Abstract
Knudson's two-hit hypothesis postulates that genetic alterations in both alleles are required for the inactivation of tumor-suppressor genes. Genetic alterations include small or large deletions and mutations. Over the past years, it has become clear that epigenetic alterations such as DNA methylation are additional mechanisms for gene silencing. Restriction Landmark Genomic Scanning (RLGS) is a two-dimensional gel electrophoresis that assesses the methylation status of thousands of CpG islands. RLGS has been applied successfully to scan cancer genomes for aberrant DNA methylation patterns. So far, the majority of this work was done using NotI as the restriction landmark site. Here, we describe the development of RLGS using AscI as the restriction landmark site for genome-wide scans of cancer genomes. The availability of AscI as a restriction landmark for RLGS allows for scanning almost twice as many CpG islands in the human genome compared with using NotI only. We describe the development of an AscI–EcoRV boundary library that supports the cloning of novel methylated genes. Feasibility of this system is shown in three tumor types, medulloblastomas, lung cancers, and head and neck cancers. We report the cloning of 178 AscI RLGS fragments via two methods by use of this library.
[Supplemental material is available online at http://www.genome.org.]
Multiple genome scanning approaches have been developed in the past years to study genetic and epigenetic alterations in cancer (Gray and Collins 2000). The majority of those techniques target genetic alterations such as deletions, insertions, and copy number changes. Restriction Landmark Genomic Scanning (RLGS), a highly reproducible two-dimensional gel electrophoresis, allows scanning of genomes for DNA polymorphisms, DNA amplification, and DNA methylation (Hayashizaki et al. 1994a,b; Plass et al. 1996; Costello et al. 2000). The use of RLGS to study human cancers resulted in the identification of several novel genes that were amplified and overexpressed in malignant tissues (Costello et al. 1997; Frühwald et al. 2000). Furthermore, the use of methylation-sensitive restriction enzymes as landmark enzymes makes scanning of genomes for changes in the DNA methylation patterns possible (Dai et al. 2001; Frühwald et al. 2001b; Rush et al. 2001; Rush and Plass 2002). This is of particular interest in cancer genetics, because promoter methylation has been shown to be involved in the silencing of tumor suppressor genes (Jones and Laird 1999; Baylin et al. 2001; Costello and Plass 2001). The methylation-sensitive restriction landmark enzyme NotI has a GC-rich recognition sequence, which is preferentially located in CpG islands sequences, found mainly in promoter regions of genes (Costello et al. 2000). In normal tissue DNAs, these sites are unmethylated (Bird 1986). However, in tumors, methylation of a NotI site results in the absence of an RLGS fragment in the respective profile.
Although RLGS profiles can be generated from any high-quality genomic DNA without prior sequence information, subsequent cloning of RLGS fragments is essential for future studies. Several PCR-based protocols have been developed allowing the identification of RLGS sequences (Ohsumi et al. 1995). More efficient, however, is a cloning strategy that uses an arrayed human library of NotI–EcoRV clones and RLGS mixing gel catalogs (Smiraglia et al. 1999). This protocol circumvents the need for PCR-based amplification, which could be problematic with GC-rich sequences. Successful use of this library system resulted in the identification of many methylation targets in several human tumors (Smiraglia and Plass 2002).
The use of the NotI–EcoRV boundary library as a cloning tool for RLGS is restricted to RLGS profiles that use the enzyme combination NotI and EcoRV as the first and second restriction enzymes. To increase the potential coverage of CpG islands, we developed reaction conditions for the use of AscI as the restriction landmark enzyme in RLGS. In addition, we prepared an AscI–EcoRV library and RLGS mixing gels that allow the efficient recovery of cloned RLGS fragments. We estimate that this novel resource, together with the NotI–EcoRV library, will greatly increase the utility of RLGS and, in addition, provide access to up to 15,000 of the estimated 29,000 CpG islands in the human genome (Venter et al. 2001).
RESULTS AND DISCUSSION
AscI and NotI Restriction Sites Predicted in the Human Genome
The majority of RLGS gels generated to study DNA methylation profiles in human malignancies have used NotI as the restriction landmark enzyme. Many of these studies were supported by a NotI–EcoRV library (Smiraglia et al. 1999). To develop an additional landmark enzyme for the purpose of CpG island identification by RLGS, we analyzed the human genome sequence for the frequency and location of restriction sites for rare cutting, methylation-sensitive restriction endonucleases. AscI is a restriction enzyme that recognizes the target sequence GGCGCGCC and does not cut the methylated recognition sequence. Some characteristics of the loci cut by AscI obtained from the human genomic sequence (August 6, 2001 draft assembly of UCSC) are listed in Table 1 and compared with those cut by NotI. Surprisingly, the human genome possesses only half the number of AscI sites (4935) as compared with NotI sites (9628), although both recognition sequences are composed of four guanines and four cytosines each, and both contain 2 CG dinucleotides. Nevertheless, NotI and AscI are highly comparable in terms of the types of loci they assess. Of particular note is the fact that 86% and 83% of these sites, respectively, are found in CpG islands, whereas only 5% and 7% are found in repetitive elements not associated with CpG islands. This strong bias of representation of CpG islands over repetitive elements is a major strength of RLGS using these two enzymes. Furthermore, 86% and 83% of these CpG islands, respectively, are associated with known genes or ESTs. These data indicate that AscI is an excellent choice of landmark enzyme to complement RLGS studies performed using NotI. In addition, because NotI and AscI sites colocalize in only 3.7% of CpG islands, by using AscI as a second landmark enzyme, we are able to almost double the number of CpG islands whose methylation status can be analyzed.
Table 1.
Characteristics of NotI and AscI Restriction Sites
| NotI | AscI | |
|---|---|---|
| Total number of restriction sites in the human genomea | 9628 | 4935 |
| Number of restriction sites in CpG islands | 8239 (86%) | 4071 (83%) |
| Number of restriction sites in repetitive elements, not CpG islands | 520 (5%) | 332 (7%) |
| Frequency of restriction sites near 5′ end of a known gene | 3357 (34.9%) | 1612 (32.7%) |
| Frequency of restriction sites near 3′ end of a known gene | 1328 (13.8%) | 725 (15%) |
| Frequency of restriction sites inside a gene | 1392 (14.5%) | 738 (15%) |
| Frequency of restriction sites falling near ESTs | 2.221 (23.1%) | 1001 (20.3%) |
| Frequency of CpG islands with both NotI and AscI sites | 1100 (3.7%) | |
August 6, 2001 draft assembly of UCSC.
