Abstract
New genes that originated by various molecular mechanisms are an essential component in understanding the evolution of genetic systems. We investigated the pattern of origin of the genes created by retroposition in Drosophila. We surveyed the whole Drosophila melanogaster genome for such new retrogenes and experimentally analyzed their functionality and evolutionary process. These retrogenes, functional as revealed by the analysis of expression, substitution, and population genetics, show a surprisingly asymmetric pattern in their origin. There is a significant excess of retrogenes that originate from the X chromosome and retropose to autosomes; new genes retroposed from autosomes are scarce. Further, we found that most of these X-derived autosomal retrogenes had evolved a testis expression pattern. These observations may be explained by natural selection favoring those new retrogenes that moved to autosomes and avoided the spermatogenesis X inactivation, and suggest the important role of genome position for the origin of new genes.
[The sequence data from this study have been submitted to GenBank under accession nos. AY150701–AY150797. The following individuals kindly provided reagents, samples, or unpublished information as indicated in the paper: M.-L. Wu, F. Lemeunier, and P. Gibert.]
New genes that originated by various molecular mechanisms are an essential component in understanding the evolution of genetic systems (Long 2001). These mechanisms include the classic mechanism of duplication (Ohno 1970), exon shuffling (Gilbert 1978), retroposition (Brosius 1991), and gene fusion through deletions or recruitment of new regions (Nurminsky et al. 1998), or a combination of these mechanisms (Long and Langley 1993; Begun 1997; Nurminsky et al. 1998). Despite the progress in recent years (Long 2001), little is known about the general pattern of new gene origination, because of the challenge to identify new genes in adequate numbers for pattern analysis.
There is increasing evidence, fortunately, that retroposition, which generates new genes in new genomic positions via reverse transcription of mRNA from a parental gene, is important for the origin of new gene functions (Brosius 1999). In mammalian systems, a classic example is the human retrogene Pgk-2 with male specific function (McCarrey and Thomas 1987). Pgk-2 is autosomal (chromosome 19) whereas the parental copy Pgk-1 is X-linked. Pgk-2 evolved late spermatogenesis-specific expression. This new expression pattern is related to the fact that late spermatogenesis cells are the only ones that do not express Pgk-1 because of male germline X inactivation (McCarrey 1994). Subsequent analyses of retroposed genes in mammalian genomes suggested that retroposition had efficiently sown the seeds of evolution in genomes (Brosius 1991). Among invertebrate systems, Drosophila genomes have been found containing a number of young genes recently created by retroposition. For example, the sphinx gene in Drosophila melanogaster and the jingwei gene in the Drosophila yakuba clade were created within 2–3 Myr by retroposition from parental genes encoding ATP synthase and alcohol dehydrogenase, respectively (Long and Langley 1993; Long et al. 1999; Wang et al. 2000, 2002). In general, recently completed genome sequences in humans (Lander et al. 2001; Venter et al. 2001) and Drosophila melanogaster (Adams et al. 2000) contain new genes created by retroposition which provide opportunities to examine the pattern of origin of new genes.
We investigated the pattern of new genes created by retroposition in the Drosophila genome. New retroposed gene copies are identified by examining hallmarks of retroposition (Li 1997): (1) one member of the pair is intronless in the coding region of sequence similarity (new copy), whereas the other has introns (parental copy); (2) one of them contains a polyA tract (new copy), if both copies are intronless; (3) the new copy may still be flanked by short duplicate sequences. The analyses of these Drosophila retrogenes (analysis of expression, substitution, and population genetics) revealed that these genes are functional. The study of the direction of retroposition showed a surprising asymmetric pattern. There is a significant excess of retrogenes that originate from the X chromosome and retropose to autosomes. These retrogenes evolved a testis expression pattern. We discuss possible explanations and conclude that these observations may be explained by natural selection favoring those new retrogenes that moved to autosomes and avoided the spermatogenesis X inactivation. Our results support the important role of genome position in new genes evolution.
RESULTS AND DISCUSSION
We have identified, from the annotated genes in the D. melanogaster genome, all pairs of homologs (70% amino acid identity or more) that are located on different chromosomes with hallmarks of retroposition (Table 1). Twenty-four young paralogous pairs fulfilled these criteria: 23 pairs in which the new copy lost the introns (CG12628, one of the 23, is additionally flanked by short repeats), and one pair with no introns in either copy but with the new copy retaining a degenerated poly-A tract (CG 12324/Rp515A). Interestingly, CG12628, which seems to be the youngest of the described retrogenes, is the only one that retains the direct repeats, a hallmark of the recent insertion event. Some other retrogenes also retained a degenerated poly-A tract: CG12628, CG10174, and CG13732. The parental genes have diverse functions, consistent with results from the human genome (Gonçalves et al. 2000).
Table 1.
