 expression domains (Plachov et al. 1990), including the developing kidney, ear, thyroid gland and midbrain-hindbrain boundary region (data not shown). Homozygous Pax8neo/neo mice were born at a Mendelian frequency, then became severely growth-retarded and died at weaning age of agenesis of the thyroid gland (data not shown). As the same phenotype was described previously for another Pax8 null mutation (Mansouri et al. 1998), we refer to the Pax8neo gene in all subsequent experiments as Pax8− allele.
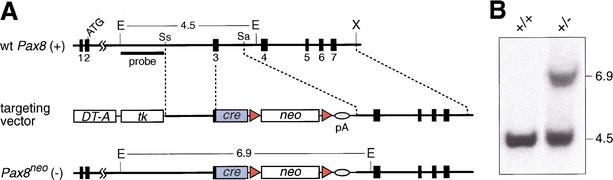
Figure 1.
Inactivation of the Pax8 locus by insertion of a cre gene. (A) Structure of the wild-type and targeted Pax8 loci. The cre gene was fused in frame to Pax8 exon 3 followed by a neomycin (neo) resistance gene and an SV40 polyadenylation site (pA). The herpes simplex virus thymidine kinase (tk) and diphteria toxin A (DT-A) genes were used for counterselection against random integration in ES cells. The neo expression cassette was flanked by frt sites (red arrowheads) that mediate deletion by the FLP recombinase. Correct targeting was verified by Southern blot analysis of EcoRI-digested DNA with the indicated probe. The lengths of DNA fragments are indicated in kilobases. The Pax8 exons are numbered according to Okladnova et al. (1997) with exon 3 coding for the N-terminal part of the paired domain. The Pax8neo allele codes for a fusion protein consisting of the first 12 amino acids of Pax8 (underlined) linked to the SV40 nuclear localization signal and the N-terminal Cre protein sequences (boldface type): MPHNSIRSGHGGPKKKRKVSNLL. E, EcoRI; N, NcoI; Sa, SacI; Ss, SspI. (B) Southern blot analysis of EcoRI-digested tail DNA from wild-type (+/+) and heterozygous (+/−) Pax8 mutant mice.
Cooperation of Pax2 and Pax8 in the development of the urogenital system
The Pax8 gene is known to be expressed together with Pax2 during mouse kidney development (Plachov et al. 1990; Dressler et al. 1990). Nevertheless, Pax8 mutant embryos develop a normal urogenital system (Fig. 2B) (Mansouri et al. 1998), whereas Pax2 mutant embryos fail to form a metanephros and genital tracts because of a defect in caudal elongation of the nephric duct (Torres et al. 1995; Favor et al. 1996; Bouchard et al. 2000). Therefore, it is conceivable that Pax2 may compensate for the loss of Pax8 in kidney development of Pax8−/− embryos. To test this hypothesis, we crossed Pax8+/− mice with Pax2+/− mice (Bouchard et al. 2000) to generate an allelic series of Pax2,Pax8 double-mutant embryos. Analysis of the urogenital system of 18.5-d embryos demonstrated that the metanephros of Pax2+/−Pax8+/− embryos was bilaterally reduced to only ∼25% of the size of control littermates (Fig. 2A,C). This reduction is more severe than in Pax2+/− embryos, which develop kidneys at ∼60% of the normal size (Porteous et al. 2000; data not shown). Apart from the hypoplastic kidney, all other components of the urogenital system developed normally in compound heterozygous embryos (Fig. 2C). Histological examination of Pax2+/−Pax8+/− kidneys revealed a perturbed architecture characterized by a reduced number and irregular arrangement of nephric tubules and glomeruli (Fig. 2E,F). The uninduced mesenchyme at the cortex and the mesenchymal stroma throughout the kidney were increased compared with control embryos, therefore demonstrating inefficient induction of tubules in compound heterozygous embryos. The remaining nephrons of Pax2+/−Pax8+/− kidneys were, however, functional as double-heterozygous animals survived for more than 18 mo.
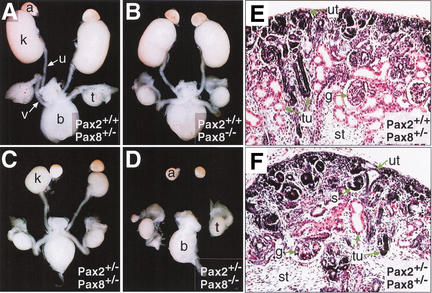
Figure 2.
Development of the urogenital system in Pax2,Pax8 mutant embryos. (A–D) The urogenital system of male E18.5 embryos of the indicated genotypes was dissected and photographed. The hypoplastic kidneys of Pax2+/−Pax8+/− embryos (C) were approximately fourfold smaller than control kidneys (A,B). Pax2+/−Pax8−/− embryos (D) failed to develop a kidney, ureter, and genital tract (vas deferens), whereas the adrenal gland, testis, and bladder formed normally. (E,F) Kidney sections stained with hematoxylin and eosin. The nephric tubules and glomeruli were reduced in number and the stromal component was increased in the hypoplastic Pax2+/−Pax8+/− kidney (F) compared with the control embryo (E) at E18.5. a, adrenal gland; b, bladder; g, glomerulus; k, kidney; s, S-shaped body; st, stroma; t, testis; tu, tubule; u, ureter; ut, ureteric tip; v, vas deferens.
A recurrent phenotype of Pax2+/−Pax8+/− females was vaginal atresia, which is characterized by the presence of a blind-ending vagina. The penetrance of this phenotype was 100% on a mixed C57BL/6x129/Sv genetic background, whereas it was 43% in C3H/He females (data not shown). Minor malformations of the open vagina were, however, still observed in compound heterozygous C3H/He females. A significant proportion of the Pax2+/−Pax8+/−males also failed to give rise to any progeny. Ductal obstruction of the genital tracts rather than a defect in sperm formation is the likely cause of this phenotype, as Pax2+/−Pax8+/− sperm could be used for in vitro fertilization of Pax2+/−Pax8+/− oocytes to generate viable off-spring after transplantation into foster mothers (data not shown). As a consequence of these genital phenotypes, Pax2,Pax8 double-mutant embryos could be generated only by crossing compound heterozygous C3H/He mice.
Pax2+/−Pax8−/− embryos entirely failed to form a metanephros, ureter, and genital tracts, indicating that a single wild-type Pax2 allele in a Pax8 mutant background is not sufficient to support the development of an adult kidney (Fig. 2D). This phenotype is similar to that of Pax2 mutant embryos (Torres et al. 1995; Bouchard et al. 2000) and is also caused by degeneration of the nephric duct during mesonephros development (data not shown). In summary, the analysis of compound Pax2,Pax8 mutant embryos unequivocally demonstrated that the transcription factors Pax2 and Pax8 cooperatively control the development of the urogenital system.
