Abstract
The βγ subunits of GTP-binding proteins (Gβγ) activate the muscarinic K+ channel (KACh) in heart by direct binding to both of its component subunits. KACh channels can also be gated by internal Na+ ions. Both activation mechanisms show dependence on hydrolysis of intracellular ATP. We report that phosphatidylinositol 4,5-bisphosphate (PIP2) mimics the ATP effects and that depletion or block of PIP2 retards the stimulatory effects of Gβγ subunits or Na+ ions on channel activity, effects that can be reversed by restoring PIP2. Thus, regulation of KACh channel activity may be crucially dependent on PIP2 and phosphatidylinositol signaling. These striking functional results are in agreement with in vitro biochemical studies on the PIP2 requirement for Gβγ stimulation of G protein receptor kinase activity, thus implicating phosphatidylinositol phospholipids as a potential control point for Gβγ-mediated signal transduction.
Keywords: G protein-gated K+ channel; phosphatidylinositol 4,5-bisphosphate
Muscarinic potassium (KACh) channels are targets of parasympathetic control in the heart. Acetylcholine is released upon vagal stimulation and binds m2 receptors in pacemaker and atrial cells to cause an increase in KACh current that leads to a decrease in heart rate (1).
The molecular mechanism of KACh activation has been the subject of intense research for more than a decade. KACh provided the first example of a “membrane-delimited pathway,” where signaling to the final target protein occurs in the plasma membrane, not proceeding through cytoplasmic second messengers (2). βγ subunits of GTP-binding proteins (Gβγ) activate native atrial (KACh) channels (3) and recombinant G protein-gated inwardly rectifying K+ (GIRK) channels (4, 5), when applied to inside-out patches. KAch channels are heterotetramers composed of two of each of the component subunits (GIRK1/GIRK4) (4, 6). Binding studies have revealed similar affinities of the native or recombinant channels for Gβγ subunits, confirming the direct nature of the interaction of these proteins (7–12). Na+ ions have been shown to regulate KACh channel activity (13). In the absence of G protein signaling, magnesium-dependent hydrolysis of ATP alters the KACh residence time in the open state and sensitizes the channel to gating by intracellular Na+ ions (13). We report herein that G protein gating of KACh is also dependent on ATP, because it becomes progressively ineffective (runs down) when internal ATP is removed. We proceed to explore the molecular mechanism by which ATP hydrolysis affects KACh gating by the Gβγ subunits or intracellular Na+ ions.
The role of phosphatidylinositol phosphates as membrane-delimited second messengers is beginning to be appreciated (for review, see ref. 14). Phosphatidylinositol 4,5-bisphosphate (PIP2) has been shown to activate not only channels related to KACh, such as KATP, but also transporters, such as the sodium-calcium exchanger (15, 16). Moreover, anionic phospholipids, and PIP2 in particular, have been shown to directly bind to proteins as diverse as phospholipases, kinases, and cytoskeletal proteins, but the functional importance of such interactions has not been fully appreciated.
Our study shows that the ATP dependence of KACh activity is mediated via phosphoinositol phosphates. Surprisingly, the presence of PIP2 was essential not only for Na+ ion gating but also for efficient Gβγ signaling. These studies reveal a clear functional dependence of G protein signaling on phosphoinositol phospholipids. In light of the dynamic state of phosphoinositol phosphates in plasma membranes, an additional level of regulation may control the function of this channel and its influence on the electrical activity of the heart.
MATERIALS AND METHODS
Expression of Recombinant Channels in Xenopus Oocytes.
Recombinant channel subunits (GIRK1, GenBank accession no. U39196; GIRK4, GenBank accession no. U39195) were expressed in Xenopus oocytes as described (17). Briefly, channel subunit coexpression was accomplished by coinjection of equal amounts of each cRNA (∼4 ng). cRNA concentrations were estimated from two successive dilutions that were electrophoresed in parallel on formaldehyde gels and compared with known concentrations of RNA marker (GIBCO/BRL). Oocytes were isolated and microinjected as described (18). All the oocytes were maintained at 18°C, and electrophysiological recordings were performed 2–6 days after injection.
Preparation of Chicken Atrial Myocytes.
The procedure used for isolating cardiac myocytes from chicken embryos has been described (13). Briefly, atrial tissue was selected from chicken embryos from eggs incubated 14–19 days. Atrial tissue (from four to six eggs) was incubated for 20–30 min at 37°C in a 15-ml Falcon tube containing 5 ml of Mg2+- and Ca2+-free Hanks’ solution supplemented with 1–2% of trypsin/EDTA solution (10×, GIBCO/BRL). Isolated myocytes were collected by triturating the digested tissue in 5 ml of trypsin-free solutions and stored at 4°C for up to 36 h. The cells were allowed to settle on poly-lysine-coated coverslips in the recording chamber before recording.
Reagents.
