Abstract
Exploration is the primary way in which rodents gather information about their spatial surroundings. Thus, spatial theories propose that damage to the hippocampus, a structure thought to play a fundamental role in spatial behavior, should disrupt exploration. Exploration in rats is organized. The animals create home bases that are central to exploratory excursions and returns, and hippocampal formation damage alters the organization of exploration by disrupting returns. Mice do not appear to readily establish home bases in novel environments, thus, for this species, it is more difficult to establish the contribution of the hippocampus to exploration. The purpose of the present study was threefold: develop a task in which mice center their exploration from a home base, determine whether the exploratory behavior is organized, and evaluate the role of fimbria-fornix lesions on exploration. Mice were given a novel exploratory task in which their nesting material was placed on a large circular table. Video records of control and fimbria-fornix mice were made in both light and dark (infrared light) conditions. Exploration patterns (outward trips, stops, and homeward trips) were reconstructed from the video records. Control mice centered their activity on their bedding, from which they made circuitous outward trips marked by many stops, and periodic direct returns. The bedding-centered behavior and outward trips of the fimbria-fornix mice were similar to those of the control mice, but significantly fewer direct return trips occurred. The direct homeward trips observed under light and dark conditions were consistent with a dead-reckoning strategy, in which an animal computes its present position and homeward trajectory from self-movement cues generated on the outward trip. Because the fimbria-fornix lesions disrupted the homeward component of exploratory trips, we conclude that the fimbria-fornix may contribute to dead reckoning in mice. The results also show that the home-bedding methodology facilitates the establishment of a home base by mice, thus providing a useful methodology for studies with mice.
One of the most interesting predictions of the spatial mapping theory of O'Keefe and Nadel (1978) is that “exploration is a direct response of the animal to the detection of a mismatch by the locale system; in the absence of the hippocampus all forms of exploratory behavior should disappear from the animal's repertoire”. Whereas their monograph reviews an extensive list of alterations in the exploratory behavior of rodents, which is, in general, consistent with the idea that exploration and the hippocampus are important for spatial behavior, the understanding of the effects of hippocampal formation lesions on spatial behavior has been hampered by a lack of understanding of the organization of rodent spatial behavior. Certainly, the typical increase in the activity of exploring hippocampal-lesioned animals (Jarrard 1968; Whishaw et al. 1994), and the tendency of these animals to investigate novel as opposed to familiar objects in the environment (Sutherland 1985; Mumby et al. 2002) does not seem consistent with the idea that all forms of exploratory behavior disappear from a hippocampectomized animal's repertoire.
A deficiency in understanding rat exploratory behavior has been readdressed by recent studies revealing that exploratory behavior is organized (Whishaw et al. 1982; Eilam and Golani 1989; Golani et al. 1993; Tchernichovski et al. 1996; Tchernichovski and Benjamini 1998; Drai and Golani 2001; Drai et al. 2000, 2001). When placed in a large open field, rats adopt certain locations as home bases, in which they linger, turn, rear, and groom. In addition, they make periodic outward trips from a home base that are slow, circuitous, and marked by a number of stops, and rapid return trips back to the home base. Their locomotor behavior also consists of movements at a number of speeds (first, second, and third gear). Specifically, movements in first gear, or lingering, occur in the vicinity of the home base. With extended exposure to an environment, exploratory trips grow in length; however, the number of stops and excursion duration are constrained by an upper bound.
The outward and homeward portions of a rat's exploratory bout may be mediated by different spatial navigational strategies, piloting and dead reckoning, respectively (Whishaw et al. 2001; Wallace et al. 2002a). Piloting is a form of navigation in which guidance is derived from external (allothetic) cues, such as visual or olfactory cues. Dead reckoning is a navigational strategy that uses self-movement (idiothetic) cues generated by vestibular input, proprioception, efference copies of commands, or sensory flows that occur in the process of movement (Gallistel 1990). Accordingly, on the outward portion of an exploratory trip, an animal may be learning about its piloting space, whereas the course for its homeward trip is set by computations derived from cues generated on the outward trip. The suggestion that the homeward trip is mediated by dead reckoning is confirmed by the persistence of direct rapid return trips under conditions in which visual cues are not available, such as testing under infrared light conditions (Whishaw et al. 2001; Wallace et al. 2002a). Examination of the organization of exploratory behavior in rats shows that the dead reckoning (homeward) component of exploration is altered, following fimbria-fornix (hippocampal) lesions (Whishaw et al. 2001). In both the light and dark (infrared), control and fimbria-fornix lesion rats leave a home cage and make circuitous trips marked by periodic stops; however, only control rats make direct return trips to the home base. Rats with fimbria-fornix lesions do return to the home base, but they do so by using a more indirect and slower approach.