Pax2-independent initiation and maintenance of Pax8 expression during early kidney development
To better understand the cooperation of Pax2 and Pax8 in kidney patterning, we investigated the early expression pattern of the two Pax genes during pro- and mesonephros development by whole-mount in situ hybridization. Pax2 expression was initiated at the 8–9-somite stage in the intermediate mesoderm corresponding to the pronephric anlage (Fig. 3A). The expression of Pax8 was detected even earlier at the 6–7-somite stage in the same region of the intermediate mesoderm (Fig. 3B). Therefore, these data identify Pax8 as the earliest known marker of mouse pronephros development in analogy to our previous finding that Pax8 is the earliest gene to be expressed in the developing kidney of zebrafish embryos (Pfeffer et al. 1998). Moreover, the Pax2 and Pax8 genes were coexpressed in the pronephric anlage, as shown by double staining of 10-somite embryos (Fig. 3C). At the same stage, the Pax2/8-positive cells in the intermediate mesoderm did not yet express the epithelial marker laminin, indicating that these cells have not yet undergone the mesenchymal-epithelial transition leading to the formation of the pronephros (Fig. 3D).
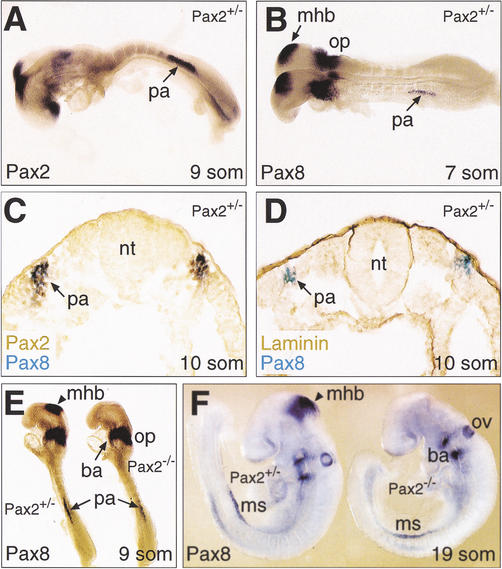
Figure 3.
Pax2-independent expression of Pax8 at the onset of kidney development. (A,B) Initiation of Pax gene expression in the pronephric anlage of Pax2+/− embryos. As shown by whole-mount in situ hybridization, Pax8 expression (B) was first detected at the 7-somite stage in the intermediate mesoderm at the level of the fifth and sixth somites corresponding to the pronephric anlage. At the 9-somite stage, the Pax2 gene (A) was also expressed in the pronephric anlage, where its expression was first observed at 8 somites (data not shown). (C) Coexpression of Pax2 and Pax8 in the pronephric anlage. Pax2 protein (brown) and Pax8 transcripts (blue) were simultaneously detected on a transverse section of a 10-somite Pax2+/− embryo by immunostaining and in situ hybridization, respectively. (D) Absence of epithelial cells in the pronephric anlage at 10 somites. An adjacent section of the same embryo shown in (C) was stained with an anti-laminin antibody (brown) in combination with Pax8 in situ hybridization (blue). (E,F) Pax2-independent initiation and maintenance of Pax8 expression during kidney development. Pax8 transcripts were detected by whole-mount in situ hybridization of Pax2+/− and Pax2−/− embryos at 9 (E) and 19 (F) somites. ba, branchial arch; mhb, midbrain-hindbrain boundary; ms, mesonephros; nt, neural tube; op, otic placode; ov, otic vesicle; pa, pronephric anlage; som, somite.
Pax2 is known to cross-regulate the Pax8 gene during midbrain-hindbrain boundary development (Ye et al. 2001; Fig. 3E). In contrast, the initiation of Pax8 expression in the pronephric anlage occurred independently of Pax2, as shown by in situ hybridization analysis of Pax2−/− embryos (Fig. 3E). The absence of Pax2 did also not affect the maintenance of Pax8 expression during mesonephros formation (Fig. 3F). Conversely, the kidney-specific expression of Pax2 must be independent of Pax8, as kidney morphogenesis is entirely normal in Pax8−/− mice (Fig. 2B) in contrast with Pax2−/− mice (Torres et al. 1995; Favor et al. 1996). We conclude, therefore, that the Pax2 and Pax8 genes are regulated independently of each other during early kidney development.
Absence of pro- and mesonephros development in Pax2−/−Pax8−/− embryos
The formation of a normal mesonephros in Pax2−/− embryos (Torres et al. 1995) suggests that the early and Pax2-independent expression of Pax8 may compensate for the loss of Pax2 at the onset of kidney development. To test this hypothesis, we analyzed the formation of the pro- and mesonephros in Pax2−/−Pax8−/− embryos. As the mutant Pax2− allele contained an in-frame lacZ gene insertion in the Pax2 locus (Bouchard et al. 2000), we used the expression of β-galactosidase as a kidney-specific marker to visualized the pro- and mesonephros. At the 15-somite stage, the β-galactosidase expression domain extended from the ninth somite to just beyond the last forming somite in the intermediate mesoderm of Pax2+/− or Pax2−/−embryos (Fig. 4A,B). This domain corresponds to the pronephros and newly forming mesonephros with its caudally extending nephric duct (Fig. 4A,B). Strong β-galactosidase expression in this region was still observed in the presence of a single wild-type Pax8 allele in Pax2−/−Pax8+/− embryos (Fig. 4C). In contrast, only weak β-galactosidase activity could be detected in the intermediate mesoderm of Pax2−/−Pax8−/− embryos, indicating that the pro- and mesonephros failed to properly form in the absence of any Pax2/8 protein.
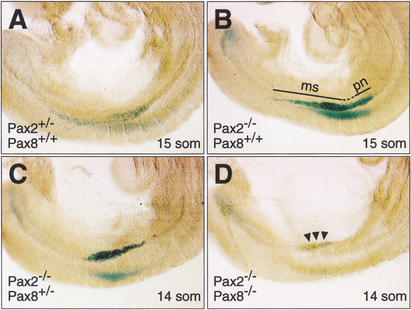
Figure 4.