Young Retroposed Genes in the Drosophila melanogaster Genome Compared to Its Parental Genes
| # | New genes | Parental genes | Gene type | KA/Ks | Ks | ||||
|---|---|---|---|---|---|---|---|---|---|
| Locus | Position | Expression | Locus | Position | Expression | ||||
| 1 | CG12628 | 2L_40D | — | Mgst1 | X_19E | GH/LP | Glutathion transferase | 0.5370 | 0.03015 |
| 2 | CG12324 | 2R_47C | LP | RpS15A | X_11E | AT/GM | Ribosomal protein | 0.2488 | 0.04035 |
| 3 | CG10174 | 2L_36F | at | Dntf2 | X_19E | GH/LP | Transporter | 0.3426 | 0.16811 |
| 4 | CG13732 | 3L_74C | at | CG15645 | X_13E | a | Unknown | 0.7951 | 0.19015 |
| 5 | CG4960 | 3R_96F | AT | CG8331 | 2R_50E | GM/GH/LP | Membrane protein | 0.3630 | 0.32681 |
| 6 | Act5C | X_5C | LD/LP/GH | Act57B | 2R_57B | AT/GM/HL | Actin | 0.0744 | 0.33619 |
| 7 | CG17856 | 3R_98C | at | CG3560 | X_14B | GH/LP | NAD dehydrogenase | 0.1767 | 0.67448 |
| 8 | CG11825 | 2R_47A | — | CG17734 | 3R_86D | LP | Unknown | 0.1691 | 0.73976 |
| 9 | CG12334 | 3R_90C | AT | CG1534 | X_9E | GM/LP/LD | Unknown | 0.1405 | 0.73999 |
| 10 | Act42A | 2R_42A | AT | Act79B | 3L_79B | GH/LP | Actin | 0.0400 | 0.74529 |
| 11 | Vha36 | 2R_52A | AT/GM/GH | CG8310 | X_3A | — | Transporter | 0.1583 | 0.80580 |
| 12 | Trxr2 | 3L_79E | AT | Trxr1 | X_7D | AT/GM/LD | Glutathion reductase | 0.2102 | 0.89926 |
| 13 | CG7768 | 3L_70D | AT | Cyp33 | 2R_54C | LD | Chaperone | 0.2940 | 0.90764 |
| 14 | Ef1α48E | 2R_48D | LD/HL/SD | Ef1α100E | 3R_100E | HL/LP | Translation Ef. | 0.0654 | 0.91664 |
| 15 | CG7235 | 2L_25F | AT/GH | Hsp60 | X_10A | AT/GM/LD | Chaperone | 0.1362 | 0.92378 |
| 16 | Pros28.1A | 3R_92F | AT/LP | Pros28.1 | X_14B | LD | Endopeptidase | 0.1637 | 0.95103 |
| 17 | CanB | X_4F | at/gdm | CanB2 | 2R_43E | SD | Protein phosphatase | 0.0177 | 1.03241 |
| 18 | CG9819 | X_14F | LP | CanA1 | 3R_100B | GH | Protein phosphatase | 0.1007 | 1.16483 |
| 19 | CG9873 | 2R_59C | at | CG9091 | X_13B | GM/SD/LP | Ribosomal protein | 0.1693 | 1.22348 |
| 20 | Sep5 | 2R_43F | LD | Sep2 | 3R_92E | GM/LD/SD | Cytoskeletal protein | 0.1647 | 1.23927 |
| 21 | CG13340 | 2R_50C | AT | CG8040 | 3L_67D | AT/GH/LP | Peptidase | 0.1256 | 1.31892 |
| 22 | Cdlc2 | 2L_22A | AT/GH/LP | ctp | X_4C | AT/GM/LP | Dynein light chain | 0.0030 | 1.43721 |
| 23 | CG4706 | 3R_86D | AT | Acon | 2L_39B | GM/LD/HL | Aconitase | 0.0789 | 1.52027 |
| 24 | CG8602 | 3L_65F | GH/LD/SD | CG12194 | 2L_25B | — | Sugar transporter | 0.1099 | 1.56227 |
This is a subsample of young retroposed copies whose parental gene lies in a different chromosome (see text for details). The KA/KS ratio is in bold when it is significantly smaller than 0.5. We have checked expression experimentally for some genes in adult males and females (a), gonadectomized males (gdm), and testis (at) (see Fig. 1 for details) and used information from Berkeley Drosophila Genome Project (BDGP) EST libraries for the other genes. Tissues in this latter case are named following BDGP nonmenclature: LD (embryos), LP (larvae and early pupae), HL and GH (both adult heads), SD (Schneider L2 cells), AT (adult testis), and GM (ovaries). When the gene is expressed in more than three tissues, only the three in which the gene is most highly expressed or more relevant for discussion, i.e., AT and GM, have been listed. The lowercase letters indicate the data from our expression experiments, and the uppercase letters the data from the BDGP EST library.