RLGS Profiles Using AscI as the Landmark Enzyme Display up to 2000 Distinct CpG Islands
We established the reaction conditions for the use of AscI as a restriction landmark site (see Fig. 1 for an outline of the procedure). RLGS profiles with AscI show a lower fragment density than NotI profiles (Fig. 2A), as expected from the genome sequence survey that identified fewer AscI restriction sites in the human genome (Table 1). An AscI master profile was prepared using total genomic DNA from three donors to maximize coverage of polymorphic spots. The master profile was labeled with a coordinate system of spot numbering (a portion is shown in Fig. 2D) as was done for the NotI master profile described previously (Costello et al. 2000). The lower density of RLGS fragments in an AscI profile allows the scoring of more fragments in the higher molecular weight sections. These sections are difficult to score in the NotI profiles due to the high density of spots and are frequently excluded from the analysis. Thus, although the number of fragments on an AscI profile is less than on a NotI profile, a similar number of ∼2500 fragments can be analyzed on both.
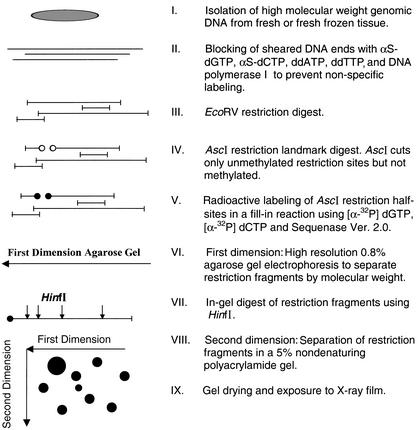
Figure 1.
Outline of the RLGS procedure using AscI as a restriction landmark enzyme.
Figure 2.
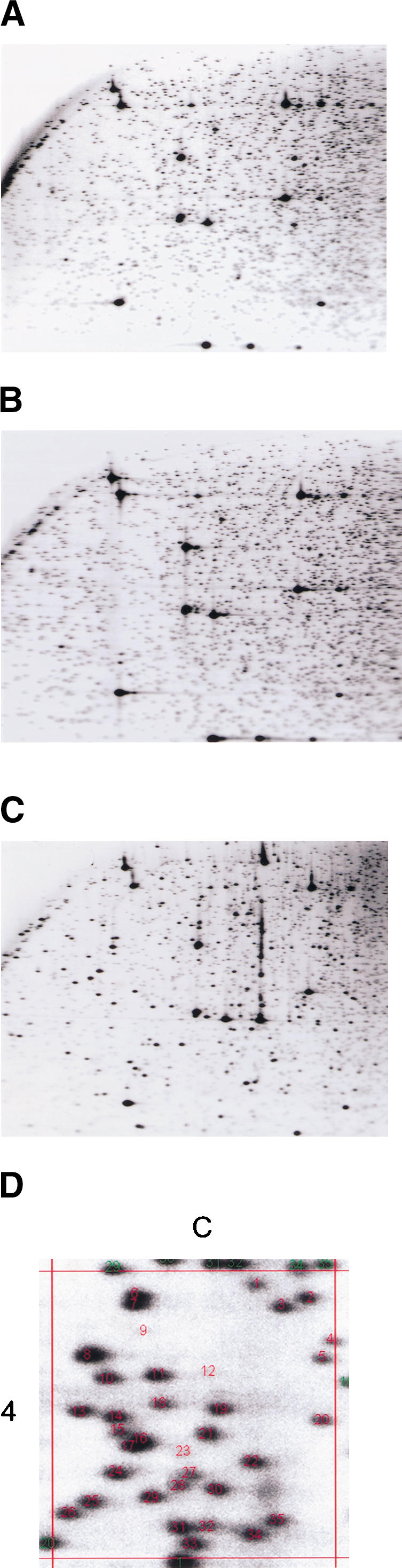
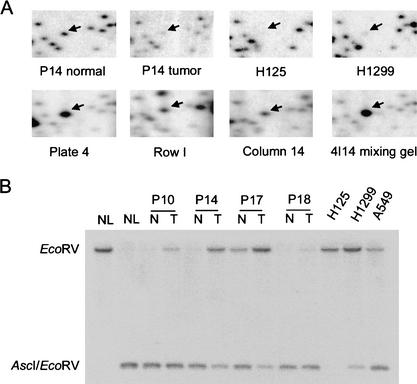
RLGS profiles using AscI–EcoRV–HinfI restriction enzyme combination. (A) RLGS profile of normal lung DNA. (B) RLGS profile using restriction trapper purified AscI–EcoRV fragments derived from peripheral blood lymphocyte DNAs. (C) RLGS mixing gel generated with normal lung DNA as the genomic background and clones from plate 3 pool in the A-RV1 library. (D) Section 4C of the AscI Master RLGS profile, showing the numbers assigned to each AscI fragment.
Because AscI is methylation sensitive, we compared methylation frequencies detected by NotI and AscI in the same samples to determine whether both recognition sequences are equal targets for aberrant methylation in human malignancies. Table 2 summarizes the data obtained for nine lung cancers, six medulloblastomas, and three head and neck cancers. The number of methylated sequences detected with both restriction enzymes is not statistically different (P ⩽ 0.05). These data indicate that although these enzymes assess different loci, they are similar in their abilities to detect aberrant methylation in human malignancies.
Table 2.