General chemicals were purchased from Sigma, including GTP, ATP, and catalytic subunit of protein kinase A. PIP and PIP2 were from Boehringer Mannheim. PIP2 was sonicated in cool water or on ice for more than 30 min before application. PIP and PIP2 antibodies were from Perseptive Biosystems (Cambridge, MA). Phosphatidylinositol-specific phospholipase C (PLC) was purchased from Boehringer Mannheim and was diluted in Mg2+-free high potassium bath solution with 0.5 mM MgCl2 added (because the high concentration of Mg2+ inhibits enzyme activation). Purified PLC-β2 protein was provided by R. Iyengar (Mount Sinai School of Medicine). The stock of PLC-β2 (0.3 μg/μl) was dissolved in 10 mM Hepes/100 mM KCl/10 mM NaCl/2 mM EGTA/1 mM MgCl2/1 mM DTT/1.25 mM CaCl2, pH 7.2. This stock solution was made 60×, at a final PLC-β2 concentration of 5 μg/ml. Okadaic acid was purchased from Research Biochemicals. H-7 was purchased form Calbiochem. GIRK4 antibody was purchased from Upstate Biotechnology. GIRK1 antibodies were provided by W. Thornhill (Mount Sinai School of Medicine) and used as described (17).
Single-Channel Recording and Analysis.
Single-channel activity was recorded in the cell-attached or inside-out patch configurations (19, 20) by using an Axopatch 200A amplifier (Axon Instruments, Foster City, CA). All microelectrodes used in the experiments were pulled from WPI-K borosilicate glass (World Precision Instruments, Sarasota, FL) and gave resistances of 3–8 MΩ. All experiments were performed at room temperature (20–22°C). Single-channel recordings were performed at a membrane potential of −80 mV and in the absence of agonist in the pipette, unless otherwise indicated. Single-channel currents were filtered at 1–2 KHz with a 6-pole low-pass Bessel filter, sampled at 5–10 kHz, and stored directly into the computer’s hard disk through the DIGIDATA 1200 interface (Axon Instruments). pclamp (version 6.01, Axon Instruments) software was used for data acquisition.
For Xenopus oocytes, the vitelline membrane was removed by placing the oocytes in a hypertonic solution (21) for 5–10 min. Shrunk oocytes were first transferred into a V-shaped recording chamber and the vitelline membrane was partially removed, exposing just enough plasma membrane for access with a patch pipette. This procedure increased the success rate of forming gigaseals and reduced automatic detaching in long-term cell-attached recordings. The pipette solution contained 96 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes (pH 7.35). The bath solution contained 96 mM KCl, 5 mM EGTA, and 10 mM Hepes (pH 7.35). Gadolinium at 100 μM was routinely added to the pipette solution to suppress native stretch channel activity in the oocyte membrane.
For chicken atrial cells, the experimental solutions were the same as those used with oocyte recordings except that 140 mM KCl (for mammalian cells) instead of 96 mM KCl (for Xenopus oocytes) was used and that recordings were without gadolinium because native atrial stretch channels did not present a problem.
Free Mg2+ and ATP concentrations were estimated as described (22). We generally applied purified G protein subunits to inside-out patches within 1–2 min after patch excision or withdrawal of MgATP, unless otherwise indicated. Under these conditions activation began within 1 min and reached steady-state levels within 2–3 min of application.
Single-channel records were analyzed by using pclamp software, complemented with our own analysis routine, as described (13). Parameters used for single-channel analysis include activity of all the channels in the patch (or the total open probability, NPo), and the mean open time (MTo). Results are displayed as averages over 5-s bins.
RESULTS
GTP applied at the intracellular side of an atrial inside-out patch, in the presence of acetylcholine in the pipette, activates KACh (e.g., ref. 3). In the absence of internal ATP however, the GTP-induced activation is transient, showing rundown characteristics with variable kinetics (Fig. 1A, n = 14). Internal ATP has been shown to have additional effects on KACh activity. Hydrolysis of ATP has been shown to enhance GTP-induced activity (23, 24) and also to sensitize native and recombinant KACh channels to gating by internal Na+ ions (13). The precise mechanism underlying the ATP effects on KACh activity has not been elucidated, although mechanisms such as nucleoside diphosphate kinase mediating phosphotransfer to the G protein from ATP or a membrane-bound kinase phosphorylating the channel protein have been proposed (23, 24). At first we explored the possibility that protein phosphorylation underlies the ATP-dependent effects on channel activity. H7 (100 μM), a nonselective kinase inhibitor, applied before or during MgATP/Na-stimulated activity had no apparent effects on channel activity (n = 7, data not shown). Internal treatment of inside-out patches with the catalytic subunit of protein kinase A (40–100 units/ml) failed to activate KACh (n = 6, data not shown). Okadaic acid (1 μM), an inhibitor of phosphatases 1 and 2A, did not affect MgATP/Na activation nor did it maintain channel activity, after washout of MgATP (n = 6, data not shown). These discouraging preliminary results turned our attention to a different direction.