Early defects of pro- and mesonephros development in Pax2−/−Pax8−/− embryos. (A–D) The expression of β-galactosidase from the mutant Pax2− allele (Bouchard et al. 2000) was used as a kidney-specific marker to visualize pro- and mesonephros development in Pax2,Pax8 mutant embryos of the indicated genotypes. Embryos of the same litter, which developed to the 14- (C,D) or 15- (A,B) somite stage, were stained with X-gal for the same period of time (15 h). Pax2+/− (A) and Pax2−/− (B) embryos expressed β-galactosidase activity in the pronephros (pn) and mesonephros (ms) extending from somite 9 to just beyond the last somite, 15. The β-galactosidase expression in Pax2−/−Pax8+/− embryos (C) was restricted to a region between somites 9 and 13. The absence of any functional Pax2/8 allele led to the down-regulation of β-galactosidase expression (arrowheads) in Pax2−/−Pax8−/− embryos (D). It is important to note that the presence of a single Pax2− (lacZ) allele resulted in a disproportionately lower X-gal staining signal in Pax2+/− embryos (A) compared with Pax2−/− embryos (B,C), which expressed the lacZ gene only at a twofold higher level because of the presence of two lacZ alleles. This nonlinearity of the β-galactosidase assay is also the reason why the reduced lacZ expression in Pax2−/−Pax8−/− embryos (D) generated a low X-gal staining signal, although it could be readily detected by the more sensitive, but nonquantitative antibody staining method (Fig. 6H).
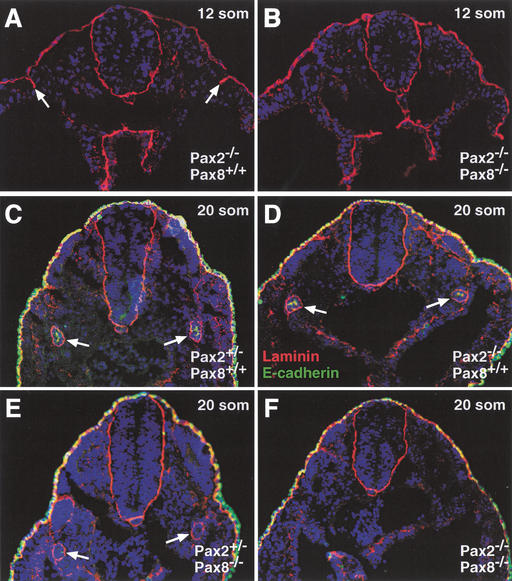
The onset of pronephros formation is characterized by the mesenchymal-epithelial transition of specific mesodermal cells, which leads to the formation of the nephric duct (Saxén 1987). We therefore investigated the developmental defect in Pax2−/−Pax8−/− embryos by analyzing the expression of the epithelial marker laminin in the intermediate mesoderm. Although laminin expression was not yet observed at 10 somites (Fig. 3D), a stripe of laminin-positive cells could be detected at 12 somites in the intermediate mesoderm of “control” Pax2−/− embryos (Fig. 5A). Later, at the 20-somite stage, laminin expression was detected in a ring consisting of the basement membranes of the nephric duct in all embryos that carried at least one functional Pax2/8 allele (Fig. 5C–E). In marked contrast, laminin expression was detected neither at the 12- nor 20-somite stage in the intermediate mesoderm of Pax2−/−Pax8−/− embryos (Fig. 5B,F). Moreover, E-cadherin, a second epithelial marker, was expressed near the luminal surface of the nephric duct in Pax2+/− and Pax2−/− embryos at 20 somites (Fig. 5C,D). Although E-cadherin expression was already reduced by lowering the Pax protein dose in Pax2+/−Pax8−/− embryos (Fig. 5E), it was never detected in the intermediate mesoderm of Pax2−/−Pax8−/− embryos (Fig. 5F). We conclude therefore that Pax2 and Pax8 together control the onset of pronephros development by regulating the mesenchymal-epithelial transition of intermediate mesoderm cells.
Figure 5.
Absence of mesenchymal-epithelial transitions in the intermediate mesoderm of Pax2−/−Pax8−/− embryos. The expression of the epithelial markers laminin (red) and E-cadherin (green) was analyzed in embryos of the indicated genotypes at 12 (A,B) or 20 (C–F) somites by immunostaining of transverse sections. At 12 somites, epithelial cells were bilaterally present in the pronephric region of Pax2−/−Pax8+/+ embryos (arrows in A) in contrast with Pax2−/−Pax8−/− embryos (B). At the 20-somite stage, a distinct ring of laminin expression demarcates the nephric duct of all mutant embryos (arrows in C–E) except in Pax2−/−Pax8−/− embryos (F). E-cadherin expression was reduced in the nephric duct of Pax2+/−Pax8−/− embryos (E; additional data not shown) and absent in Pax2−/−Pax8−/− embryos (F).
Absence of nephric gene expression in Pax2−/−Pax8−/− embryos
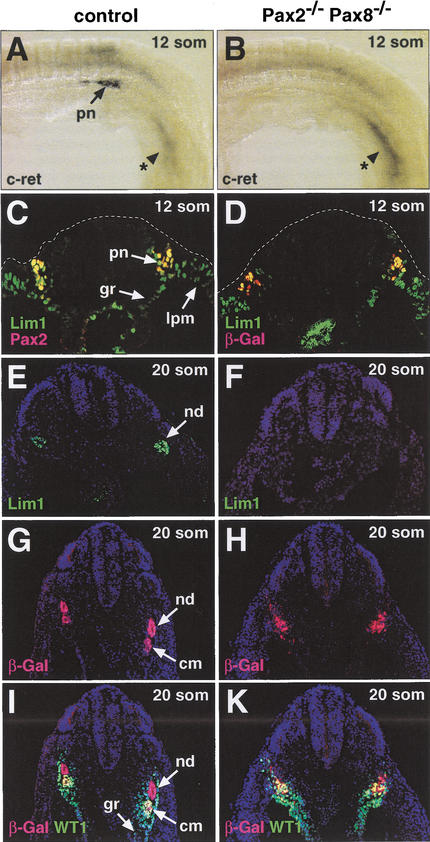
We next examined the expression of early kidney-specific genes in Pax2,Pax8 double-mutant embryos. The c-ret gene is one of the first genes to be specifically expressed in the nephric duct of the pro- and mesonephros (Pachnis et al. 1993). At 12 somites, high c-ret expression was detected at the caudal end of the pronephros adjacent to the last forming somite in wild-type embryos (Fig. 6A). In contrast, the Pax2−/−Pax8−/− embryos entirely failed to express the c-ret gene in the intermediate mesoderm at this early stage (Fig. 6B) as well as at 20 somites (data not shown). The Lim1 gene is initially transcribed throughout the lateral mesoderm (Barnes et al. 1994; Fujii et al. 1994; Tsang et al. 2000), as shown by its expression in the lateral plate mesoderm, intermediate mesoderm, and genital ridge at the 12-somite stage (Fig. 6C). At this early time point, Lim1 is coexpressed with Pax2 or β-galactosidase in the pronephric anlage of control or Pax2−/−Pax8−/− embryos, respectively (Fig. 6C,D). Thereafter, the broad Lim1 expression becomes restricted to the mesonephric duct at 20 somites (Fig. 6E) and is subsequently also observed in the condensing mesenchyme and nephric tubules (Barnes et al. 1994; Fujii et al. 1994; Tsang et al. 2000). At 20 somites, Lim1 expression could, however, not be detected in the intermediate mesoderm of Pax2−/−Pax8−/− embryos (Fig. 6F). In contrast, β-galactosidase-positive cells were readily identified in double-mutant embryos both at 12 and 20 somites (Fig. 6D,H). Therefore, the lack of c-ret and Lim1 expression at these early stages was not caused by a selective loss of intermediate mesoderm cells, but rather indicates a complete failure of pro- and mesonephros formation in the combined absence of Pax2 and Pax8.