Methylation Frequencies in Various Tumor Samples Determined by Either NotI or AscI as a Restriction Enzyme
| Tumor typea | NotI RLGS gels | AscI RLGS gels | Z-test statistic for proportionsb | ||||
|---|---|---|---|---|---|---|---|
| No. of methylated CpG islands | No. of RLGS fragments analyzed | Methylation frequency in NotI gels | No. of methylated CpG islands | No. of RLGS fragments analyzed | Methylation frequency in AscI gels | ||
| Lung | 63 | 1184 | 5.3% | 78 | 1614 | 4.8% | 0.5833 |
| Lung | 59 | 1184 | 5.0% | 62 | 1614 | 3.8% | 1.4669 |
| Lung | 28 | 1184 | 2.4% | 49 | 1690 | 2.9% | −0.8735 |
| Lung | 8 | 1184 | 0.7% | 8 | 1008 | 0.8% | −0.3234 |
| Lung | 7 | 1184 | 0.6% | 13 | 1205 | 1.0% | −1.3079 |
| Lung | 5 | 1184 | 0.3% | 18 | 1734 | 1.0% | −1.8470 |
| Lung | 3 | 1184 | 0.3% | 7 | 1734 | 0.4% | −0.6822 |
| Lung | 1 | 1184 | 0.1% | 3 | 1380 | 0.2% | −0.8503 |
| Lung | 0 | 1184 | 0.0% | 5 | 1614 | 0.3% | −1.9169 |
| MB | 53 | 1702 | 3.1% | 20 | 923 | 2.1% | 1.4091 |
| MB | 32 | 1768 | 1.8% | 22 | 1246 | 1.8% | 0.0903 |
| MB | 31 | 1825 | 1.7% | 21 | 1093 | 1.9% | −0.4401 |
| MB | 32 | 2016 | 1.6% | 19 | 1172 | 1.6% | −0.0735 |
| MB | 15 | 1741 | 0.9% | 19 | 1327 | 1.4% | −1.4947 |
| MB | 14 | 2018 | 0.7% | 9 | 1421 | 0.6% | 0.2140 |
| HNSCC | 13 | 1703 | 0.8% | 14 | 1739 | 0.8% | −0.1387 |
| HNSCC | 3 | 1839 | 0.2% | 6 | 1009 | 0.6% | −1.9625 |
| HNSCC | 0 | 2126 | 0.0% | 1 | 1243 | 0.1% | −1.3080 |
HNSCC, head and neck squamous cell carcinomas; MB, medulloblastoma.
Z-test static value for testing the significant difference in methylation frequencies in NotI and AscI gels. All of the Z-static values are between −1.96 to +1.96, which suggest that there is no significant (p ≤ 0.05) difference in methylation frequencies in NotI and AscI gels.
Establishment and Initial Characterization of an AscI–EcoRV Library
The initial step in the construction of the AscI–EcoRV boundary library was the purification of AscI–EcoRV fragments from total genomic DNA using the BssHII/AscI restriction trapper. This procedure results in the enrichment of AscI–EcoRV fragments and eliminates EcoRV–EcoRV fragments (see Methods for details). The quality of the purified AscI–EcoRV fragments was tested by using an aliquot of these fragments for RLGS separation. A portion of the purified AscI–EcoRV fragments was labeled and subjected to two-dimensional separation in the RLGS system. The resulting RLGS profile showed the same set of fragments as the original profile without prior purification (Fig. 2A,B), indicating that the purification did not result in loss or gain of certain fragments. The remaining purified material was used for cloning into pBluescript KS-AscI. The AscI–EcoRV library (A-RV-1) consists of 19,200 clones picked into fifty 384 well plates. The average insert size was 2.48 kb (n = 75) ranging from 0.3 to 10 kb. Accordingly, the library has an expected bias toward smaller fragments, reflecting the cloning bias of the plasmid vector. Clones from 48 plates were spotted onto filters for hybridization-based screening, providing an additional resource for studies of CpG islands. Each filter contains the entire set of clones from the 48 plates spotted in duplicate. The availability of these filters allows for rapid identification of plasmid clones with 5′ end sequences for known genes. In addition, these clones provide a unique resource for array-based studies.
AscI–EcoRV Library Clone Sequence Characteristics are Similar to Predicted
CpG islands are mainly located in the promoter region of genes and are less frequently found in the body or 3′ end of genes. The survey of the human genome for AscI sites described above indicated that the recognition sequence of AscI (GGCGCGCC) has a preferential localization to CpG islands. To determine whether our library has a similar representation, we sequenced 178 AscI–EcoRV fragments cloned from this library by the two methods described below. A total of 158 sequences (89%) showed CpG island features (see Methods). We mapped all 178 sequences to the human genome draft sequence (August 6, 2001 draft assembly of UCSC) and found that 137 (77%) mapped to known genes or ESTs. In 84 cases in which the CpG island could be mapped within the context of a known gene, 66 (79%) were found in the 5′ end of a gene, 12 (14%) in the body, and 6 (7%) in the 3′ end (Table 3). This further supports the assumption that AscI sites are preferentially located in CpG islands near genes and assures that our library is a faithful representation of this.
Table 3.