The organization of exploratory behavior in mice may differ from that of rats. Drai and Golani (2001) note that mice differ from rats in that their lingering episodes are not restricted to several locations. In preliminary studies with mice, we have observed similarly that the mice do not appear to set up home bases as readily as rats (J.H. Gorny, B. Gorny, D.G. Wallace, and I.Q. Whishaw, unpubl.). A failure to establish home bases may explain the difficulty in obtaining spontaneous escape responses from mice in open field tests (Pompl et al. 1999). Pompl et al (1999) used both a light and a fan to force the mice to escape (for review, see Bach et al. 1999); whereas, rats displayed rapid spontaneous escape behavior in a similar task (Barnes 1979).
The purpose of the present study was threefold, (1) to develop a task in which mice establish a home base, (2) to determine whether mouse exploratory behavior is organized, and (3) to determine whether hippocampal formation lesions disrupt exploration, especially the homeward component of exploration. A technique of using a real home base, a mouse's nesting material rather than the unstructured exploratory environment typically used for rats (Eliam and Golani 1989; Drai et al. 2000) was introduced. A nest box has proven to be useful in previous studies of dead reckoning, in which mice retrieved pups or hamsters retrieved food (Etienne 1980; Alyan 1996). The growing number of studies using transgenic mice models demands a better understanding of mouse spatial behavior. The current analysis of mouse spontaneous exploration will provide valuable insights to mouse spatial behavior.
RESULTS
Histological Results
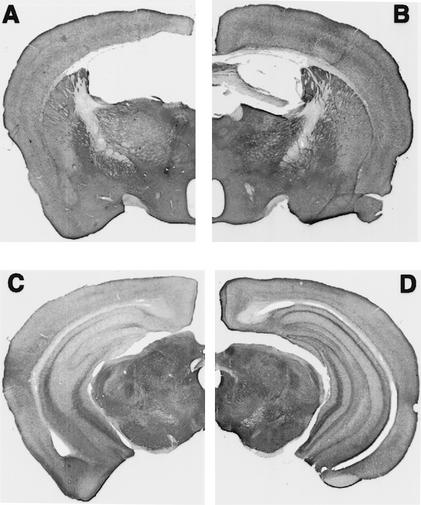
The fimbria-fornix was completely sectioned in the mice that were given lesions (Fig. 1A,B). The tract made by the electrode only slightly damaged the supracallosal septohippocampal pathways and cortex. Previous work with rats has demonstrated that the supracallosal damage does not produce additional impairments on spatial tasks (Sutherland and Rodriguez 1989; Jeltsch et al. 1994). Stains for acetylcholinesterase (AChE) revealed extensive depletion of AChE in the hippocampus (Fig. 1C,D), a marker that serves to confirm the completeness of the lesion. Shute and Lewis (1963) and Fibiger (1982) have concluded that AChE-intense neurons in the medial septal and diagonal band nuclei project via the fimbria-fornix to the hippocampus. Further evidence that the fimbria-fornix provides major cholinergic input to the hippocampus was demonstrated by Jeltsch et al. (1994), in which fimbria-fornix lesions reduced cholinergic markers by ∼70% in the dorsal hippocampus.
Figure 1.
Coronal hemisections of a fimbria-fornix brain (A,C) and a control brain (B,D) stained for acetylcholinesterase (AChE). Note: in A the fimbria-fornix is absent, and in C the AChE staining in the hippocampus is absent.