Figure 6.
Absence of Lim1 and c-Ret expression in the intermediate mesoderm of Pax2−/−Pax8−/− embryos. (A–D) c-Ret and Lim1 expression at the 12-somite stage. c-Ret transcripts were detected by whole-mount in situ hybridization in the pronephros of wild-type control embryos (A) in contrast to Pax2−/−Pax8−/− embryos (B) that, however, still expressed c-ret in the tail region (indicated by asterisk). Antibody staining of transverse sections was used to reveal the expression of Lim1 and Pax2 or β-galactosidase (β-Gal) in control Pax8+/− (C) and Pax2−/−Pax8−/− (D) embryos, respectively. (E–K) Lim1 and WT1 expression at the 20-somite stage. Adjacent transverse sections of control Pax2+/−and Pax2−/−Pax8−/− embryos were analyzed by immunostaining for Lim1 (E,F), β-Gal (G–K) and WT1 (I,K) protein expression. cm, condensing mesenchyme; gr, genital ridge; lpm, lateral plate mesoderm; nd, nephric duct; pn, pronephros.
At 20 somites, the nephrogenic mesenchyme has just started to condense to form the tubules of the mesonephros in a process that is initiated by signals from the nephric duct (Saxén 1987). We have visualized this process by double staining of control Pax2+/− embryos for β-galactosidase (Pax2) and WT1 expression, as the WT1 gene is expressed in the intermediate mesoderm and genital ridge (Fig. 6I) and Pax2 (β-Gal) in the nephric duct and condensing mesenchyme (Fig. 6I). Three distinct cell populations could be visualized in control Pax2+/− embryos by this procedure: the WT1−β-Gal+ nephric duct (red), the WT1+β-Gal+ condensing mesenchyme (yellow), and the WT1+β-Gal− genital ridge and intermediate mesoderm (green; Fig. 6I). Although WT1−β-Gal+ (red) and WT1+β-Gal+ (yellow) cells were also present in Pax2−/−Pax8−/− embryos, they were intermingled without any apparent patterning (Fig. 6K), indicating that these cells neither formed a nephric duct nor condensing mesenchyme in the absence of Pax2 and Pax8. Therefore, the lack of kidney-specific gene expression and nephric structures in Pax2−/−Pax8−/− embryos points to an essential role of Pax2 and Pax8 in committing mesodermal cells to the kidney fate.
Late apoptosis of mesodermal cells in the absence of nephric induction
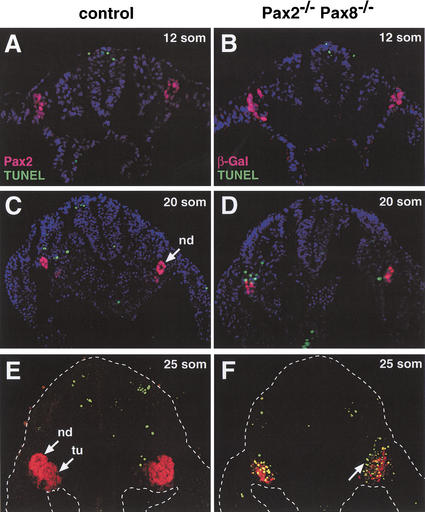
Several studies have implicated Pax2 in the control of cell survival during metanephros development (Bouchard et al. 2000; Ostrom et al. 2000; Porteous et al. 2000; Torban et al. 2000). We therefore assessed by combined TUNEL and Pax2 (β-Gal) staining whether increased apoptosis may also contribute to the early kidney developmental defects in Pax2−/−Pax8−/− embryos. No apoptotic cells could be detected, however, within or near the Pax2 (β-Gal) expression domain in the intermediate mesoderm of control and Pax2−/−Pax8−/− embryos at the 12-somite stage (Fig. 7A,B), when the pronephros just starts to form in wild-type embryos (Fig. 5A). At 20 somites, a slight increase in apoptotic cells was observed in Pax2−/−Pax8−/− embryos (Fig. 7C,D), whereas apoptosis was massively induced in these embryos at 25 somites (Fig. 7E,F). In the absence of Pax2 and Pax8, almost all β-galactosidase-positive cells were undergoing apoptosis (Fig. 7F) in striking contrast with control embryos, where apoptotic cells could hardly be detected in the mesonephros and surrounding mesoderm at 25 somites (Fig. 7E). Interestingly, ∼20% of the apoptotic signals in Pax2−/−Pax8−/− embryos were observed outside of the β-galactosidase expression domain (Fig. 7D,F), thereby raising the possibility that Pax2 and Pax8 may control the survival of adjacent mesodermal cells by a non-cell-autonomous mechanism. Importantly, however, the cells of the intermediate mesoderm continued to proliferate normally during early kidney development even in the absence of Pax2 and Pax8 (data not shown). Together, these data indicate that a relatively long time period (∼24 h) elapses from the onset of Pax2/8 expression (at 6–8 somites) to the activation of fulminant apoptosis (at 25 somites) in Pax2−/−Pax8−/− embryos. This late induction of apoptosis therefore points to a more indirect role of Pax2 and Pax8 in controlling the survival of intermediate mesoderm cells. Importantly, the loss of intermediate mesoderm cells did not affect normal development of the gonads and limbs in Pax2−/−Pax8−/− embryos (data not shown).
Figure 7.