Cloned AscI Spots (See Supplemental Data Online for a Complete List of Cloned RGLS Fragmentsf)
| RLGS spot | Chr. positiona | Chr. band | CpG islandb | Methylationc | Gene or EST homologyd | Contexte | |
|---|---|---|---|---|---|---|---|
| Primary | Cell lines | ||||||
| A2D16g | chrNA_rdm:9890705-9892163 | ? | Y | L | L HN LU MB | DRD4/PTDSS2 | 5′end/Body |
| A5E46 | chrX:17627774-17629763 | Xp22.13 | Y | L | L HN LU | – | – |
| A4G14 | chr1:256787415-256787746 | 1q41 | Y | L | L LU | – | – |
| A2D08 | chr1:68251887-68253584 | 1p32.1 | Y | L | LU | JUN | Body |
| A3F22 | chr1:108979505-108982150 | 1p22.1 | Y | L | L | – | – |
| A3F38 | chr1:201154777-201155644 | 1q24.2 | N | L HN MB | L LU | PMX1 | 5′ end |
| A6E17 | chr1:133792275-133794522 | 1p13.2 | Y | L | L LU | – | – |
| A3F30 | chr1:8129740-8132794 | 1p36.23 | N | L | LU | EST | – |
| A5E43 | chr1:127726863-127728875 | 1p13.3 | Y | L HN MB | L HN LU MB | ALX3 | 5′ end |
| A4E32 | chr1:22646645-22648732 | 1p36.32 | Y | L | L LU | – | – |
| A3H43 | chr1:2844351-2849754 | 1p36.32 | Y | L | L HN LU | – | – |
| A2F45 | chr2:117320895-117322735 | 2q13 | N | L HN MB | L HN LU MB | EST | – |
| A3E38 | chr2:179015764-179017423 | 2q31.1 | N | L | L | – | – |
| A4D36 | chr2:138670622-138671469 | 2q21.2 | N | L | L LU | – | – |
| A4G44 | chr3:220718143-220722411 | 3q29 | Y | L | HN LU | EST | – |
| A5F32 | chr3:153653591-153655489 | 3q22.1 | N | L MB | L LU MB | – | – |
| A5G07 | chr3:8664656-8665380 | 3p26.1 | Y | L HN | L HN LU | GRM7 | 5′ end |
| A5E28 | chr3:3902165-3903025 | 3p26.1 | Y | L | L HN LU | – | – |
| A4B07 | chr3:146695171-146695918 | 3q21.3 | Y | L | HN LU | EST | – |
| A2E14 | chr4:5349208-5351766 | 4p16.1 | Y | L MB | L LU | HSA250839 | 5′ end |
| A2D23 | chr4:171849449-171850978 | 4q32.1 | Y | L HN | L HN LU | GLRB | 5′ end |
| A3E31 | chr4:181006489-181008945 | 4q32.3 | Y | L | L LU | TLL1 | 5′ end |
| A6E16 | chr5:49418697-49420952 | 5p11 | Y | L | L LU | EST | – |
| A4F02 | chr5:187652008-187654787 | 5q35.1 | Y | L | EST | – | |
| A6D30 | chr5:195984026-195984768 | 5q35.2 | Y | MB | MB | B4GALT7 | 5′ end |
| A3G33 | chr5:83731413-83731663 | 5q13.3 | Y | L | L HN LU | MGC15435 | 3′ end |
| A4C25 | chr6:103613999-103614941 | 6q15 | Y | L | LU | BACH2 | 5′ end |
| A3E39 | chr6:86748700-86751107 | 6q14.1 | Y | L HN | L HN LU | EST | – |
BLAT search results based on http://genome.ucsc.edu/cgi-bin/hgBlat, Aug. 2001 Freeze.
Y indicates that the AscI site is within a CpG island, N indicates that it is not.
Indicates methylation found in a primary tumor or cell line as indicated: (L) Lung carcinoma; (MB) medulloblastoma; (HN) head and neck squamous cell carcinoma; (LU) leukemia.
Known genes found in the Refseq database or spliced ESTs.
5′ end indicates that the CpG island includes the region immediately upstream of exon 1 and/or exon 1. The 3′ end indicates that the CpG island is found in 3′ most exon. Body indicates that the CpG island is found within the genomic structure of the gene excluding the 5′-most and 3′-most known exons.
Genes share same CpG island in their 5′ end and are transcribed in opposite directions.
Bold indicates the RLGS fragments that were cloned directly using the mixing gels.
Establishment of Mixing Gels as a Cloning Tool for RLGS Fragments
To generate a novel tool that will aid the cloning of RLGS fragments from profiles generated with AscI as the landmark enzyme and EcoRV as the second restriction enzyme, we prepared RLGS mixing gels from plates 1 to 32 of the A-RV-1 library. In addition, the rows and columns from these 32 plates were individually pooled to produce 16-row pool (A–P) and 24 column pool (1–24) mixing gels. The procedure followed the strategy used previously for the generation of the NotI–EcoRV mixing gel catalog (Smiraglia et al. 1999). In RLGS mixing gels, fragments for which a corresponding clone is present in the pool of clones mixed with the genomic DNA will show enhancement. Determination of the plate, row, and column mixing gels in which the RLGS spot of interest is enhanced indicates the unique library address in which the corresponding RLGS fragment is cloned. An example of an RLGS mixing gel is shown in Figure 2C, with clones from a 384-well plate of the library. The average number of enhanced RLGS fragments per plate is 153, as many of the clone insert sizes fall outside of the window of resolution of a standard RLGS profile. In the 32-plate mixing gels, there are 1468 unique RLGS fragments represented.
Use of the AscI–EcoRV Cloning Gel Catalog to Identify Hypermethylated Sequences in Various Cancers
AscI is preferentially located within CpG islands and is methylation sensitive. Thus, AscI is useful as a restriction landmark enzyme in RLGS studies to identify methylation changes in two different samples. We used the methylation scanning properties of AscI to determine methylation changes in medulloblastoma (MB), lung cancer, and head and neck cancer (HNSCC) primary tumors relative to adjacent normal tissue, as well as cell lines representing all three tumor types and a leukemia cell line. RLGS fragment loss in tumor profiles and cell line profiles is the most prominent observation and is indicative of hypermethylation of those fragments. Less frequently, newly appearing RLGS spots are found on the tumor profiles that may represent hypomethylation. Unfortunately, however, such rare RLGS spots cannot be cloned using this library, because they are not present in the RLGS profiles of the DNAs used to create the library. Figure 3 shows an example of an RLGS fragment (RLGS fragment A2E54; in which A indicates the AscI profile and 2E54 indicates spot No. 54 in section 2E) that is present in normal adjacent lung tissue DNA, but absent from the lung tumor and two lung cancer cell lines. The corresponding library clone was identified in the plate 4, row I, and column 14 mixing gels. Clone 4I14 was isolated from the library and used in a single-clone mixing gel to confirm that the clone represents the intended RLGS spot (Fig. 3A). Insert DNA from this clone was used as a hybridization probe for Southern blot analysis to confirm methylation of the AscI site. Lung tumor and normal genomic DNA was digested with both EcoRV and AscI. Control DNA in lane 1 of Figure 3B was digested with EcoRV only. In the Southern analysis, the probe detects either a small (AscI–EcoRV) fragment or a larger (EcoRV–EcoRV) fragment. The presence of the large fragment indicates that the AscI site was protected from restriction digestion by DNA methylation. The Southern data for patient 14 confirms the RLGS result. Similarly, 17 additional RLGS fragments have been cloned using this targeted approach and are shown in bold in Table 3.