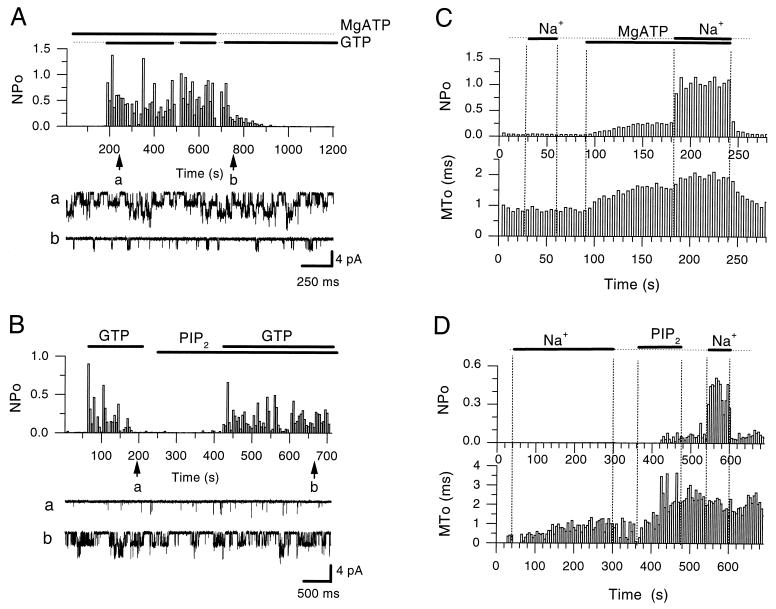
Figure 1.
PIP2 mimics the MgATP dependence of the rundown of G protein-mediated activation as well as channel sensitization to gating by internal Na+ ions. (A) NPo plot of KACh channel activity in an inside-out patch from a chicken embryonic atrial myocyte, showing that in the presence of ATP (2 mM, sodium salt, in the presence of 0.6 mM Mg2+ in the bath solution), GTP (100 μM) produced sustained activity (10 μM acetylcholine was present in the pipette). Withdrawal of ATP caused a gradual rundown of the GTP-induced activity. As can be seen at the beginning of the record ATP perfusion alone did not activate the channel. Application of each nucleotide is indicated by the bars. Representative single-channel currents are shown before and after the rundown of the activity, as indicated at time points a and b during the course of the experiment. (B) Plot similar to that in A, showing that PIP2 restored the ability of GTP to stimulate channel activity and mimicked ATP in its ability to prevent rundown of the GTP-induced channel activity. Acetylcholine at 5 μM was present in the pipette solution. Perfusion of the inside-out patch with GTP (100 μM) and PIP2 (5 μM) in the bath solution is indicated by bars. The arrows labeled a and b show time points during which rundown and restored single channel activity is shown, respectively. PIP2, like ATP, did not stimulate channel activity when perfused alone. (C) NPo and MTo plots of KACh channel activity in inside-out patches from Xenopus oocyte coinjected with GIRK1/GIRK4 cRNAs. In the absence of MgATP, the channels did not respond to Na+ stimulation (20 mM). MgATP (5 mM) modified channels to a longer-lived open state but produced little activation. After this effect, channels responded to Na+ by increasing NPo with no further significant change in MTo. (D) Experiment similar to that in A from another patch showing that PIP2 (1 μM) produced similar effects on MTo and sensitization to gating by intracellular Na+ (20 mM). PIP2 application, unlike ATP, had long lasting effects and, therefore, it was not necessary to apply it with Na+ ions for maximal effects.
PIP2 could mimic the ATP-dependent effects. Fig. 1B (n = 4) shows a representative experiment in which 5 μM PIP2, just like MgATP (Fig. 1A), prevented rundown of the GTP-induced channel activity (NPo).
Similarly, PIP2 mimicked the MgATP sensitization to gating by Na+ ions of both native atrial and recombinant KACh channels (coexpressed GIRK1/GIRK4 subunits in Xenopus oocytes). Fig. 1C (n > 10) compares the effects of the MgATP exposure with that of PIP2 (Fig. 1D, n = 9) on recombinant channels. Application of Na+ ions to inside-out patches in solutions lacking MgATP or PIP2 did not produce appreciable activity or alter the open time kinetics. Openings in both cases were short-lived with mean open times in the order of 1 ms or less (MTo). In contrast, treatment with MgATP or PIP2 produced longer-lived openings and a small corresponding effect on activity. Such modification sensitized the channel to gating by internal Na+ ions, application of which produced a large increase in activity. The PIP2 treatment, unlike the corresponding MgATP perfusion, did not require concurrent application with Na+ ions for maximal effects. The PIP2 effect was longer lasting on the channel mean open time and sensitization to Na+ ions (Fig. 1D) than the MgATP treatment (ref. 13 and Fig. 1C).