Late apoptosis of intermediate mesoderm cells in Pax2−/−Pax8−/− embryos. Cells undergoing apoptosis (green dots) were detected on transverse sections of control or Pax2−/−Pax8−/− embryos by TUNEL assay in combination with immunohistochemical analysis of Pax2 (red; A,C,E) or β-galactosidase (red; B,D,F) expression. Apoptotic cells within or adjacent to the β-Gal (Pax2) expression domain were absent in Pax2−/−Pax8−/− embryos at 12 somites (B), slightly increased at 20 somites (D) and abundant at 25 somites (F) compared with the control Pax2+/−(C) and Pax8+/− (A,E) embryos. An arrow in F points to apoptotic cells surrounding the β-Gal expression domain. nd, nephric duct; tu, tubules.
Ectopic kidney formation by Pax2 misexpression
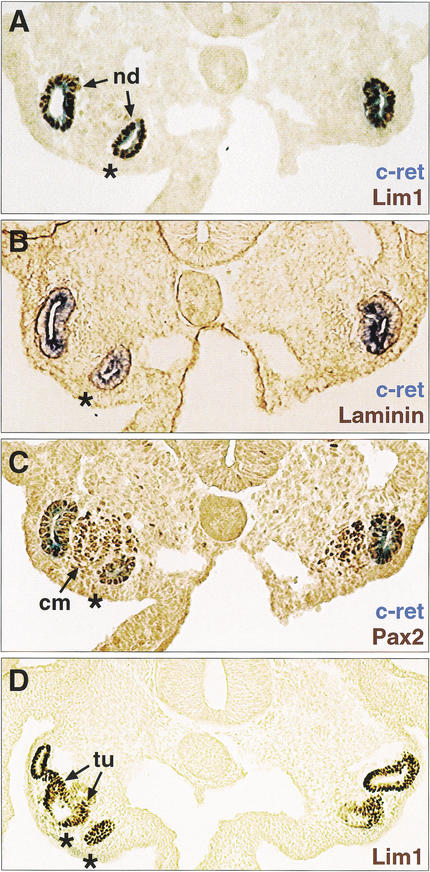
So far we have shown by loss-of-function analysis in mouse embryos that Pax2 and Pax8 are necessary for pro- and mesonephros development. We next performed gain-of-function experiments in chick embryos to investigate whether Pax2 expression is also sufficient to promote early kidney development. To this end, a replication-competent retrovirus expressing the mouse Pax2b protein (RCAS-mPax2) was unilaterally injected at HH stages 4–6 into the mid-streak tissue of chick embryos, which subsequently gave rise, among other tissues, to the intermediate mesoderm. Two days after injection, the nephric structures were visualized by analyzing the kidney-specific expression of c-ret, Lim1, laminin, and Pax2 (Fig. 8). The formation of an ectopic nephric duct with its associated condensing mesenchyme was observed in 19% (n = 13) of 70 embryos that survived the injection of the RCAS-mPax2 virus (Fig. 8). The ectopic nephric duct developed in a mirror image-like fashion in the region of the genital ridge (Fig. 8) or intermediate mesoderm (data not shown) and extended on average for a length of 50–150 μm, as shown by serial sectioning. Importantly, we could never detect any ectopic nephric structures in embryos (n = 32) that were injected with the control virus RCAS-AP (Fekete and Cepko 1993; data not shown). We therefore conclude that the Pax2/8 proteins are not only necessary, but also sufficient for committing mesodermal cells to the nephric lineage.
Figure 8.
Ectopic kidney formation on Pax2 misexpression in the intermediate mesoderm. A chicken retrovirus expressing the mouse Pax2 protein (RCAS–mPax2) was injected into the mid-streak tissue adjacent to Hensen's node on one side of chick embryos at HH stage 4–6. (A–C) Ectopic kidney formation in a representative embryo at 48 h after injection (HH stage 17–18). Adjacent sections were stained by in situ hybridization for c-ret mRNA expression (blue; A–C) and by immunohistochemistry (brown) for Lim1 (A), laminin (B) or Pax2 (C) protein expression. (D) Lim1 staining of an embryo that progressed further in kidney development. Asterisks denote ectopic nephric ducts and tubules that formed in the region of the genital ridge on the injected side (left). cm, condensing mesenchyme; nd, nephric duct; tu, tubules.
Discussion
Although a large number of genes have been identified as regulators of kidney organogenesis, targeted mutagenesis has failed to demonstrate an essential role for any of these genes in controlling the earliest steps of kidney development, that is, the formation of the pro- and mesonephros (Davies and Brändli 2002). Most notably, mutation of the Pax2 gene interfered with metanephros development (Torres et al. 1995; Favor et al. 1996), whereas kidney morphogenesis was entirely normal in Pax8-deficient mice (Mansouri et al. 1998). Here we demonstrate that Pax2 and Pax8 have redundant functions in kidney organogenesis in agreement with the fact that members of the Pax2/5/8 family can substitute for each other in mouse development because of their equivalent transcriptional activity (Bouchard et al. 2000). Pax2 and Pax8 are not only coexpressed at the onset of kidney development, but together are also required for the formation of the pro- and mesonephros. In the absence of both transcription factors, the intermediate mesoderm was unable to undergo the mesenchymal-epithelial transition necessary for nephric duct formation and failed to express early markers of nephric identity such as c-Ret and Lim1. Conversely, misexpression of Pax2 was sufficient to induce ectopic nephric structures in the intermediate mesoderm and genital ridge. Therefore, both gain- and loss-of-function analyses identified Pax2 and Pax8 as critical regulators of nephric lineage specification.
The first morphological sign of pronephros development is the conversion of mesenchymal cells in the intermediate mesoderm to epithelial cells developing into the pronephric duct (Saxén 1987). This initial step of kidney development completely failed in Pax2,Pax8 double-mutant embryos, thereby preventing the formation of the nephric duct and its associated tubules. Pax2 also has an important role in controlling mesenchymal-epithelial transitions during adult kidney morphogenesis, as the inhibition of Pax2 expression by antisense oligonucleotides prevented the condensation and epithelial conversion of mesenchymal cells in mouse kidney organ cultures (Rothenpieler and Dressler 1993). Likewise, the epithelial differentiation of the pronephric duct was abnormal in Pax2.1-deficient (noi) zebrafish embryos (Majumdar et al. 2000). Therefore, the Pax2/8 proteins appear to control the gene expression program responsible for mesenchymal-epithelial conversion not only at the onset but also throughout kidney development.