Figure 3.
RLGS identifies DNA methylation in primary lung cancer. (A) Sections from RLGS profiles including RLGS fragment A2E54 (arrow). Sections from normal and tumor profiles from patient 14 as well as two lung cancer cell lines (H1299 and H125) are shown. The corresponding AscI–EcoRV clone was found in plate 4, row I, and column 14, and this clone was confirmed by use in a mixing gel. (B) DNA from AscI clone 4I14 corresponding to RLGS spot A2E54 was used for Southern analysis. DNAs from normal lung (NL), lung tumors (T), and adjacent normal tissue (N) from patients 10, 14, 17, and 18, as well as from three lung cancer cell lines H125, H1299, and A549 were digested with AscI and EcoRV. DNA in the first lane was digested only with EcoRV and shows the size of the EcoRV fragment. In the double digests, hybridization to the large EcoRV band is indicative of protection of the AscI site digestion by methylation. The smaller band is indicative of cutting by AscI.
Large-Scale Identification of RLGS Fragment Sequences
A second nontargeted strategy to identify the sequences of RLGS fragments in AscI gels was also used. AscI–EcoRV clones derived from the A-RV-1 were sequenced from the AscI end. This sequence was used to determine the full-length AscI–EcoRV and the AscI–HinfI restriction fragment sizes from the August 2001 freeze of the human genome. DNAs of clones with insert sizes of 0.5–5 kb and an AscI–HinfI fragment >100 bp were pooled into groups of up to 15 plasmid DNAs and used for RLGS mixing gels. These mixing gels resulted in the enhancement of exactly the same number of RLGS fragments as the number of plasmid clones in the pool. Because the sequence, and thus the predicted mobility of these clones in RLGS gels was known, it was possible to unambiguously identify which clone and sequence corresponds with each RLGS fragment. By use of this strategy, it was possible to identify 160 additional RLGS fragments and their sequences. This data is summarized in Table 3, which is sorted by methylation status and chromosomal location. Of the total number (178) of AscI fragments cloned by the two methods described, we found 70 that were methylated in primary tumors and 119 methylated in cancer cell lines (Table 3; see supplemental data online for the complete list of cloned RLGS fragments).
Conclusion
We have developed a valuable resource for isolating and studying CpG-rich regulatory human sequences using AscI as a restriction landmark enzyme. AscI is as suitable as NotI to determine methylation patterns in human malignancies and nearly doubles the set of loci that can be studied. The AscI recognition sequence occurs less frequently in the genome than the NotI sequence, but its location is similarly biased toward CpG islands. The cloning gel catalogs now available for NotI–EcoRV and AscI–EcoRV allow for the targeted cloning of up to 3257 RLGS fragments [1789 from NotI (Smiraglia et al. 1999), 1468 from AscI], >90% of which are expected to represent CpG islands (Smiraglia and Plass 2002). In addition, we have developed a nontargeted, but higher throughput strategy for using these libraries to clone RLGS fragments.
By applying these cloning strategies, we have created a novel resource, which when used in conjunction with RLGS analysis of tumor profiles, allows for the identification of large numbers of methylation targets. Even in this limited study of 18 analyzed primary tumor profiles, we have already identified 70 targets of hypermethylation in cancer. Three of the genes that were identified, HOXA11, NELL1, and ALX3 have been identified previously by others as methylation targets in lung adenocarcinomas (Shiraishi et al. 2002a,b) or neuroblastomas (Wimmer et al. 2002), respectively. As we increase the number of tumor profiles analyzed and go through multiple iterations of the cloning strategies describe in this article, we will significantly increase the number of targets of hypermethylation that we can identify. This is a requisite step to begin to understand the mechanisms and consequences of such hypermethylation.
Together, there are a little over 15,000 NotI and AscI restriction sites in the human genome. Considering the total number of 29,000 CpG islands in the human genome (Venter et al. 2001), these libraries provide access to nearly half of the CpG islands. Therefore, these libraries will prove to be excellent tools for the study of aberrant CpG island methylation when used in combination with various methylation-scanning techniques such as RLGS and differential methylation hybridization (Frühwald and Plass 2002; Smiraglia and Plass 2002). Although standard RLGS running conditions only resolve a set of ∼2500 CpG islands with a first dimension size of 5 kb–500 bp, these conditions can be altered to resolve a similar number of fragments with first dimension size ranging from 10 to 5 kb (Hughes et al. 1998). Thus, by modifying RLGS electrophoresis conditions and by utilizing other technologies that do not require electrophoresis, the full potential of these libraries may be achieved.
METHODS
Tissue Samples and Cell Lines
Frozen non-small cell lung tumors paired with normal adjacent tissues were collected through the Cooperative Human Tissue Network (CHTN). Nine paired samples (patient nos. 2, 3, 5, 7, 10, 11, 13, 14, and 17) and clinical characteristics were described previously (Dai et al. 2001). Six medulloblastoma samples were described previously (Frühwald et al. 2001b). Three head and neck cancer tissues were collected at The Ohio State University through the CHTN. All sample collection was performed in accordance with NIH guidelines. Non-small cell-line lung cancer lines A549 (from ATCC), H125, H1299, and H2086, head and neck cancer cell line SCC-9, leukemia cell lines HL-60, ML-1, and K-562, medulloblastoma cell lines Daoy, D425 MED, MHH-MED-1, and MHH-PNET-5, used in this study were described previously (Dai et al. 2001; Frühwald et al. 2001a; Rush et al. 2001, 2002; Smiraglia et al. 2001).
Isolation of Plasmid and Genomic DNAs
High molecular weight DNA for the RLGS procedure was isolated according to our previously published protocol (Smiraglia et al. 1999). Plasmid DNA was isolated using QIAprep Spin Miniprep kit (QIAGEN) and the manufacturer's recommended protocols.