Several lines of evidence indicate that these newly derived genes are functional. First, many of them are known genes with identified bona fide proteins (Table 1). Second, we examined functional constraints on these new genes by comparative analysis of the rates of nonsynonymous substitutions per site (KA) and synonymous substitutions per site (KS) between the members of each gene pair. In general, a KA/KS ratio that is significantly lower than unity is considered to indicate functional constraint. However, the expected KA/KS ratio for divergence between a functionless new retrogene duplicate and a functional parental gene should be smaller than unity but higher than 0.5, dependent upon the selective constraint on the parental gene (Li 1997). In a conservative test, we considered KA/KS significantly lower than 0.5 to indicate functional constraint on both genes. We found that the KA/KS ratios of 20 of the 24 gene pairs are significantly lower than 0.5 (Table 1); the ratios of four genes are not significantly lower than 0.5.
We surveyed nucleotide polymorphism in these four genes by sequencing 12 to 36 alleles for each gene, which suggested strong selective constraints (Table 2). First, in these genes, nonsynonymous polymorphism is significantly lower than synonymous polymorphism (χ2 = 21.25, P < 0.00001). Second, variation in these genes does not significantly differ from the values for average functional genes in Drosophila (πs = 0.0135, πtotal = 0.0040), whereas one could predict that functionless DNA should have higher variation (Powell 1997). Finally, none of the alleles, with the exception of some alleles of CG12628, contain a frameshift mutation and/or premature stop codon. Although CG12628 shows a premature stop codon or one base pair deletion in some alleles, a large proportion (60.61%) of alleles maintain an intact reading frame. Furthermore, nonsynonymous polymorphism is lower than synonymous polymorphism in both the normal alleles and the truncated alleles in which a shorter predicted open reading frame (ORF) remains. Thus, the functional role for this retrogene cannot be ruled out. These polymorphism data together with KA/KS values significantly lower than 0.5 in the rest of the genes suggest that almost all new retrogenes identified are subject to strong functional constraints. Furthermore, in RT-PCR experiments and BDGP EST libraries (Fig. 1, Table 1), we observed that most new retrogenes are expressed in one or more of the investigated tissues, further suggesting that these genes are functional. Population genetic analyses of the gene sequences with newly evolved expression patterns suggest that some of these new genes may have evolved functions that did not exist previously (E. Betrán and M. Long, unpubl.).
Table 2.
Polymorphism Analysis of the Retroposed Copies of Genes With Lower KS (see Table 1)
| New gene | L | N | S | MS | MN | πS | θS | πN | θN |
|---|---|---|---|---|---|---|---|---|---|
| CG12628 | 456 bp | 33 | 5 | 2 | 3 | 0.0047 | 0.0042 | 0.0030 | 0.0022 |
| CG12324 | 390 bp | 16 | 8 | 7 | 1 | 0.0182 | 0.0232 | 0.0004 | 0.0010 |
| CG10174 | 390 bp | 36 | 7 | 5 | 2 | 0.0125 | 0.0134 | 0.0015 | 0.0016 |
| CG13732 | 630 bp | 12 | 8 | 4 | 5 | 0.0098 | 0.0103 | 0.0019 | 0.0033 |
L, length of the gene; N, number of alleles sequenced; S, segregating sites; M, number of mutations; π, average nucleotide pairwise differences, and θ, estimator of 4Neμ, where Nc and μ are effective population size and neutral mutation rate, respectively. The subscripts N and S refer to nonsynonymous sites and silent sites, respectively. Stop codon position or codons with deletions for CG12628 were excluded from the analysis. Values were calculated using DNAsp software (Rozas and Rozas 1999).
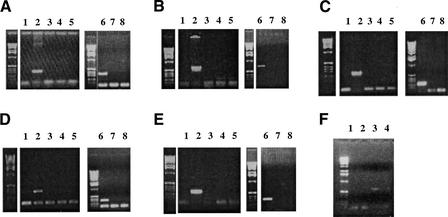
Figure 1.
RT-PCR for several genes. (A) CG10174, (B) CG13732, (C) CG17856, (D) CanB, and (E) CG9873. Lane 1 corresponds to gonadectomized male cDNA, lane 2 is testis + accessory glands cDNA; lanes 3 and 4 are the negative controls after DNA digestion for the experiments of lanes 1 and 2, respectively, and lane 5 is the negative control of the PCR. Lane 6 is the PCR experiment using testis cDNA; lane 7 is the negative control after DNA digestion, and lane 8 is the negative control of the PCR. (F) Lane 1 is CG15645 RT-PCR using cDNA from polyA selected RNA from a mixed sample of males and females; lane 2 is the PCR from this mRNA without being reverse-transcribed from the mixed sample; lanes 3 and 4 are the nested PCR experiments using the PCR products of lanes 1 and 2 as templates. The DNA marker, as shown here, is a 1-kb DNA ladder (Gibco).