Stops
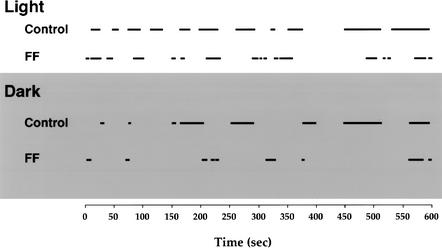
Upon being placed on the apparatus, the animals began making a number of exits and returns from the nesting material (Fig. 2). Placing bedding material on the table proved effective in establishing a home base, as both the control and fimbria-fornix mice made many visits to the bedding material (bars in Fig. 2).
Figure 2.
An ethogram of the time spent in a home base location (indicated by black bars) for a representative control and a representative fimbria-fornix mouse in the light (top) and the dark (bottom) condition.
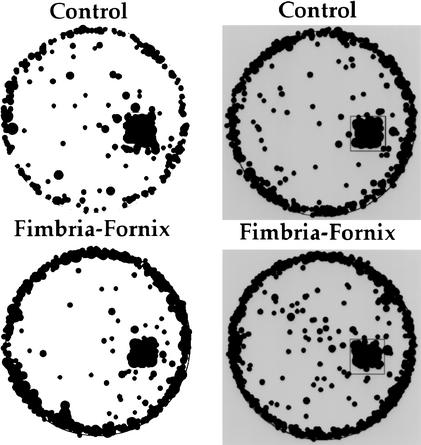
Stops other than at the bedding material were distributed primarily around the perimeter of the apparatus (Fig. 3). Both control and fimbria-fornix mice stopped more frequently in the dark condition than in the light condition [F(1,12) =26.217, P <0.05]. There was no significant effect of group or Group by Lighting Condition interaction.
Figure 3.
Location of stops for control and fimbria-fornix mice under light (left) and dark (right) conditions in a 10-min test. Both groups of mice demonstrated similar stopping locations, characterized by a high incidence of stops around the edge of the circular table and within the home location.
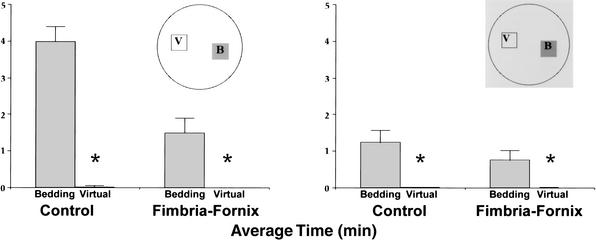
In addition, the mice spent substantial time in the bedding area, with control mice spending a mean of 3.9 min at this location in the light and 1.5 min there in the dark. Comparable times for the fimbria-fornix mice were 1.3 and 0.7 min, respectively. An ANOVA on time spent at the bedding location gave a significant effect of group [F(1,12) = 11.6, P < 0.05], lighting condition [F(1,12) = 33.7, P < 0.05], and Group by Lighting Condition interaction, [F(1,12) = 11.3, P < 0.05]. Despite group and lighting condition differences, the bedding location was the most visited site. To confirm that the home base was a focus for stops that did not occur in the periphery, stops made in a virtual nesting area diagonally opposite to the area of nesting material were compared with stops made in the area of the nesting material (Fig. 4). The ANOVA conducted on mean time resulted in significant main effects of group [F(1,12) = 11.6, P < 0.05], lighting condition [F(1,12) = 34.1, P < 0.05], and location [F(1,12) = 72.85, P < 0.05]. The ANOVA also revealed that the Group by Lighting condition interaction [F(1,2) =11.53, P < 0.05], the Group by Location interaction [F(1,12) =11.63, P < 0.05], the Lighting Condition by Location interaction [F(1,12) = 33.46, P < 0.05], and Group by Lighting Condition by Location interaction [F(1,12) =11.09, P < 0.05] were significant. Follow-up tests indicated that both the control and the fimbria-fornix rats spent more time in both light and dark conditions in the real home base as opposed to the virtual home base.
Figure 4.
Time (mean and standard error) spent in the home and a virtual home location (diagonal to the actual home) in the light (left) and the dark (right) condition. Time within the home bedding location (B) in comparison to the virtual home location (V) was significantly different in both light and dark conditions for both control and fimbria-fornix groups. (*) P < 0.05.