RLGS
RLGS was performed according to published protocols (Okazaki et al. 1994) with modifications for the use of AscI as the restriction landmark enzyme. Briefly, to prevent nonspecific labeling, the sheared ends of ∼7 μg of genomic DNA were blocked in a 10-μL reaction by the addition of nucleotide analogs (αS-dGTP, αS-dCTP, ddATP, ddTTP) using 2.5 U of DNA polymerase I (Boehringer Mannheim) (37°C, 20 min) followed by enzyme inactivation (65°C, 30 min). The DNA was digested (37°C, 2 h) with 20 U of EcoRV (New England Biolabs), followed by 20 U of AscI (New England Biolabs) in NEB buffer 4 (37°C, 2 h). The resulting restriction sites from AscI were labeled in a fill-in reaction using Sequenase Ver. 2.0 (USB) in the presence of [α-32P]dGTP (6000 Ci/mmole, NEN Life Science Products) and [α-32P]dCTP (3000 Ci/mmole, NEN) for 30 min and stopped by adding buffer that included dCTP and dGTP. A portion of the reaction was electrophoresed through a 60-cm long, 0.8 % agarose tube gel (first dimension separation). The agarose gel was equilibrated in restriction buffer and the DNA was digested in the gel with 750 U of HinfI (New England Biolabs) at 37°C for 2 h. The agarose gel was placed horizontally across the top of a nondenaturing 5% polyacrylamide gel, the two gels were connected with molten agarose, and the DNA was electrophoresed in the second dimension. The gels were dried and exposed to Kodak X-OMAT AR film in the presence of one intensifying screen (Quanta 111, DuPont) for 2–10 d.
AscI Restriction Trapper Purification
A mix of 500 μg of total human genomic DNA from three donors was digested with 1500 U of AscI (37°C for 3 h), and subsequently with 500 U of EcoRV (37°C overnight), extracted with phenol/chloroform/isoamyl alcohol (PCI), precipitated, and resuspended in H2O at a concentration of 2 mg/mL. Aliquots of 100 μg of restriction-digested DNA were ligated in 150-μL volume to a 0.67% (w/v) DNA Trapper R–BssHII (Japan Synthetic Rubber Co.) in the presence of 10% PEG 6000 using 1400 U of T4 DNA ligase (New England Biolabs) at 18°C overnight. The DNA trapper-ligated DNA was digested twice with 100 U of EcoRV, centrifuged to remove nonligated EcoRV fragments, and then digested with 100 U of AscI to release AscI–EcoRV fragments. DNA fragments were PCI purified, precipitated in the presence of glycogen (Boehringer Mannheim), and dissolved in 13 μL of TE buffer. A total of 11.8 μg of purified DNA was recovered. To determine the quality and purity of the AscI–EcoRV fragments, 1 μg was endlabeled with a fill-in reaction using Sequenase Ver. 2.0 in the presence of [α-32P]dGTP (6000 Ci/mmole, DuPont) and [α-32P]dCTP (3000 Ci/mmole, DuPont) and subjected to the two-dimensional separation in the RLGS system. The resulting profile was compared with the RLGS profile prepared from total genomic DNA.
Construction of Vector KSII+–AscI
To insert an AscI site (GGCGCGCC) into vector Bluescript KSII+ (Stratagene), 50 pmole of each primer AscI-1 (CCACCGCGGTGGGCGCGCCT) and AscI-2 (CTAGAGGCGCGCCCACCGCGGTGGAGCT) (custom made by MWG Biotech) were annealed and subsequently ligated with 100 ng of SacI–XbaI cut vector DNA. Appropriate insertion of the annealed primers would not disturb the ORF of the multiple cloning site and, hence, the vector's capability for blue/white selection on agar medium containing X-GAL. Escherichia coli DH10B (Life Technologies) were transformed with the ligation mixture and plated onto LB agar containing ampicillin, IPTG, and X-GAL. Blue colonies were tested for the presence of KSII+ harboring an AscI site. One of such plasmids, designated KSII+–AscI, was selected for subsequent library construction.
Library Construction
To facilitate reliable double digestions of vector KSII+–AscI with AscI plus EcoV, we first shotgun cloned AscI–EcoRV genomic fragments of mouse DNA into the vector. A clone with a 1.6-kb AscI–EcoRV insert was then used to prepare the vector for library construction. Two micrograms of the recombinant plasmid were AscI–EcoRV digested and separated on a gel. The vector band was sliced out and run a second time on a gel to improve purity. The band was eluted and dissolved in H2O at a concentration of 10 ng/μL. Self ligation of 10 ng and subsequent electroporation of electrocompetent E. coli DH10B cells (Life Technologies; transformation efficiency ∼7–× 109 transformants/μg pUC19) yielded in 45 clones. This figure indicated the expected nonrecombinants when 10 ng of vector DNA were ligated with insert DNA at similar conditions. For library construction, two 10-μL ligation mixtures, each containing 10 ng of vector DNA, 3 μL of human restriction trapper purified DNA and 0.5 U of T4 DNA ligase (Roche Diagnostics) were incubated at 16°C for 16 h. After addition of 2.5 M NH4-acetate (final concentration) and 1 μL of glycogen (stock: 20 mg/mL; Roche Diagnostics) as carrier, the DNA was precipitated and redissolved in a total of 5 μL of 0.5× TE. A total of 1 μL of ligated DNA was used per transformation.
Library Picking, Replication, and Preparation of High-Density Hybridization Filters
Transformed cells were spread onto LB/Agar plates containing ampicillin (100 μg/mL), IPTG, and X-gal, and grown at 37°C for 18–20 h. Clones were picked manually and arrayed into 384-well microtiter plates containing LB/ampicillin (50 μg/mL)/glycerol (7.5%). The arrayed clones were incubated at 37°C for 18 h, and then frozen at −80°C. Five additional copies of each plate were made using a 384-pin replicating tool (V & P Scientific) for inocculation. The replicas were grown for 18 h at 37°C and then frozen at −80°C. High-density hybridization filters were prepared using a Q-Bot colony picker/high-density filter gridder. All clones from plate 1 to 48 were used to spot onto three 22.25 × 22.25-cm nylon membranes by use of protocols identical to the one used for BAC clones (Osoegawa et al. 2000).