Examination of the physical positions of these newly evolved functional genes revealed an unexpected pattern. We observed that 12 pairs (50%) originated from parental genes located on the X chromosome despite its low gene number (17% of the genes in the genome), whereas we found only 12 from autosomes, 3 to X and 9 to autosomes (Tables 1, 3). This pattern is significantly different from the expected (P = 0.0084; Table 3). If every gene in the genome is retroposed with equal probability, a sample of 24 parental genes should include only 5.6 (23.3%) from the X chromosome and 18.4 (76.7%) from autosomes (see Methods). Therefore, there is an excess of new genes retroposed from the X-linked parental genes to autosome; correspondingly, there is a deficiency of retroposed genes originated from autosomes (Table 3).
Table 3.
Analysis of the Pattern of Retroposition
| Direction of the gene formation event | Expectation | Observed No. | Excess (%) | ||
|---|---|---|---|---|---|
| % | No. | ||||
| X→A | 23.3 | 5.6 | 12 | 114 | |
| A→X | 20.3 | 4.9 | 3 | −39 | |
| A→A | 56.4 | 13.5 | 9 | −33 | |
| X2 = 9.55, df = 2, P = 0.0084 | |||||
X, X chromosome; A, autosome; Excess = [O − E)/E] × 100; E, expected; O, observed.
Although this result suggests that many new genes originated from the X chromosome, it is unclear whether or not this observation is limited to the identified new genes in the group defined by 70% amino acid identity. Thus, we extended a similar analysis (see Methods) to the new retrogenes of 50% or higher identity at the amino acid level with their parental genes and observed a similar phenomenon. Of 159 putative interchromosomal retroposition events, 63 (40%) originated from X-linked genes, indicating a highly significant excess of X-linked origination events over the 23.3% expected under the assumption of random retroposition (P < 0.0001, χ2 = 23.81, df = 1). Therefore, the pattern that we observed is not limited to a certain subset of genes.
We had ignored retroposed copies from the X chromosome that inserted elsewhere in the same chromosome in all previous analyses, to ensure that we were not looking at tandem duplicates or at ancient tandem duplicates now separated by paracentric inversions within the same chromosome (Powell 1997). However, we examined the frequency of retroposition among different sections within the X chromosome. In the retrogenes with 50% or higher amino acid identity with parental genes, we found that of 67 putatively retroposed copies from the X chromosome, only four inserted into different X chromosomal sections. The expected value of within-X transpositions is 10.1, which is significantly higher than the observed value (P = 0.039, χ2 = 4.33, df = 1).
Four possible explanations could account for the observed pattern: (1) nonrandom generation of retrogenes by a disproportionate number of X-linked genes that express in the germline cells; (2) negative selection against insertions in the X chromosome; (3) different recombination rates (or possibly deletion rates) between the autosomes and the X chromosome; and (4) positive Darwinian selection favoring retrogenes generated from the X chromosome to the autosomes.
We found similar proportions of X-linked and autosomal genes expressed in germline cells in the Berkley EST libraries of ovary and adult testis (E. Betrán, K. Thornton, and M. Long, unpubl.), ruling out the first possible explanation that a disproportionate number of genes that express in the germline are X-linked resulting in the larger number of X-originated retrogenes. Alternatively, if insertions are slightly deleterious because of possible disruption of the regulation of gene activity, there will be stronger selection against X-linked than autosomal insertions because of male hemizygosity for the X (Charlesworth et al. 1987). This selection would reduce the number of insertions surviving in the X chromosome by a small proportion, e.g., lower than 2%, under the assumptions that the selection intensity is an order of magnitude lower than the inverse of effective population size and that the fitness effects of insertions are recessive (see Methods). This can account only for a negligible part of the deficiency of new gene insertions in the X chromosome. Therefore, the negative selection from this hypothetical process cannot explain the excess of retroposition from X-linked parent genes.
The ectopic exchange model predicts that insertion elements will be more abundant in regions of low recombination because they are less likely to be deleted by unequal recombination (Langley et al. 1988). Hence, under this model, different recombination rates of the autosomes and the X chromosome would be likely to be associated with different deletion rates, thus yielding different rates of new retrogenes between the X and the autosomes, as we observed. However, there is no evidence for different recombination rates between autosomes and the X chromosome. Recombination rates per base pair in these chromosomes are similar (Ashburner 1989), and the product between the population size and the time spent in females (recombining sex) is the same for X chromosomes
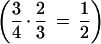 |
and autosomes
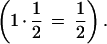 |
The fourth hypothesis, positive selection, seems more parsimonious to interpret the excess of retroposition from X to autosomes. X inactivation during early spermatogenesis could produce a selective advantage for the retroposed genes with novel functions that escape X linkage and become expressed in testis, as previously suggested (Lifschytz and Lindsley 1972; McCarrey 1994). X inactivation early in spermatogenesis is well documented in Drosophila, mouse, and human (Lifschytz and Lindsley 1972; Richler et al. 1992). Thus, a mutant with a newly retroposed gene on autosomes will have some advantage over an X-linked form, because the mutant can carry out a new function putatively required in male germline cells after the X chromosome becomes inactivated. This hypothesis assumes that retroposition occurs from genes on all chromosomes with the same probability but natural selection favors the ones that avoid X-linkage by moving to an autosome and developing expression in testis.