A striking, but late aspect of the Pax2,Pax8 double-mutant phenotype was the fulminant apoptosis of the intermediate mesoderm in 25-somite embryos (E9.5). In agreement with this, Pax2 has been implicated previously in the survival control of nephric cells, as its twofold reduced expression in Pax2+/− embryos led to a moderate increase in apoptosis during metanephros development (Bouchard et al. 2000; Ostrom et al. 2000; Porteous et al. 2000). In contrast, the complete absence of Pax2 and Pax8 resulted in the death of all β-galactosidase-positive cells in the intermediate mesoderm, although this process was delayed by ∼24 h compared with the initiation of Pax2 (β-Gal) and Pax8 expression in the pronephric anlage at 6–8 somites. Interestingly, the region undergoing cell death extended beyond the domain of Pax2 (β-Gal) expression, indicating that the Pax2/8 proteins control the survival of the adjacent mesoderm in an non-cell-autonomous manner. Candidates for such a non-cell-autonomous signal could be secreted molecules of the EGF, FGF, or BMP families, which are known to maintain the survival of the nephrogenic mesenchyme (Coles et al. 1993; Perantoni et al. 1995; Godin et al. 1998; Dudley et al. 1999).
As each Pax protein fulfills multiple roles in development, it is able to initiate an organ-specific gene expression program only in cooperation with local transcription factors (. Misexpression of Pax5 throughout the entire hematopoietic system was able to bias lineage commitment toward the B cell pathway only within the lymphoid progenitor cell compartment (Bouchard et al. Souabni et al. 2002). Similarly, the kidney-inducing potential of Pax2 was temporally and spatially restricted, as the Pax2 retrovirus had to be delivered to the lateral mesoderm before somitogenesis and could then induce ectopic kidneys only in a competence region corresponding to the intermediate mesoderm and genital ridge (data not shown). Homeodomain transcription factors of the Lim protein family are likely cofactors that cooperate with the Pax2/8 proteins in the specification of the nephric lineage. During early somitogenesis, the Lim1 gene is expressed throughout the lateral mesoderm including the genital ridge and intermediate mesoderm (Barnes et al. 1994; Fujii et al. 1994; Tsang et al. 2000) and is initially expressed in the pronephric anlage independently of Pax2 and Pax8. Thereafter, the widespread Lim1 expression is rapidly restricted to the nephrogenic cord (Barnes et al. 1994; Fujii et al. 1994; Tsang et al. 2000), where it overlaps with and becomes dependent on Pax2 and Pax8. Importantly, enforced expression of Lim1 or Pax8 alone was able to induce ectopic pronephric structures only at a low frequency in Xenopus embryos (Carroll and Vize 1999). In contrast, ectopic expression of Lim1 together with Pax8 synergistically activated pronephros development in frog embryos (Carroll and Vize 1999). This finding, together with our data, suggests that Lim1 acts as a competence factor to determine the nephric field, within which the local induction of Pax2 and Pax8 specifies the kidney fate.
To date, little is known about Pax2/8 target genes that mediate the function of these two transcription factors during early kidney development. One of them codes for the secreted molecule GDNF (Brophy et al. 2001), which is essential for normal morphogenesis of the adult kidney (Moore et al. 1996; Pichel et al. 1996; Sánchez et al. 1996). Interestingly, the expression of c-Ret, the signaling receptor for GDNF, was never initiated in the intermediate mesoderm of Pax2−/−Pax8−/− embryos, suggesting that the GDNF–Ret pathway is regulated at multiple levels by the Pax2/8 proteins. GDNF signaling proved to be sufficient for guiding the caudal migration of the pronephric duct in Axolotl embryos (Drawbridge et al. 2000), consistent with the early expression of c-ret during kidney development (Pachnis et al. 1993). In contrast, targeted mutagenesis in the mouse embryo has identified only a later role of the GDNF-Ret signaling pathway in controlling the outgrowth and branching of the ureter during metanephros development (Schuchardt et al. 1994; Moore et al. 1996; Pichel et al. 1996; Sánchez et al. 1996).
The role of Pax2 and Pax8 in nephric lineage specification appears to be conserved in vertebrate evolution, as these two genes are expressed as the earliest kidney-specific markers in the intermediate mesoderm during mouse, frog, and zebrafish development (Fig. 3; Pfeffer et al. 1998; Heller and Brändli 1999). Moreover, the mutually independent initiation of Pax2 and Pax8 expression in the pronephric anlage has also been conserved between mouse and zebrafish (Fig. 3; Pfeffer et al. 1998), indicating that the two genes respond to the same signals from the surrounding tissue. Using microsurgical manipulation of the chick embryo, Obara-Ishihara et al. (1999) identified the surface ectoderm as the source of a BMP4 signal that activates Pax2 expression and pronephros development in the underlying intermediate mesoderm. The study of Mauch et al. (2000) demonstrated that yet unidentified signals from the paraxial mesoderm are both necessary and sufficient for Pax2 activation and pronephros induction. Based on these data, it is conceivable that signals emanating from perpendicular sources (ectoderm vs. somite) may induce at their intersection the expression of Pax2 and Pax8 in a discrete group of mesodermal cells. As the specification of the nephric lineage is a direct consequence of the initiation of Pax2/8 expression (as shown by this study), it will be important to gain molecular insight into the regulation of these two genes by identifying their essential kidney-specific enhancers and upstream regulatory factors, using transgenic approaches (Pfeffer et al. 2002).
The functional analyses of other Pax genes have revealed a fundamental role for this class of transcription factors in cell lineage specification during organ development (for review, see . For instance, Pax5 is essential for the formation of B-lymphocytes within the hematopoietic system (Bouchard et al. Nutt et al. 1999), Pax8 for follicular cells in the thyroid gland (Mansouri et al. 1998), Pax4 for insulin-producing β-cells, and somatostatin-expressing δ-cells in the endocrine pancreas (Sosa-Pineda et al. 1997), Pax6 for the glucagon-synthesizing α-cells (St-Onge et al. 1997; Sander et al. 1997), and Pax7 for myogenic progenitor (satellite) cells in adult skeletal muscle (Seale et al. 2000). The specification of the nephric lineage by Pax2 and Pax8 is therefore consistent with the more general role of Pax proteins in cell fate determination during organogenesis. It will be interesting to see whether Pax2 and Pax8 restrict the fate of the mesodermal progenitor cells to the nephric lineage by simultaneously activating the expression of kidney-specific genes and repressing the transcriptional program of alternative cell lineages similar to the role of Pax5 in B cell commitment (Nutt et al. 1999).