Number of Trips and Trip Duration
The ANOVA revealed no significant difference in the number of exploratory trips (as defined by excursions that left the home base) between the two groups, nor were there significant differences between lighting conditions, or a Group by Lighting Condition interaction. Although the ANOVA based on trip duration resulted in no effect of group, trip duration in the dark was observed to be significantly longer than under the light condition [F(1,12) = 56.959, P < 0.05]. In addition, a significant Group by Lighting Condition interaction [F(1,12) = 10.378, P < 0.05] revealed that the excursions for fimbria-fornix mice were longer in duration under the dark condition in contrast to the light condition.
Outward and Homeward Trips
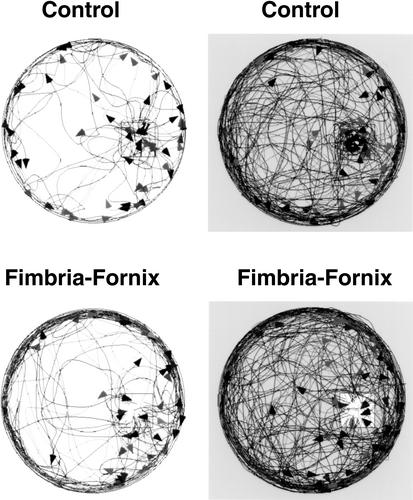
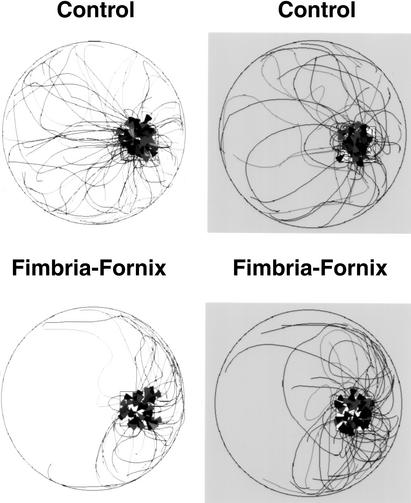
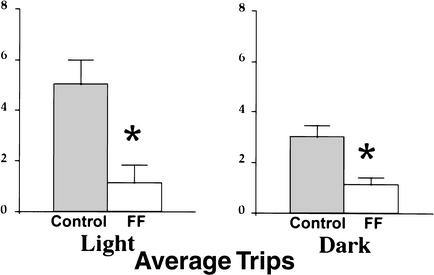
During the outward portion of trips from the bedding area, both control and FF mice traveled across the center of the table or around its periphery. Counts of trips made across the center of the table versus around the periphery indicated that there were no significant effects of group, lighting condition, or Group by Lighting Condition interaction. Figure 5 plots the outward trip segments for all control and FF mice under light and dark conditions. There were differences in the trajectory of homeward trips between the control and FF groups in both lighting conditions (Fig. 6). Control mice made many long return trips that carried them across the center of the table compared with the FF animals. FF mice tended to travel around the periphery of the table until they reached the proximity of the nesting material. Thus, the control mice made many long return trips, whereas the fimbria-fornix mice made few long return trips, but many short returns from points that were in close proximity to the nest material. To analyze these differences, trips were divided into long and short trips (Fig. 7). The ANOVA conducted on the number of long trips observed under light and dark conditions indicated a significant group effect [F(1,12) = 16.842, P < 0.05], whereas lighting condition and Group by Lighting Condition interaction were not significant. The control group made significantly more long trips in both the light and dark conditions.
Figure 5.
Outward paths recorded for all control and fimbria-fornix mice over a 10-min time period in the light and in the dark. Both control and fimbria-fornix mice made longer and more complex outward trips in the dark conditions, but travel distances did not differ between groups. Arrowheads indicate the location of the last stop for a single exploratory trip.
Figure 6.
Return trips recorded over a 10-min time period in light and dark conditions. Note: the control mice made more long direct returns to the home location in both light and dark conditions than did the fimbria-fornix mice. Arrowheads indicate the completion for a single return trip.
Figure 7.
Number of long trips (mean and standard error) returning to the home location by control and fimbria-fornix mice in light and dark conditions. The control group made significantly more long trips than did the fimbria-fornix group in both light and dark conditions. (*) P < 0.05.