The hypothesis of selective advantage by avoiding X linkage predicts that most of the new retrogenes that evolved from X-linked parent genes would be expressed in the male germline, nonexclusively. The new genes can also develop or retain additional functions in other tissues (McCarrey 1994). Data in Table 1 and Figure 1 confirm this prediction, showing that 10 of the 11 genes retroposed from the X chromosome, for which expression information is available, are expressed in adult male testis. Such a high percentage (91%) of retrogenes expressed in the testis is unlikely to be a random pattern, considering that transcripts of only ∼10% of the ∼13,600 genes of the Drosophila genome have been detected in testis (Andrews et al. 2000), and it is in agreement with the prediction of the hypothesis of positive selection. Nevertheless, it is also possible that the expression pattern of a new copy could be a by-product of the region into which it fortuitously inserted (Bownes 1990; Pasyukova et al. 1997). However, these explanations predict such elements to be nonfunctional pseudogenes, against our observations above and the fact that these retrogenes have been kept, according to our phylogenetic data (see Methods), far longer than the half-life of pseudogenes in Drosophila (Watterson 1983; Petrov et al. 2000).
Here we observed that new functional retrogenes, mostly with newly evolved testis expression, tend to avoid X-linkage by moving to an autosome. Consistently, it was observed that, in Drosophila, autosomal mutations for male sterility have mostly late spermatogenesis effects (Castrillon et al. 1993) and, in the nematode C. elegans, X-linked sperm-enriched and germline-intrinsic genes are scarce (Reinke et al. 2000). This pattern reveals a possible role of Darwinian selection for the retroposed new genes that escape from the spermatogenesis X inactivation, although there may be additional mechanisms contributing to the retroposition process, for example, the hypothetical sexual antagonism that genetic variants are advantageous for one sex but disadvantageous for the other sex (Rice 1984; C.-I. Wu, pers. comm.). The pattern also supports the view that genomic location matters for gene function (Hurst and Randerson 1999). Genes that escape X-linkage by retroposing to an autosome and are expressed in the male germline have been found in mammals (Dahl et al. 1990; McCarrey 1994), although a comparable general pattern has not been detected in the human genome (Venter et al. 2001). If this pattern exists in the human genome, it could be obscured by the enormous number of degenerating retroposed copies in this genome (Gonçalves et al. 2000). A large number of X-linked genes expressed in spermatogonia have been reported in the mouse (Wang et al. 2001). Our finding is not necessarily contradictory to this interesting observation. These mouse genes, observed from the early stage (mitotic cells) of spermatogenesis, are expressed prior to X inactivation. When we analyzed locations of the known mammalian genes that are expressed exclusively during male meiosis (Eddy and O'Brien 1998), we found that all 26 genes are located on autosomes and none are on the X chromosome (E. Betrán and M. Long, unpubl.). This result, revealing a different pattern from that of Wang et al. (2001) in a different spermatogenesis stage, suggests that the mammalian late spermatogenesis was likely subject to selection as we observed in Drosophila.
METHODS
Genome Analysis of Retroposed Copies of Genes
Sequence data (Adams et al. 2000) were obtained from the BDGP Web site (www.fruitfly.org). The database of real and predicted amino acid sequences of Release 2 was first purged of peptides resulting from alternative transcription, retaining only the longest peptide sequence. Paralogous pairs were identified from the fasta33_t program (Pearson 1990) alignments of this entire database with a criterion of at least 70% amino acid identity or ≥50% amino acid identity in a minimum overlap of 35 amino acids in the region of local alignment (Thornton and Long 2002).
The coding regions of the pairs with 70% amino acid identity were aligned with the corresponding genomic region and inspected for retroposition features: (1) one pair member was intronless in the region of sequence similarity whereas the other had introns; (2) one of them had a poly-A tail when both copies were intronless; and/or (3) one copy was flanked by short repeats. All three hallmarks of retroposition can be found in a retrogene, sometimes two, sometimes only one. Only pairs that were on different chromosomes were considered. The retroposition features plus the fact that all pairs are in different chromosomes ensure that we are not looking at tandem duplicates or at tandem duplicates that are separated by paracentric or pericentric inversions (Powell 1997); they are instead retroposed copies of genes. In the case of families (more than two homologs), the parental gene was considered to be the one with the smaller KS. Pairs with homology to mobile elements were discarded.