Materials and methods
Generation of Pax8 mutant mice
The Pax8 targeting vector was assembled in a pSP64 plasmid containing a polylinker with appropriate restriction sites. A 1.6-kb SspI–NcoI fragment (partially digested with NcoI; intron 2) and a 3.8-kb SacI–XbaI fragment (exon 4–7) were cloned as 5′ and 3′ homology regions from a mouse Pax8 cosmid into the modified pSP64 vector. The cre gene was inserted as a 1.1-kb EarI–MluI fragment from pMC–Cre by the use of an adaptor sequence restoring the Pax8 reading frame at the NcoI site in exon 3. A 1.9-kb SacI–SalI DNA fragment containing the PGK–neo expression cassette (flanked by frt sites) from pM30 (Meyers et al. 1998) was cloned downstream of the cre gene, whereas the HSV-tk and DT-A genes (negative selection) were inserted upstream of the 5′ homology region. NotI-linearized DNA (15 μg) was electroporated into E14.1 ES cells (1 × 107) followed by selection with 250 μg/mL G418 and 2 μM gancyclovir. Individual clones were screened for homologous recombination by nested PCR, and positive clones were verified by Southern blot analysis of EcoRI-digested DNA with an external 1.4-kb EcoRI–SspI probe (intron 2). Three correctly targeted ES cell clones were injected into C57BL/6 blastocysts, and chimeric males were mated with C57BL/6 females to obtain Pax8neo/+ offspring. The different Pax8 alleles were genotyped by PCR with the following primers: 5′-TCTCCACTCCAACATGTCTGC-3′ (Pax8 intron 2), 5′-CCCTCCTAGTTGATTCAGCCC-3′ (Pax8 exon 3), and 5′-AGCTGGCCCAAATGTTGCTGG-3′ (cre gene). The wild-type and Pax8 mutant alleles gave rise to PCR products of 389 and 673 bp, respectively. The Pax8neo allele (referred to as Pax8− allele) was backcrossed into the C3H/He genetic background for at least four generations and then crossed into Pax2+/− C3H/He mice (Bouchard et al. 2000) to generate double-mutant mice.
In situ hybridization analysis
Mouse and chick embryos were processed for whole-mount in situ hybridization with digoxigenin-labeled antisense RNA probes as described (Henrique et al. 1995). The mouse Pax2, Pax8, and c-ret probes have been described (Adams et al. 1992; Pachnis et al. 1993). The chicken c-ret probe was generated by RT-PCR cloning of a 1.4-kb cDNA from embryonic head RNA, using the primers 5′-GCGGGGCTTCCTTTGGTCT GT-3′ and 5′-ATGTTTCCTGCTCTGCTTGTC-3′ (accession no. Z49898). In situ hybridization was combined with immunohistochemical analysis by first performing the in situ hybridization reaction on whole-mount embryos followed by cryosectioning and processing of the 10 μm sections for antibody staining.
Immunohistochemical analysis
Embryos or dissected tissues were fixed and processed for histological or immunohistochemical analysis as described (Bouchard et al. 2000). The following antibodies were used for immunostaining: rabbit anti-Pax2 Ab (1:100 dilution; Covance); rabbit anti-laminin Ab (1:500; Sigma); rabbit anti-β-galactosidase Ab (1:1000; Cortex Biochem); rat anti-E-cadherin Ab (1:200; Zymed Laboratories); mouse anti-Lim1/2 Ab (1:1000; Developmental Studies Hybridoma Bank); and mouse anti-WT1 Ab (1:100; Dako). Secondary reagents used for detection were the anti-rabbit Vectastain kit (Vector Labs) or Alexa488- or Alexa546-labeled anti-mouse, anti-rabbit, or anti-rat antibodies (1:200; Molecular Probes). DAPI was used for counterstaining at 50 μg/mL in Slow Fade Light mounting medium (Molecular Probes).
β-Galactosidase and TUNEL staining
β-Galactosidase activity was detected by X-gal staining of whole-mount embryos as described (Pfeffer et al. 2000). The fluorescein in situ cell death detection kit (Roche, Mannheim) was used for TUNEL analysis of cryosections that were stained before with a rabbit anti-Pax2 or rabbit anti-β-galactosidase antibody.
Retroviral infection of chick embryos
The mouse Pax2 gene was ectopically expressed in chick embryos by infection with the replication-competent retrovirus RCASBP(A) (Morgan and Fekete 1996) carrying the mPax2b cDNA. The mPax2 coding sequence was PCR-modified by converting the sequence containing the translation start codon into an NcoI site, cloned as a NcoI–HindIII fragment into the shuttle vector Cla12Nco (Hughes et al. 1987) and transferred as a ClaI fragment into the retroviral vector RCASBP(A). The RCAS–mPax2 virus was produced at a titer of 2 × 109 infectious units/mL as described (Morgan and Fekete 1996) and was injected unilaterally next to Hensen's node into the mid-streak tissue of embryos at HH stages 4–6. Embryos were harvested 48 h post-injection (at HH stages 17–18), and the expression of kidney-specific genes was analyzed by whole-mount in situ hybridization and immunohistochemistry. The viral infection and ectopic Pax2 expression was monitored by immunostaining with the anti-Gag antibody AMV3C2 (1:1000; Developmental Studies Hybridoma Bank) and an anti-mouse Pax2 antibody (1:200; Covance), respectively. Pathogen-free fertilized White Leghorn eggs (SPAFAS) were obtained from Charles River (Sulzfeld, Germany) and incubated at 37.5°C in a rocking incubator. Embryos were staged according to Hamburger and Hamilton (1992).
Accession number
The mouse Pax8 gene sequences were submitted to GenBank (accession no. AY157583).
Acknowledgments
We thank A. Mansouri and Z. Kozmik for Pax8 DNA clones; C. Theuβl for blastocyst injection; G. Schaffner for DNA sequencing; and C. Hartmann for critical reading of the manuscript. This research was supported by Boehringer Ingelheim and by the Austrian Science Foundation (grant P13601-GEN).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL busslinger@nt.imp.univie.ac.at; FAX 43-1-798-9370.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.240102.
References
- Adams B, Dörfler P, Aguzzi A, Kozmik Z, Urbánek P, Maurer-Fogy I, Busslinger M. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 1992;6:1589–1607. doi: 10.1101/gad.6.9.1589. [DOI] [PubMed] [Google Scholar]
- Barnes JD, Crosby JL, Jones CM, Wright CVE, Hogan BLM. Embryonic expression of Lim-1, the mouse homolog of Xenopus XLim-1, suggests a role in lateral mesoderm differentiation and neurogenesis. Dev Biol. 1994;161:168–178. doi: 10.1006/dbio.1994.1018. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Pfeffer P, Busslinger M. Functional equivalence of the transcription factors Pax2 and Pax5 in mouse development. Development. 2000;127:3703–3713. doi: 10.1242/dev.127.17.3703. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Schleiffer A, Eisenhaber F, Busslinger M. Evolution and function of Pax genes. In: Cooper D, editor. Encyclopedia of the Human Genome. UK: Nature Publishing Group; 2003. . (In Press.) [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–4756. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Vize PD. Synergism between Pax-8 and lim-1 in embryonic kidney development. Dev Biol. 1999;214:46–59. doi: 10.1006/dbio.1999.9414. [DOI] [PubMed] [Google Scholar]
- Coles HS, Burne JF, Raff MC. Large-scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: Bipartite structure of the paired domain and its binding site. Genes & Dev. 1993;7:2048–2061. doi: 10.1101/gad.7.10.2048. [DOI] [PubMed] [Google Scholar]
- Davies, J.A. and Brändli, A.W. 2002. The Kidney Development Database. http://golgi.ana.ed.ac.uk/kidhome.html.