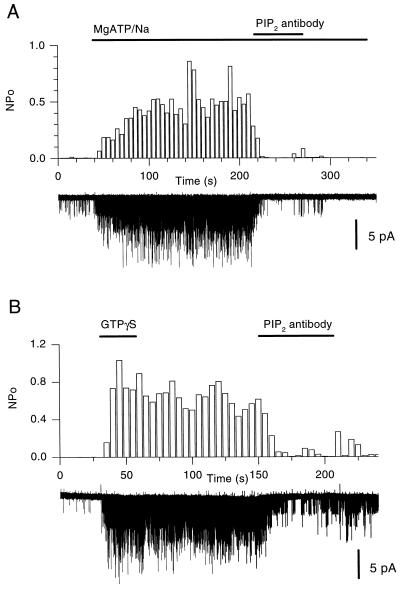
We reasoned that block of PIP2 ought to abolish the MgATP effects on channel activity, if these effects were due to formation of PIP2 from ATP-mediated phosphorylation of phosphatidylinositol. Fig. 2A (n = 11) shows that treatment of a Xenopus oocyte inside-out patch expressing recombinant KACh with an anti-PIP2 antibody (PIP2-Ab) blocked the ability of MgATP and Na+ ions to sustain channel activity. The effect of the PIP2-Ab was rapid and robust. An anti-PIP antibody also inhibited KACh activity but was not as effective as the PIP2-Ab (n = 6, data not shown). Similar experiments using antibodies to either the Kv1.1 or the GIRK4 (N-terminal antigen) channels were without effect (n = 4, data not shown). A GIRK1 (C-terminal antigen) antibody caused a small decrease in channel activity but much less than the consecutive treatment with PIP2-Ab (n = 4, data not shown). Similar results were obtained with native KACh channels from atrial cells (n = 7, data not shown). The nonhydrolyzable analog of GTP, guanosine 5′-[γ-thio]triphosphate (GTP[γS]) causes persistent KAch channel activation (e.g., ref. 3). PIP2-Ab treatment of an atrial inside-out patch preactivated by GTP[γS], blocked KACh activity in a manner similar to that seen with the MgATP/Na-activated channels (Fig. 2B, n = 4). To rule out that the PIP2-Ab block was due to a nonprotein impurity, boiled PIP2-Ab (30 min) was compared in the same patch to unboiled PIP2-Ab. In all cases boiled PIP2-Ab was without effect, whereas unboiled PIP2-Ab potently blocked channel activity (n = 7, data not shown). Similar inhibitory actions of the PIP2-Ab were obtained with recombinant GTP[γS]-stimulated KACh channels. However, the ability of GTP[γS] to stimulate recombinant KAch channels was not as reproducible, probably in part due to the limiting endogenous expression of G protein subunits relative to the overexpressed channel (data not shown). PIP2-Ab was effective also in blocking recombinant KACh channels, previously activated with Gβγ subunits (n = 3, data not shown).
Figure 2.
PIP2-Ab blocks the MgATP/Na or GTP[γS] activation of KACh channels. (A) Single-channel records and analyzed activity (NPo) plots at the same compressed time scale, from an inside-out patch obtained from a Xenopus oocyte expressing KACh (GIRK1/GIRK4). KACh activation with MgATP/Na (5/20 mM) was blocked by PIP2-antibody (titer, 1:1 or 1:500 dilution from manufacturer’s stock) rendering further MgATP/Na perfusion ineffective. (B) Plot similar to that in A showing GTP[γS]-mediated persistent activation (10 μM) and PIP2-Ab (titer, 1:2 or 1:250 dilution from manufacturer’s stock) block from an inside-out patch obtained from an atrial cell (5 μM acetylcholine was present in the pipette solution).