The velocity of homeward trips made by control and fimbria-fornix mice was obtained from the measures of travel distance and time. The ANOVA conducted on homeward trip velocities resulted in no effect of group, lighting condition, or Group by Lighting Condition interaction.
DISCUSSION
The present study developed a behavioral test in which exploratory behavior was organized, and fimbria-fornix lesions disrupted this organization. We observed that when mice were given bedding material, they preferred that location, suggesting that they used the site as a home base. Their behavior was organized into outward and homeward components centered on the bedding site; however, fimbria-fornix lesions disrupted the homeward component of the exploratory behavior. Thus, the results are consistent with a role for the hippocampal formation in exploratory behavior, by mediating the dead reckoning component of exploration used by mice to return to their home base.
Exploratory behavior in rats, which may initially appear unstructured, is organized and divisible into components that are organized in relation to a reference point such as a real or virtual home base (Eilam and Golani 1989). The exploratory behavior of mice is also organized, but establishment of a home base is less obvious in mice than in rats (Drai and Golani 2001). In a series of preliminary studies, we placed mice onto a large circular table to determine whether they would establish home bases from which to explore. Although in different studies the mice were left for as long as 2 h, it was not clear that they established home bases as readily as rats do; a result confirming the report of Drai and Golani (2001). When provided with a shelter, which is easily adopted by rats as a home base (Wallace et al. 2002a), mice again appeared to ignore the shelter (Pompl et al. 2000). The strategy used here of placing the mice, along with nesting material from their home cages, onto the table was successful. Mice spent a significant portion of their time in the vicinity of the nesting material and subsequently made many excursions away and back to this home base. In addition, this method allowed us to collect a sufficient number of exploratory trips for analysis within only a 10-min test. Thus, the present study demonstrated that mice explore from, and return to, a home base, as do rats.
Following the establishment of a home base, rat exploratory behavior can be fragmented into two components as follows: circuitous outward trips marked by stops, and more direct rapid returns to the home base (Whishaw et al. 2001; Wallace et al. 2002a). Here, we show that exploratory behavior of mice is similar, although not identical, to that of rats. To be specific, in both light and dark conditions, the mice made circuitous outward trips, which were clustered around the perimeter of the circular table and characterized by many stops. In addition, the mice made many direct return trips, usually directly across the center of the table, back to the home base in both light and dark conditions. Previous studies have reported that a rat progresses slowly and intermittently away from a home location and fast and continuously back to the home base (Tchernichovski and Benjamini 1998; Whishaw et al. 2001). In the present analysis of mouse exploratory behavior, the homeward velocity was not noticeably faster than was the locomotor portion of the outward trip. Nevertheless, the fact that the mice made direct homeward trips in both the light and the dark is consistent with the return trips, like those of the rats, mediated by dead reckoning (Whishaw et al. 2001; Wallace et al. 2002a).
Although mice with fimbria-fornix lesions utilized the nesting material as a home base and explored the table making frequent stops, their homeward trips appeared to be a continuation of their outward excursion. Specifically, in both light and dark conditions, fimbria-fornix mice made significantly fewer long direct return trips to their home location than control mice, and the return trips that they did make were circuitous and seemed to occur simply because the animals accidentally encountered their home base as they traveled around the perimeter of the apparatus. In this respect, their return trips are similar to those described for rats with fimbria-fornix lesions (Whishaw et al. 2001; Wallace et al. 2002b). Thus, as has been suggested for rats, the failure of these mice to display direct home trips is consistent with impaired dead reckoning, and that this dead reckoning strategy was disrupted by lesions to the fimbria-fornix.
We have considered the possibility that the long direct homeward trips made by the mice may be mediated by olfactory cues emanating from the nesting material, but this seems unlikely for two reasons. First, control mice initiated long direct return trips to the home base from points that were at a considerable distance from the nest, a distance at which they may have been unable to detect directional olfactory cues from the bedding material. Second, if olfactory cues guided homeward trips, there is no reason to suspect that fimbria-fornix lesions would disrupt such behavior in the lesion group. Studies of tracking rats demonstrate that fimbria-fornix rats are more likely to follow odor trails than are control rats (Whishaw and Gorny 1999). Thus, parsimony suggests that direct return trips in the control mice may be mediated by dead reckoning, and that this behavior is disrupted in mice with fimbria-fornix lesions.