In the case of paralogous pairs with amino acid identity ≥50%, we obtained the numbers of exons for each gene in each paralogous pair from the BDGP annotation. We only included gene pairs where one member is predicted to contain introns (parental gene) and the member has no predicted introns (new gene) that locate in different chromosomes, that is, the duplication arose by a retroposition event. Tandem duplicated members of gene families would look like many events but, for our purpose, they were considered a single retroposition event.
KA and KS estimation and KA/KS ratio test
KA and KS were estimated in the region of sequence similarity using K-estimator software (Comeron 1999). We used a likelihood ratio test to determine whether KA/KS between pairs of duplicates was smaller than 0.5. The Codeml program of PAML 3.1 (Yang 1998) was run twice for every gene pair; first fixing ω = 0.5 and second estimating omega. The log likelihood value of the 0.5 model (l0) was compared to the free model (l1). We considered the ratio significantly smaller than 0.5 if the free model was significantly more likely than the 0.5 model. Significance at the 5% level was tested by comparing twice the log likelihood difference, 2Δl = 2(l1 − l0), to a χ2 distribution with one degree of freedom (Yang 1998).
Expected Number of Retropositions
Considering the number of genes per chromosome and the size (euchromatin) of the chromosome as the source and target of insertion, respectively, the fact that X-linked genes are dosage-compensated, and assuming independent generation and landing on a chromosome site and equal numbers of males and females in the population, we calculated the expected frequency (PKL) (i.e., Px→A, PA→x, and P A→A, where “→” indicates the direction of retroposition, from the parental gene to the new gene [A→A includes A2→A3 and A3→A2]).
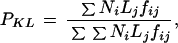 |
where Ni and Lj are the proportions of gene number at the source chromosome i and the euchromatic size of the targeted chromosome, respectively, and fij is the frequency of occurrence of this type of retroposition to a given chromosome in the population. According to genome data (Adams et al. 2000) and the existence of males and females in the population, i, j: X, 2 and 3, Ni: 0.17, 0.38, 0.45; Lj: 0.19, 0.36, 0.44 (chromosome 4 ignored for its minuscule size); and fij: 0.75 for j = X and 1 for j = 2 or 3; reflecting the relative population sizes of the X chromosome and autosomes. When i = j, the expectation within chromosomes is calculated. The expected percentage of interchromosomal retroposition events that originate from the X chromosome to autosomes is 23.3% (see Table 3 for the other expected values). The expected percentage of copies originated from X chromosome that become inserted in the X chromosome is 15%.
Relative Fixation Rates of X Chromosome and Autosomes
The difference of relative fixation rates between X chromosome (KX) and autosome (KA) for a slightly deleterious mutation model with selection in one or both sexes and dosage compensation is given by KA/KX = 1 + 1/3Nes(h − 1/2) (Charlesworth et al. 1987); where h is the dominance coefficient, Ne the effective population size, and s the selection coefficient. When considering reasonable magnitudes of these parameters, e.g., NeS = −0.1 and h = 0, we have Kx = 0.98KA, indicating that X-linked genes would evolve at slightly slower rates than autosomal genes.
Population Genetic Analysis and Worldwide Samples
Genes were PCR-amplified from single Drosophila individuals from a worldwide sample of D. melanogaster. D. melanogaster strains used were: OK17, HG84, and Z(s)56 from Africa; yep3, yep18, yep25, Cof3, BLI5, cal4, y10, and y2 from Australia; 253.4, 253.27, 253.30, and 253.38 from Taiwan; Closs3, Closs10, Closs16, Closs19, and Seattle from USA; Rio from Brazil; Rinanga, Bdx, Besançon, Prunay, and Capri from France.
Primers used to amplify genes for sequencing were: 5′ATTCCGGATTGCAAGTATGAGC3′ / 5′GAACCCAAGATCC GGATTTATTTT3′ for CG12628; 5′GCTGCCAACTCGCTTC ATAA3′ / 5′AACGTAGGAAATGTTGAAGCTG3′ for CG12324; 5′TGCAGGGCGCATTGTTCAG3′ / 5′CATACGCCTGCCAA TACGAGT3′ for CG10174; and 5′TTACGCAATTCAAT GGCACCT3′ / 5′GAGAAGCAGCAGCGGGAGAT3′ for CG13732. Sequence was obtained for both strands and haplotypes determined directly or by subcloning and sequencing individual clones. Sequences were aligned and revised by eye considering the information from the literature (Adams et al. 2000).