RLGS Mixing Gels With Clones From the AscI–EcoRV Library (A-RV-1)
Plates 1 to 32 from A-RV-1 were chosen for the RLGS mixing gels. Clone pool DNAs for each of the 32 plates, all 16 rows (A–P) and 24 columns (1–24) were prepared as described earlier (Smiraglia et al. 1999). Individual clones were grown in microtiter plates, overnight cultures were combined, and plasmid DNAs for each pool of clones were isolated using spin columns (QIAGEN). Genomic DNA from normal lung was labeled by a fill-in reaction using Sequenase Ver. 2.0 (USB) in the presence of [α-32P]dCTP (6000 Ci/mmole, NEN) and [α-32P]dGTP (3000 Ci/mmole, NEN) for 30 min. Pooled clone DNA was digested by EcoRV (Promega) and AscI (NEB) sequentially and labeled following the same procedure for standard RLGS. Ten picogram DNA per clone of labeled pooled clone DNA was mixed with the appropriate amount of labeled genomic DNA and loaded on the first dimension RLGS agarose gel followed by the standard RLGS procedure. The amount of labeled genomic DNA was optimized to obtain a 4-day exposure of the RLGS gel on X-ray film.
Sequencing and Database Analysis
All sequence analyses were performed in the Core Facility of the Division of Human Cancer Genetics using an ABI PRISM 377 DNA sequencer. For CG-rich sequences high-annealing temperatures were employed using an ABI PRISM BigDye Terminator Cycle Sequencing kit. AscI–EcoRV clones were sequenced with M13 forward primer. DNA sequence files were analyzed using DNAstar and Chromas software. For homology searches, sequences were submitted to the publicly available databases.
Bioinformatics
The standard two-sided Z-test was used to compare the methylation frequencies in NotI and AscI test.
We downloaded the assembled sequences (August 6, 2001 draft assembly of UCSC) of the 24 chromosomes from the UCSC Human Genome Project working draft (http://genome.ucsc.edu). We scanned each of the chromosomes for NotI (GCGGCCGC) and AscI (GGCGCGCC) sites, and retrieved the sequences that contain these sites. Each sequence is of a 1008-bp length (−500 to +500 of the site). We used a sliding window 201 bp in length, and counted the percentage of CpG dinucleotides (CpG score) and GC% for each window. The sequence is considered a CpG island if there exists a sliding window with CpG score ⩾ 60% and GC% ⩾ 50. We retrieved all of the 30,095 CpG islands mapped in the human genome and counted the number of CpG islands that have NotI and AscI sites. To determine whether these sites fall in a gene region or not, we used the public human genome annotations available at UCSC genome server. We counted the number of sites that fall in and around (within the 5-kb region of the annotated gene ends) known genes and ESTs.
Web Site References
http://genome.ucsc.edu; Web site offers free access to the human genomic sequence.
Acknowledgments
We thank Barbara Swiatkiewcz for her help with the clone spotting. The authors thank Drs. Hayashizaki and Okazaki for the generous gift of the restriction trapper. This work was supported in part by grants P30 CA16058, RO1 CA93548, and R01 DE13123 (CP). L.J.R. was supported by a T32 training grant CA09338 (PI, Michael Caligiuri) and grant CA089317 from NCI. M.F., D.K., and J.M. were supported by the Deutsche Krebshilfe (10-1699-Fr 1), the Deutsche Forschungsgemeinschaft (FR1516/1-1). C.P. is a Leukemia and Lymphoma Society Scholar.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Plass-1@medctr.osu.edu; FAX (614) 688-4761.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.197402.
REFERENCES
- Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001;10:687–692. doi: 10.1093/hmg/10.7.687. [DOI] [PubMed] [Google Scholar]
- Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello JF, Fr̈hwald MC, Smiraglia DJ, Rush LJ, Robertson GP, Gao X, Wright FA, Feramisco JD, Peltomaki P, Lang JC, et al. Aberrant CpG-island methylation has non-random and tumour-type-specific patterns. Nat Genet. 2000;24:132–138. doi: 10.1038/72785. [DOI] [PubMed] [Google Scholar]
- Costello JF, Plass C, Arap W, Chapman VM, Held WA, Berger MS, Su Huang HJ, Cavenee WK. Cyclin-dependent kinase 6 (CDK6) amplification in human gliomas identified using two-dimensional separation of genomic DNA. Cancer Res. 1997;57:1250–1254. [PubMed] [Google Scholar]
- Dai Z, Lakshmanan RR, Zhu WG, Smiraglia DJ, Rush LJ, Frühwald MC, Brena RM, Li B, Wright FA, Ross P, et al. Global methylation profiling of lung cancer identifies novel methylated genes. Neoplasia. 2001;3:314–323. doi: 10.1038/sj.neo.7900162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühwald MC, Plass C. Global and gene-specific methylation patterns in cancer: Aspects of tumor biology and clinical potential. Mol Genet Metab. 2002;75:1–16. doi: 10.1006/mgme.2001.3265. [DOI] [PubMed] [Google Scholar]
- Frühwald MC, O'Dorisio MS, Rush LJ, Reiter JL, Smiraglia DJ, Wenger G, Costello JF, White PS, Krahe R, Brodeur GM, et al. Gene amplification in PNETs/medulloblastomas: Mapping of a novel amplified gene within the MYCN amplicon. J Med Genet. 2000;37:501–509. doi: 10.1136/jmg.37.7.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühwald MC, O'Dorisio MS, Dai Z, Rush LJ, Krahe R, Smiraglia DJ, Pietsch T, Elsea SH, Plass C. Aberrant hypermethylation of the major breakpoint cluster region in 17p11.2 in medulloblastomas but not supratentorial PNETs. Genes Chromosomes Cancer. 2001a;30:38–47. [PubMed] [Google Scholar]