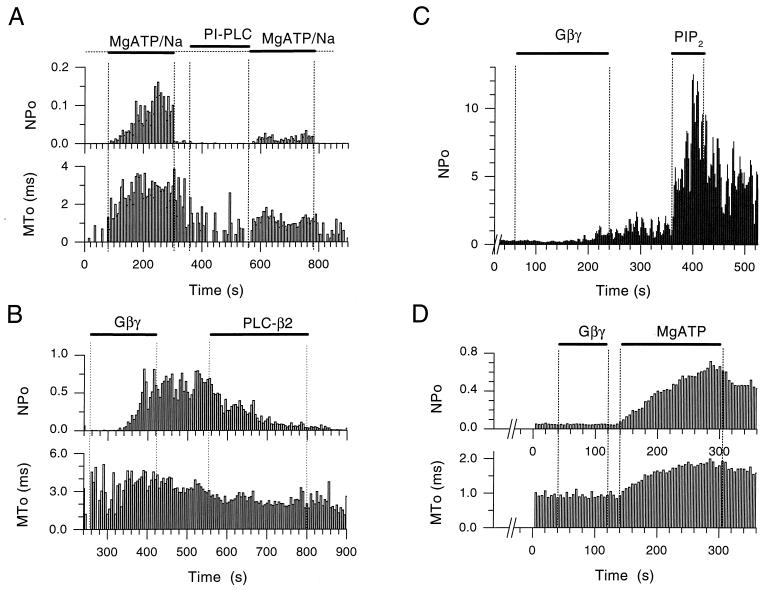
To further test the hypothesis that PIP2 plays a central role in the control of channel gating by internal Na+ ions or G protein subunits, we tested the effects of inositol-specific phospholipases on recombinant KACh channels expressed in Xenopus oocytes. We first used a phosphatidylinositol-specific PLC that hydrolyzes phosphatidylinositol, thus preventing accumulation of PIP2. Fig. 3A (n = 7) shows that such treatment greatly limited channel sensitization to Na+ by MgATP, producing channel openings with a decreased mean open time, consistent with the notion that the PIP2 levels were decreased. Gβγ subunits cause a persistent stimulation of KACh activity. Experiments in which KACh was activated by Gβγ subunits showed no significant decline in activity in the first 5 min after the end of Gβγ perfusion. In Fig. 3B (n = 7), channel activation by Gβγ subunits was followed by a 4-min treatment with PLC-β2, which hydrolyzes PIP2 to inositol trisphosphate and diacylglycerol (DAG). PLC-β2 treatment reversed channel activation by Gβγ. PLC-β2-treated patches showed on the average a 60% decrease in Gβγ-induced activity in the first 2 min of the treatment, whereas in the 2 min prior to the onset of PLC-β2 application, no significant decrease in activity was obtained (n = 3). Thus, the PLC-β2-mediated inhibition of the Gβγ-induced activity was considerably faster than the persistent unopposed Gβγ stimulated activity. Again, the PLC-β2 treatment resulted in openings with decreased open times, consistent with the effect of a reduction in PIP2 levels. It should be noted that the reduction in MTo of the Gβγ-stimulated activity after the PLC-β2 treatment was not as pronounced as that after similar treatment of Na+-stimulated activity.
Figure 3.
Depletion of PIP2 by PLCs blocks MgATP and Gβγ stimulation of KACh channel activity and restoration of PIP2 reverses these effects. (A) Single-channel NPo and MTo plots as a function of time in the experiment were obtained from an inside-out patch of a Xenopus oocyte expressing GIRK1/GIRK4. The KACh channels were first activated by application of MgATP/Na (5/20 mM). Both NPo and MTo were increased by MgATP/Na. Subsequent treatment of the inside-out patch with phosphatidylinositol-specific PLC (1 unit/ml) for approximately 2.5 min, significantly reduced activation by MgATP/Na, causing a concomitant decrease in MTo. (B) Single-channel NPo and MTo plots, obtained from an inside-out patch of a Xenopus oocyte expressing GIRK1/GIRK4. Soon after excision, Gβγ application (20 nM) caused persistent channel activation. Subsequent treatment of the patch with PLC-β2 (5 μg/ml) inhibited activity with a concomitant decrease in MTo. (C) NPo plot as a function of time in the experiment from an inside-out patch of Xenopus oocyte expressing GIRK1/GIRK4. The patch was exposed to PLC-β2 (5 μg/ml) for a period greater than 5 min, before Gβγ (20 nM) application. PLC-β2 treatment greatly retarded Gβγ effectiveness. Subsequent perfusion with PIP2 (5 μM) revealed high channel activity, thus rescuing the Gβγ action. The number of active KAch channels in the membrane was greater than 5, thus precluding analysis of MTo [see Sui et al. (13)]. (D) Single-channel NPo and MTo plots, obtained from an inside-out patch of Xenopus oocyte expressing GIRK1/GIRK4. Perfusion of the patch with ATP-free solutions for several minutes rendered Gβγ (20 nM) ineffective. Subsequent addition of MgATP (5 mM) to the membrane revealed high channel activity, thus rescuing the Gβγ action, which was presumably still bound but ineffective.
Under conditions that depleted PIP2, Gβγ treatment of inside-out patches could be rendered ineffective. Fig. 3 C and D shows two such examples together with restoration of PIP2 and rescue of the Gβγ effect. Fig. 3C (n = 6) shows that Gβγ application after pretreatment of an inside-out patch with PLC-β2 for a period greater than 5 min was without a large effect. Subsequent perfusion of the patch with 5 μM PIP2 resulted in great activity, restoring the effectiveness of Gβγ. This result suggested that the G protein complex remained bound but incapable of gating the channel efficiently, until PIP2 became available. In Fig. 3D (n = 13) after a long perfusion of the inside-out patch with MgATP-free solutions, Gβγ was unable to activate KACh. However, when MgATP was provided, it modified the channel into a longer-lived open state and revealed significant levels of activity. Because MgATP alone does not produce such high levels of activity (see Fig. 1C and ref. 13), this activation again would be attributed to the continued presence of Gβγ, presumably through its tight binding to the channel. Thus, lack of MgATP inhibited and its reperfusion rescued Gβγ effectiveness. These results corroborated the PIP2-Ab results, strongly implicating a permissive role for PIP2 in the gating of KACh channels.