- Donovan MJ, Natoli TA, Sainio K, Amstutz A, Jaenisch R, Sariola H, Kreidberg JA. Initial differentiation of the metanephric mesenchyme is independent of WT1 and the ureteric bud. Dev Genet. 1999;24:252–262. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<252::AID-DVG8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Drawbridge J, Meighan CM, Mitchell EA. GDNF and GFRa-1 are components of the axolotl pronephric duct guidance system. Dev Biol. 2000;228:116–124. doi: 10.1006/dbio.2000.9934. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes & Dev. 1999;13:1601–1613. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favor J, Sandulache R, Neuhäuser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Spörle R, et al. The mouse Pax21Neu mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci. 1996;93:13870–13875. doi: 10.1073/pnas.93.24.13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H. Expression patterns of the murine LIM class homeobox gene Lim1 in the developing brain and excretory system. Dev Dyn. 1994;199:73–83. doi: 10.1002/aja.1001990108. [DOI] [PubMed] [Google Scholar]
- Godin RE, Takaesu NT, Robertson EJ, Dudley AT. Regulation of BMP7 expression during kidney development. Development. 1998;125:3473–3482. doi: 10.1242/dev.125.17.3473. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of chick embryos. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Heller N, Brändli AW. Xenopus Pax-2/5/8 orthologues: Novel insights into Pax gene evolution and identification of Pax-8 as the earliest marker of otic and pronephric cell lineages. Dev Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: Cellular and molecular regulation. Mech Dev. 2000;92:31–45. doi: 10.1016/s0925-4773(99)00323-8. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nature Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- Mauch TJ, Yang G, Wright M, Smith D, Schoenwolf GC. Signals from trunk paraxial mesoderm induce pronephros formation in chick intermediate mesoderm. Dev Biol. 2000;220:62–75. doi: 10.1006/dbio.2000.9623. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nature Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development. 1999;126:1103–1108. doi: 10.1242/dev.126.6.1103. [DOI] [PubMed] [Google Scholar]
- Okladnova O, Poleev A, Fantes J, Lee M, Plachov D, Horst J. The genomic organization of the murine Pax 8 gene and characterization of its basal promoter. Genomics. 1997;42:452–461. doi: 10.1006/geno.1997.4735. [DOI] [PubMed] [Google Scholar]
- Ostrom L, Tang MJ, Gruss P, Dressler GR. Reduced Pax2 gene dosage increases apoptosis and slows the progression of renal cystic disease. Dev Biol. 2000;219:250–258. doi: 10.1006/dbio.2000.9618. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Perantoni AO, Dove LF, Karavanova I. Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci. 1995;92:4696–4700. doi: 10.1073/pnas.92.10.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: Dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Bouchard M, Busslinger M. Pax2 and homeodomain proteins regulate a 435 bp enhancer of the mouse Pax5 gene at the midbrain-hindbrain boundary. Development. 2000;127:1017–1028. doi: 10.1242/dev.127.5.1017. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Payer B, Reim G, Pasca di Magliano M, Busslinger M. The activation and maintenance of Pax2 expression at the mid-hindbrain boundary is controlled by separate enhancers. Development. 2002;129:307–318. doi: 10.1242/dev.129.2.307. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng H-Z, Granholm A-C, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110:643–651. doi: 10.1242/dev.110.2.643. [DOI] [PubMed] [Google Scholar]
- Porteous S, Torban E, Cho N-P, Cunliffe H, Chua L, McNoe L, Ward T, Souza C, Gus P, Giugliani R, et al. Primary renal hypoplasia in humans and mice with PAX2 mutations: Evidence of increased apoptosis in fetal kidneys of Pax21Neu +/− mutant mice. Hum Mol Genet. 2000;9:1–11. doi: 10.1093/hmg/9.1.1. [DOI] [PubMed] [Google Scholar]
- Rothenpieler UW, Dressler GR. Pax-2 is required for mesenchyme-to-epithelium conversion during kidney development. Development. 1993;119:711–720. doi: 10.1242/dev.119.3.711. [DOI] [PubMed] [Google Scholar]
- Sainio K, Hellstedt P, Kreidberg JA, Saxén L, Sariola H. Differential regulation of two sets of mesonephric tubules by WT-1. Development. 1997;124:1293–1299. doi: 10.1242/dev.124.7.1293. [DOI] [PubMed] [Google Scholar]
- Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubüser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes & Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Saxén L. Organogenesis of the kidney. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Behringer RR. Requirement for Lim1 in head-organizer function. Nature. 1995;374:425–430. doi: 10.1038/374425a0. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing b cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Souabni, A., Cobaleda, C., Schebesta, M., and Busslinger, M. 2002. Pan-hematopoietic expression of Pax5 (BSAP) promotes B cell development at the expense of T-lymphopoiesis and erythroblast formation. Immunity (In press). [DOI] [PubMed]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing a-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Torban E, Eccles MR, Favor J, Goodyer PR. PAX2 suppresses apoptosis in renal collecting duct cells. Am J Pathol. 2000;157:833–842. doi: 10.1016/S0002-9440(10)64597-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gómez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PPL. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol. 2000;223:77–90. doi: 10.1006/dbio.2000.9733. [DOI] [PubMed] [Google Scholar]
- Vainio S, Müller U. Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. Cell. 1997;90:975–978. doi: 10.1016/s0092-8674(00)80363-3. [DOI] [PubMed] [Google Scholar]
- Ye W, Bouchard M, Stone D, Luo X, Vella F, Lee J, Nakamura H, Ang S-L, Busslinger M, Rosenthal A. Distinct regulators control the induction, positioning and maintenance of the mid-hindbrain organizer signal FGF8. Nature Neurosci. 2001;4:1175–1181. doi: 10.1038/nn761. [DOI] [PubMed] [Google Scholar]