- Frühwald MC, O'Dorisio MS, Dai Z, Tanner SM, Balster DA, Gao X, Wright FA, Plass C. Aberrant promoter methylation of previously unidentified target genes is a common abnormality in medulloblastomas—implications for tumor biology and potential clinical utility. Oncogene. 2001b;20:5033–5042. doi: 10.1038/sj.onc.1204613. [DOI] [PubMed] [Google Scholar]
- Gray JW, Collins C. Genome changes and gene expression in human solid tumors. Carcinogenesis. 2000;21:443–452. doi: 10.1093/carcin/21.3.443. [DOI] [PubMed] [Google Scholar]
- Hayashizaki Y, Hirotsune S, Okazaki Y, Shibata H, Akasako A, Muramatsu M, Kawai J, Hirasawa T, Watanabe S, Shiroishi T, et al. A genetic linkage map of the mouse using restriction landmark genomic scanning (RLGS) Genetics. 1994a;138:1207–1238. doi: 10.1093/genetics/138.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashizaki Y, Shibata H, Hirotsune S, Sugino H, Okazaki Y, Sasaki N, Hirose K, Imoto H, Okuizumi H, Muramatsu M, et al. Identification of an imprinted U2af binding protein related sequence on mouse chromosome 11 using the RLGS method. Nat Genet. 1994b;6:33–40. doi: 10.1038/ng0194-33. [DOI] [PubMed] [Google Scholar]
- Hughes SJ, Glover TW, Zhu XX, Kuick R, Thoraval D, Orringer MB, Beer DG, Hanash S. A novel amplicon at 8p22–23 results in overexpression of cathepsin B in esophageal adenocarcinoma. Proc Natl Acad Sci. 1998;95:12410–12415. doi: 10.1073/pnas.95.21.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- Ohsumi T, Okazaki Y, Hirotsune S, Shibata H, Muramatsu M, Suzuki H, Taga C, Watanabe S, Hayashizaki Y. A spot cloning method for restriction landmark genomic scanning. Electrophoresis. 1995;16:203–209. doi: 10.1002/elps.1150160135. [DOI] [PubMed] [Google Scholar]
- Okazaki Y, Okuizumi H, Sasaki N, Ohsumi T, Kuromitsu J, Kataoka H, Muramatsu M, Iwadate A, Hirota N, Kitajima M, et al. A genetic linkage map of the mouse using an expanded production system of restriction landmark genomic scanning (RLGS Ver.1.8) Biochem Biophys Res Commun. 1994;205:1922–1929. doi: 10.1006/bbrc.1994.2895. [DOI] [PubMed] [Google Scholar]
- Osoegawa K, Tateno M, Woon PY, Frengen E, Mammoser AG, Catanese JJ, Hayashizaki Y, de Jong PJ. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 2000;10:116–128. [PMC free article] [PubMed] [Google Scholar]
- Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, Kato R, Sasaki H, Hirotsune S, Okazaki Y, et al. Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS-M. Nat Genet. 1996;14:106–109. doi: 10.1038/ng0996-106. [DOI] [PubMed] [Google Scholar]
- Rush, L.J. and Plass, C. 2002. Restriction landmark genomic scanning for DNA methylation in cancer: Past, present, and future applications. Analyt. Biochem. (In press). [DOI] [PubMed]
- Rush LJ, Dai Z, Smiraglia DJ, Gao X, Wright FA, Frühwald M, Costello JF, Held WA, Yu L, Krahe R, et al. Novel methylation targets in de novo acute myeloid leukemia with prevalence of chromosome 11 loci. Blood. 2001;97:3226–3233. doi: 10.1182/blood.v97.10.3226. [DOI] [PubMed] [Google Scholar]
- Rush LJ, Heinonen K, Mrozek K, Wolf BJ, Abdel-Rahman M, Szymanska J, Peltomaki P, Kapadia F, Bloomfield CD, Caligiuri MA, et al. Comprehensive cytogenetic and molecular genetic characterization of the TI-1 acute myeloid leukemia cell line reveals cross-contamination with K-562 cell line. Blood. 2002;99:1874–1876. doi: 10.1182/blood.v99.5.1874. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Sekiguchi A, Oates AJ, Terry MJ, Miyamoto Y. HOX gene clusters are hotspots of de novo methylation in CpG islands of human lung adenocarcinomas. Oncogene. 2002a;21:3659–3662. doi: 10.1038/sj.onc.1205453. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Sekiguchi A, Terry MJ, Oates AJ, Miyamoto Y, Chuu YH, Munakata M, Sekiya T. A comprehensive catalog of CpG islands methylated in human lung adenocarcinomas for the identification of tumor suppressor genes. Oncogene. 2002b;21:3804–3813. doi: 10.1038/sj.onc.1205454. [DOI] [PubMed] [Google Scholar]
- Smiraglia DJ, Plass C. The study of aberrant methylation in cancer via restriction landmark genomic scanning. Oncogene. 2002;21:5414–5426. doi: 10.1038/sj.onc.1205608. [DOI] [PubMed] [Google Scholar]
- Smiraglia DJ, Frühwald MC, Costello JF, McCormick SP, Dai Z, Peltomaki P, O'Dorisio MS, Cavenee WK, Plass C. A new tool for the rapid cloning of amplified and hypermethylated human DNA sequences from restriction landmark genome scanning gels. Genomics. 1999;58:254–262. doi: 10.1006/geno.1999.5840. [DOI] [PubMed] [Google Scholar]
- Smiraglia DJ, Rush LJ, Frühwald MC, Dai Z, Held WA, Costello JF, Lang JC, Eng C, Li B, Wright FA, et al. Excessive CpG island hypermethylation in cancer cell lines versus primary human malignancies. Hum Mol Genet. 2001;10:1413–1419. doi: 10.1093/hmg/10.13.1413. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;5507:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wimmer K, Zhu XX, Rouillard JM, Ambros PF, Lamb BJ, Kuick R, Eckart M, Weinhausl A, Fonatsch C, Hanash SM. Combined restriction landmark genomic scanning and virtual genome scans identify a novel human homeobox gene, ALX3, that is hypermethylated in neuroblastoma. Genes Chromosomes Cancer. 2002;33:285–294. doi: 10.1002/gcc.10030. [DOI] [PubMed] [Google Scholar]