Phylogenetic Inference
Chromosomes with standard arrangement of D. melanogaster (CS), D. simulans (Florida), D. yakuba (115) or D. teissieri (128.2), and D. erecta (154.1), representing different lineages in the D. melanogaster subgroup of species (Lemeunier and Ashburner 1976; Powell 1997) were hybridized with fluorescent probes (Wang et al. 2000) of the retroposed copy of the pair in most cases. Presence or absence of this copy was investigated using D. melanogaster maps cut and pasted to reconstruct the other species maps. All retroposed genes except the first four genes in Table 1 are older than the estimated age of the D. melanogaster subgroup (data not shown), 15 My (Powell 1997).
Expression Analysis
Using RT-PCR experiments (Wang et al. 2000), transcription was addressed for several genes. Analysis of expression of intronless genes is challenging because genomic contamination can produce a band the same size as that expected from the cDNA. To ensure that we were getting product from the cDNA, we obtained poly-A selected RNA or, alternatively, we obtained total RNA and digested the possible DNA contaminant by RNAse-free DNAse treatment (Gibco) and ran controls including mRNA without being reverse-transcribed. Primer sequences were: 5′TTGTCCAGCAGTACTACGCC3′ / 5′TTGGGCTTCAGCAAAAAGAT3′ for CG10174; 5′AGAAGT TGCTCGAGCAGAGC3′ / 5′CTCCGAGGCAGTTACATCCA3′ for CG13732; 5′TGTCTGGATTCAACCAATAC3′ / 5′GCTCTT CGCGCTCCTTTTGC3′ for CG17856; 5′ACTCGGGTGCGC TGAGCATA3′ / 5′CCTTGTCCGCAAAGCAAATG3′ for CG4209; 5′TGACCAAGGGAACCACTAGT3′ / 5′TCTTAGCG GCACCTCCTTCA3′ for CG9873; and 5′ATGGAATTCAAT TACCTTGCT3′ / 5′CTTGCAACTTCTGCTGTAGG3′ for CG15645.
WEB SITE REFERENCES
www.fruitfly.org; BDGP Web site.
Acknowledgments
We thank Mao-Lian Wu, Françoise Lemeunier, and Patricia Gibert for providing Drosophila strains used in this work, Josep M. Comeron, Justin Fay, Chung-I. Wu, and Ziheng Yang for valuable discussion, Janice B. Spofford for critically reading the manuscript, and anonymous reviewers for their comments that helped to improve the manuscript. K.T. was supported by an NIH training grant. This work was supported by grants from the National Science Foundation and a Packard Fellowship in Science and Engineering to M.L.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mlong@midway.uchicago.edu; FAX (773) 702-9740.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.604902. Article published online before print in November 2002.
REFERENCES
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Andrews J, Bouffard GG, Cheadle C, Lu J, Becker KG, Oliver B. Gene discovery using computational and microarray analysis of transcription in the Drosophila melanogaster testis. Genome Res. 2000;10:2030–2043. doi: 10.1101/gr.10.12.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A laboratory handbook. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Begun DJ. Origin and evolution of a new gene descended from alcohol dehydrogenase in Drosophila. Genetics. 1997;145:375–382. doi: 10.1093/genetics/145.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownes M. Preferential insertion of P elements into genes expressed in the germ-line of Drosophila melanogaster. Mol Gen Genet. 1990;222:457–460. doi: 10.1007/BF00633856. [DOI] [PubMed] [Google Scholar]
- Brosius J. Retroposons—Seeds of evolution. Science. 1991;251:753. doi: 10.1126/science.1990437. [DOI] [PubMed] [Google Scholar]
- Brosius J. RNAs from all categories generate retrosequences that may be exapted as novel genes or regulatory elements. Gene. 1999;238:115–134. doi: 10.1016/s0378-1119(99)00227-9. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Gonczy P, Alexander S, Rawson R, Eberhart CG, Viswanathan S, DiNardo S, Wasserman SA. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: Characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135(2):489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B, Coyne JA, Barton NH. The relative rates of evolution of sex chromosomes and autosomes. Am Nat. 1987;130:113–146. [Google Scholar]
- Comeron JM. K-Estimator: Calculation of the number of nucleotide substitutions per site and the confidence intervals. Bioinformatics. 1999;15:763–764. doi: 10.1093/bioinformatics/15.9.763. [DOI] [PubMed] [Google Scholar]
- Dahl H-HM, Brown R M, Hutchison WM, Maragos C, Brown GK. A testis-specific form of the human pyruvate dehydrogenase E1 α subunit is coded for by an intronless gene on chromosome 4. Genomics. 1990;8:225–232. doi: 10.1016/0888-7543(90)90275-y. [DOI] [PubMed] [Google Scholar]