Is the effector molecule PIP2 itself or its hydrolysis products (inositol trisphosphate and DAG)? Both PIP2 block (by the PIP2-Ab) and PIP2 depletion (by the PLCs) had similar inhibitory effects on channel activity. This inhibitory effect could not be due to PIP2 hydrolysis products because the action of the PIP2-Ab and the PLC treatment would have opposite effects on the levels of these metabolites. In direct tests, neither inositol trisphosphate nor DAG were successful in activating KACh, whereas MgATP/Na in the same patches caused on the average at least 10-fold higher channel activity (n = 4, data not shown). In addition, these hydrolysis products failed to inhibit activity after stimulation by MgATP/Na (n = 4, data not shown). Application of arachidonic acid to inside-out patches had no effect on activation or block of KACh activity (n = 5, data not shown), whereas MgATP/Na caused on the average a 15-fold increase in activity. Although arachidonic acid has been shown to cause KACh activation in the cell-attached mode (25, 26), Kurachi et al. (25) also found it ineffective when they tested it in inside-out patches. This indicates that the PIP2 effects were not due to hydrolysis of its metabolite DAG to arachidonic acid. Thus, these results suggest that PIP2 acted directly rather than through its hydrolysis products.
DISCUSSION
ATP-dependent regulation of protein function, requiring ATP hydrolysis distinct from that involved in protein phosphorylation, is beginning to receive its overdue attention. In particular, evidence for the generation of phosphatidylinositol phosphates by lipid kinases, acting as membrane-delimited second messengers, to regulate ion channel and transporter activity has been presented (for review, see ref. 14). KATP channels, which belong to the same family of inwardly rectifying K+ channels as KACh, were shown to be stimulated by anionic phospholipids and PIP2, in particular. PIP2 mimicked ATP in preventing channel current rundown or in rescuing activity after rundown (16). Other recombinant inwardly rectifying channels, such as ROMK1 or IRK1, that exhibit MgATP-dependent rundown of their activity were stimulated by PIP2, as well (27).
In the case of the G protein-gated K+ channel, phosphatidylinositol phospholipids seem to play a permissive role by regulating the effectiveness of gating molecules such as Gβγ subunits or Na+ ions, rather than activating the channel themselves. The effects of PIP2 cannot be explained by an indirect action of its hydrolysis products, as these did not mimic the PIP2 effects. Similarly, the PIP2 effects were not due to production of arachidonic acid from the hydrolysis of DAG. Could the PIP2 effects be the result of its conversion to PIP3? Although PIP3 effects were not tested directly, it is unlikely that this phospholipid plays a major role in the effects described, because it is a trace membrane component and also because the MgATP effect could be reversed by PIP2-specific proteins (i.e., the antibody and PLC-β2). However, the specificity of PIP2 phospholipids remains to be more carefully established.
G protein-coupled receptor kinases have been shown to interact directly with anionic phospholipids (28–31). This interaction was shown to be functionally important because it regulated the ability of such kinases to phosphorylate G protein-coupled receptors and initiate their desensitization. Moreover, Gβγ subunits bind G protein-coupled receptor kinases directly to stimulate receptor phosphorylation, a property that was found to depend on anionic phospholipids and PIP2, in particular (28). Thus, the G protein-gated K+ channels, just as G protein-coupled receptor kinases, are lipid-dependent proteins that require phosphatidylinositol phospholipids for regulation of their activity by Gβγ subunits. It is intriguing that other Gβγ-sensitive proteins are also PIP2 binding proteins, (e.g., PLCβ isoforms, phosphatidylinositol 3-kinase). A possible PIP2 dependence on the Gβγ regulation of the activity of such proteins remains to be examined. However, a PIP2 requirement for activation of Gβγ-insensitive proteins, such as phospholipase D, has been reported as well (32).
Enzymes containing pleckstrin homology domains have been found in a variety of proteins (33, 34), including protein kinases (e.g., G protein-coupled receptor kinases and protein kinase C), substrates for kinases, regulators of small G proteins, PLC isozymes, and cytoskeletal proteins. Interestingly, this domain has been reported to bind PIP2 (33, 34) but also Gβγ subunits (35, 36) and protein kinase C (37). Although specific PIP2-binding sites have been identified in proteins such as the cytoskeletal protein α-actinin (38) and in G protein-coupled receptor kinases (29–31), equivalent sites were not immediately obvious from examination of the KACh channel primary amino acid sequences.
PIP2 is a central molecule in the phosphoinositide cycle, by serving as the precursor of important signaling molecules such as inositol trisphosphate, DAG or PI(3,4,5)P3. In addition, the results from this study and others demonstrate that PIP2 can serve as a direct membrane-delimited second messenger molecule itself. Moreover, the functional dependence of Gβγ signaling on PIP2 predicts the possible cross-talk of different membrane-delimited signaling pathways, analogous to that seen with soluble second messengers.