- Eddy EM, O'Brien DA. Gene expression during mammalian meiosis. Curr Top Dev Biol. 1998;37:141–199. [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978;217:501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Gonçalves I, Duret L, Mouchiroud D. Nature and structure of human genes that generate retropseudogenes. Genome Res. 2000;10:672–678. doi: 10.1101/gr.10.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LD, Randerson JP. An eXceptional chromosome. Trends Genet. 1999;15:383–385. doi: 10.1016/s0168-9525(99)01809-0. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Langley CH, Montgomery E, Hudson R, Kaplan N, Charlesworth B. On the role of unequal exchange in the containment of transposable element copy number. Genet Res. 1988;52:223–235. doi: 10.1017/s0016672300027695. [DOI] [PubMed] [Google Scholar]
- Lemeunier F, Ashburner M. Relationships in the melanogaster species subgroup of the genus Drosophila (Sophophora). II. Phylogenetic relationships between six species based upon polytene banding sequences. Proc R Soc Lond B. 1976;193:257–294. doi: 10.1098/rspb.1976.0046. [DOI] [PubMed] [Google Scholar]
- Li W-H. Molecular evolution. Sunderland, MA: Sinauer Associates; 1997. [Google Scholar]
- Lifschytz E, Lindsley DL. The role of X-chromosome inactivation during spermatogenesis. Proc Nat Acad Sci. 1972;69:182–186. doi: 10.1073/pnas.69.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. Evolution of novel genes. Curr Opin Genet Dev. 2001;11:673–680. doi: 10.1016/s0959-437x(00)00252-5. [DOI] [PubMed] [Google Scholar]
- Long M, Langley C H. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science. 1993;260:91–95. doi: 10.1126/science.7682012. [DOI] [PubMed] [Google Scholar]
- Long M, Wang W, Zhang J. Origin of new genes and source for N-terminal domain of the chimerical gene, jingwei, in Drosophila. Gene. 1999;238:135–141. doi: 10.1016/s0378-1119(99)00229-2. [DOI] [PubMed] [Google Scholar]
- McCarrey JR. Evolution of tissue-specific gene expression in mammals. How a new phosphoglycerate kinase was formed and refined. Bioscience. 1994;44:20–27. [Google Scholar]
- McCarrey JR, Thomas K. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature. 1987;326:501–505. doi: 10.1038/326501a0. [DOI] [PubMed] [Google Scholar]
- Nurminsky DI, Nurminskaya MV, Aguilar DD, Hartl DL. Selective sweep of a newly evolved sperm-specific gene in Drosophila. Nature. 1998;396:572–575. doi: 10.1038/25126. [DOI] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. New York, NY: Springer-Verlag; 1970. [Google Scholar]
- Pasyukova E, Nuzhdin S, Li W, Flavell AJ. Germ line transposition of the copia retrotransposon in Drosophila melanogaster is restricted to males by tissue-specific control of copia RNA levels. Mol Gen Genet. 1997;255:115–124. doi: 10.1007/s004380050479. [DOI] [PubMed] [Google Scholar]
- Pearson WR. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 1990;183:63–98. doi: 10.1016/0076-6879(90)83007-v. [DOI] [PubMed] [Google Scholar]
- Petrov DA, Sangster TA, Johnston JS, Hartl DL, Shaw KL. Evidence for DNA loss as a determinant of genome size. Science. 2000;287:1060–1062. doi: 10.1126/science.287.5455.1060. [DOI] [PubMed] [Google Scholar]
- Powell JR. Progress and prospects in evolutionary biology: The Drosophila model. New York, NY: Oxford University Press; 1997. p. 355. [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJM, Davis EB, Scherer S, Ward S, et al. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6:605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Rice WR. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Richler C, Soreq H, Wahrman J. X inactivation in mammalian testis is correlated with inactive X-specific transcription. Nat Genet. 1992;2:192–195. doi: 10.1038/ng1192-192. [DOI] [PubMed] [Google Scholar]
- Rozas J, Rozas R. DnaSP version 3.52: An integrated program for molecular population genetics and molecular evolution. Bioinformatics. 1999;15:174–175. doi: 10.1093/bioinformatics/15.2.174. [DOI] [PubMed] [Google Scholar]
- Thornton K, Long M. Rapid divergence of gene duplicates on the Drosophila melanogaster X chromosome. Mol Biol Evol. 2002;19:918–925. doi: 10.1093/oxfordjournals.molbev.a004149. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–426. doi: 10.1038/86927. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang J, Alvarez C, Llopart A, Long M. The origin of the Jingwei gene and the complex modular structure of its parental gene, yellow emperor, in Drosophila melanogaster. Mol Biol Evol. 2000;17:1294–1301. doi: 10.1093/oxfordjournals.molbev.a026413. [DOI] [PubMed] [Google Scholar]
- Wang W, Brunet FG, Nevo E, Long M. Origin of sphinx, a young chimeric RNA gene in Drosophila melanogaster. Proc Nat Acad Sci. 2002;99:4448–4453. doi: 10.1073/pnas.072066399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson GA. On the time for gene silencing at duplicate loci. Genetics. 1983;105:745–766. doi: 10.1093/genetics/105.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Likelihood ratio test for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15:568–573. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]