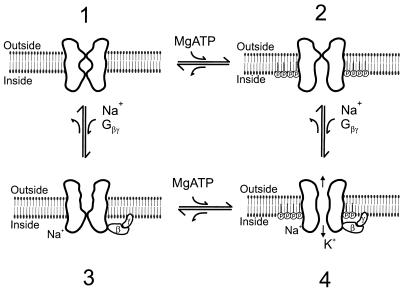
Fig. 4 shows a model depicting direct interactions of phosphorylated phosphatidylinositol phospholipids with the channel protein and their crucial role on channel activation by Gβγ subunits or Na+ ions. A two-gate model is postulated. One of the gates is sensitive to phosphorylated inositol phospholipids, and the other gate is sensitive to the channel gating molecules Gβγ or Na+ ions. In the presence of MgATP, phosphorylated phospholipids are produced but on their own are incapable of efficiently gating the channel open (state 2). In the absence of MgATP (and therefore phosphoinositides), gating molecules of KACh (such as Gβγ subunits or Na+ ions) are ineffective in activating the channel (state 3). However, when both phosphorylated phospholipids and gating molecules are present, both gates are open and the channel becomes permeable to K+ ions (state 4).
Figure 4.
Two-gate model of KACh activation. State 1. In the absence of MgATP and Na+ ions or Gβγ subunits both channel gates are closed. State 2. In the presence of MgATP, inositol phospholipids are phosphorylated by lipid kinases to produce phosphoinositides. Direct interaction of phosphoinositides with the channel protein results in opening of the bottom gate. However, K+ ion permeation is limited or absent, because the top gate is closed. State 3. In the presence of the channel gating molecules (Na+ ions or Gβγ complex) but in the absence of MgATP, the top gate is open but K+ ion permeation is limited or absent, because the bottom gate is closed. State 4. In the presence of intact phosphoinositides and gating molecules (Na+ ions or Gβγ subunits), both gates are open resulting in K+ ion permeation.
Acknowledgments
We appreciate the open-spirited discussions with Drs. Don Hilgemann and Chou-Long Huang. We thank Dr. Massimo Sassaroli for advice regarding handling of lipids, and Dr. Cheng He and Xiaying Wu for advice and technical assistance. We are grateful for the gifts of purified PLC-β2 from Dr. Ravi Iyengar, GIRK1 and Kv1.1 antibodies from Dr. William Thornhill, and purified Gβγ from John Hildebrandt. Finally, we thank Drs. Evgeny Kobrinsky, Noelle Langan, Robert Margolskee, Tooraj Mirshahi, Eitan Reuveny, Harel Weinstein, and Hailin Zhang for helpful comments on the manuscript. This work was supported by a fellowship to J.L.S. from the New York Aaron Diamond Foundation and a grant from the National Institutes of Health (HL54185).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: KACh, muscarinic K+ channel; Gβγ, βγ subunits of GTP-binding proteins; PIP2, phosphatidylinositol 4,5-bisphosphate; GIRK, G protein-gated inwardly rectifying K+ channel; PLC, phospholipase C; MTo, mean open time; NPo, activity of N channels in the patch; GTP[γS], guanosine 5′-[γ-thio]triphosphate; DAG, diacylglycerol.
References
- 1.Sui, J.-L., Chan, K., Langan, M.-N., Vivaudou, M. & Logothetis, D. E. in Advances in Second Messenger and Phosphoprotein Research, eds. Armstrong, D. & Rossie, S., in press. [DOI] [PubMed]
- 2.Soejima M, Noma A. Pflügers Arch. 1984;400:424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- 3.Logothetis D E, Kurachi Y, Galper J, Neer E J, Clapham D E. Nature (London) 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- 4.Krapivinsky G, Gordon E A, Wickman K, Velimirovic B, Krapivinsky L, Clapham D E. Nature (London) 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 5.Reuveny E, Slesinger P A, Inglese J, Morales J M, Iniguez-Lluhi J A, Lefkowitz R J, Bourne H R, Jan Y N, Jan L Y. Nature (London) 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- 6.Silverman S K, Lester H A, Dougherty D A. J Biol Chem. 1996;271:30524–30528. doi: 10.1074/jbc.271.48.30524. [DOI] [PubMed] [Google Scholar]
- 7.Krapivinsky G, Krapivinsky L, Wickman K, Clapham D E. J Biol Chem. 1995b;270:29059–29062. doi: 10.1074/jbc.270.49.29059. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel M T, Peralta E G. Cell. 1995;83:443–449. doi: 10.1016/0092-8674(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 9.Huang C-L, Slesinger P A, Casey P J, Jan Y N, Jan L Y. Neuron. 1995;15:1133–1143. doi: 10.1016/0896-6273(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 10.Huang C-L, Jan Y N, Jan L Y. FEBS Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- 11.Inanobe A, Morishige K I, Takahashi N, Ito H, Yamada M, Takumi T, Nishina H, Takahashi K, Kanaho Y, Katada T, Kurachi Y. Biochem Biophys Res Commun. 1995;212:1022–1028. doi: 10.1006/bbrc.1995.2072. [DOI] [PubMed] [Google Scholar]
- 12.Doupnik C A, Dessauer C W, Slepak V Z, Gilman A G, Davidson N, Lester H A. Neuropharmacology. 1996;35:923–931. doi: 10.1016/0028-3908(96)00125-6. [DOI] [PubMed] [Google Scholar]
- 13.Sui J L, Chan K W, Logothetis D E. J Gen Physiol. 1996;108:381–391. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilgemann D W. Annu Rev Physiol. 1997;59:193–220. doi: 10.1146/annurev.physiol.59.1.193. [DOI] [PubMed] [Google Scholar]
- 15.Hilgemann D W, Ball R. Science. 1996;273:956–959. doi: 10.1126/science.273.5277.956. [DOI] [PubMed] [Google Scholar]
- 16.Fan Z, Makielski J C. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- 17.Chan K W, Langan M N, Sui J L, Kozak J A, Pabon A, Ladias J A A, Logothetis D E. J Gen Physiol. 1996;107:381–397. doi: 10.1085/jgp.107.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logothetis D E, Movahedi S, Satler C, Lindpaintner K, Nadal-Ginard B. Neuron. 1992;8:531–540. doi: 10.1016/0896-6273(92)90281-h. [DOI] [PubMed] [Google Scholar]
- 19.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 20.Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Pflügers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 21.Stühmer W. Methods Enzymol. 1992;207:319–339. doi: 10.1016/0076-6879(92)07021-f. [DOI] [PubMed] [Google Scholar]
- 22.Vivaudou M B, Arnoult C, Villaz M. J Membr Biol. 1991;122:165–175. doi: 10.1007/BF01872639. [DOI] [PubMed] [Google Scholar]
- 23.Heidbüchel H, Callewaert G, Vereecke J, Carmeliet E. Pflügers Arch. 1990;416:213–215. doi: 10.1007/BF00370246. [DOI] [PubMed] [Google Scholar]
- 24.Kim D. J Physiol (London) 1991;437:133–155. doi: 10.1113/jphysiol.1991.sp018588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurachi Y, Ito H, Sugimoto T, Shimizu I, Miki I, Ui M. Nature (London) 1989;337:555–557. doi: 10.1038/337555a0. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Lewis L L, Graziadei L, Neer E J, Bar-Sagi D, Clapham D E. Nature (London) 1989;337:557–560. doi: 10.1038/337557a0. [DOI] [PubMed] [Google Scholar]
- 27.Huang C-L, Hilgemann D W. Biophys J. 1997;72:A250. [Google Scholar]
- 28.DebBurman S K, Ptasienski J, Benovic J L, Hosey M M. J Biol Chem. 1996;271:22552–22562. doi: 10.1074/jbc.271.37.22552. [DOI] [PubMed] [Google Scholar]
- 29.Pitcher J A, Touhara K, Payne E S, Lefkowitz R J. J Biol Chem. 1995;270:11707–11710. doi: 10.1074/jbc.270.20.11707. [DOI] [PubMed] [Google Scholar]
- 30.DebBurman S K, Ptasienski J, Boetticher E, Lomansney J W, Benovic J L, Hosey M M. J Biol Chem. 1995;270:5742–5747. doi: 10.1074/jbc.270.11.5742. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher J A, Fredericks Z L, Stone W C, Premont R T, Stoffel R H, Koch W J, Lefkowitz R J. J Biol Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- 32.Pertile P, Liscovitch M, Chalifa V, Cantley L C. J Biol Chem. 1995;270:5130–5135. doi: 10.1074/jbc.270.10.5130. [DOI] [PubMed] [Google Scholar]
- 33.Harlan J E, Hajduk P J, Yoon H S, Fesik S W. Nature (London) 1994;371:168–170. doi: 10.1038/371168a0. [DOI] [PubMed] [Google Scholar]
- 34.Mayer B J, Ren R, Clark K L, Baltimore D. Cell. 1993;73:629–630. doi: 10.1016/0092-8674(93)90244-k. [DOI] [PubMed] [Google Scholar]
- 35.Touhara K, Inglese J, Pitcher J A, Shaw G, Lefkowitz R J. J Biol Chem. 1994;269:10217–10220. [PubMed] [Google Scholar]
- 36.Tsukada S, Simon M I, Witte O N, Katz A. Proc Natl Acad Sci USA. 1994;91:11256–11260. doi: 10.1073/pnas.91.23.11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao L, Kawakami Y, Kawakami T. Proc Natl Acad Sci USA. 1994;91:9175–9179. doi: 10.1073/pnas.91.19.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukami K, Sawada N, Endo T, Takenawa T. J Biol Chem. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]