Two weaknesses in the present study relate to the brief period of time the animals were observed and that only one strain of mice was examined. With respect to the first point, if the animals were left for considerably longer periods of time, the animals' behavior may have become more organized. Because of the analysis that we performed, we digitized the behaviors of the animals manually, and so the analysis of even a 10-min period was demanding. Nevertheless, it seems unlikely that behavior would become more organized, because in the brief test that we administered, we did obtain results similar to those reported for rats that were observed for as long as a few hours (Eilam and Golani 1989). Finally, using rats, we have found a very similar pattern of impairment following fimbria-fornix lesions (Whishaw et al. 2001). For these reasons, we suggest that the technique of using the animals' bedding provides a useful way of allowing mice to organize their exploratory behavior so that it can be evaluated in relatively brief tests. With respect to the second point, there are many mice strains, and only one strain was used here. Although we suspect that the behavior of C57Bl/6 represents wild-type behavior, this could be examined in further studies.
Conclusion
The results of the present study confirm the predication of spatial theory that damage to the hippocampal formation will disrupt exploratory behavior. Here, we demonstrate that if mice are provided with bedding material, their exploratory behavior is organized into components, home base behavior, outward trips, and homeward trips. In addition, because homeward trips are relatively direct in both the light and the dark, the results are consistent with the hypothesis that the homeward trips may be mediated by dead reckoning. Our finding that fimbria-fornix lesions disrupt the homeward component of the exploratory trips is consistent with a role for the hippocampal formation in dead reckoning. We emphasize, however, that this result and conclusion should not signify that the outward component of exploration is normal in the exploring mice. It is likely that the outward component of the exploratory trip is important for gathering information concerning the relationships of the exploratory arena to ambient cues, and it is important to note that the ability of the mice to use ambient cues was not assessed. In addition, we found that the fimbria-fornix mice spent less time at the home base than did the control mice, suggesting that the lesion may disrupt other aspects of exploratory behavior. This should not detract from the successful objectives of the present study, which were to devise an exploratory task for mice, determine whether their exploration is organized, and examine whether fimbria-fornix lesions disrupt exploratory trip organization.
MATERIALS AND METHODS
Animals
Fourteen experimentally naïve, adult female mice C57BL/6 (Mus musculus domesticus) were used. The animals were housed in single plastic cages on a 12–12 h light-dark cycle under standard vivarium conditions with food and water available ad libitum.
Surgery
Seven mice were anesthetized with Isoflurane and oxygen. To produce fimbria-fornix lesions, 1.5 mA cathodal current was passed for 40 sec through 00 stainless-steel insect pins, insulated with Epoxylite except at the surface of their tips (Whishaw and Jarrard 1995). The lesions were made at one site in each hemisphere using coordinates in reference to bregma and the surface of the dura, 0.5 mm posterior, 0.5 mm lateral, and 2.0 mm ventral. Behavioral testing began 2 wk following surgery.
Apparatus
The testing apparatus (Fig. 8) consisted of a large wooden circular table (204 cm in diameter) covered with linoleum that could be washed following the testing of each mouse. The table was elevated 64 cm above the floor, and its surface was mounted on ball bearings so that it could be rotated after the testing of each mouse in order to displace any surface cues. The apparatus was located in a lightproof test room (3 × 12 m) in which many visual cues including posters, counters, and a desk with computers were present. A camera was located above the apparatus so that the behavior of the animals could be video recorded.
Figure 8.
The apparatus consists of a circular wooden table (204 cm in diameter) covered with linoleum, upon which a square piece of linoleum containing the animals bedding (home) was positioned. The mouse was free to explore the table during the test. A mouse and a typical exploratory path have been superimposed on the table.
Filming
To control the animal's use of visual cues, the exploratory trials were carried out in either light or dark (infrared light) conditions, and exploratory behavior was filmed using a camera sensitive to both normal and infrared light (Sony handycam CCD-TRV615). In the light condition, the room lights were on and the animal was able to use various visual cues within the room to locate its home or navigate. The room lights were turned off for the dark condition, thus eliminating visual cues. The experimenter wore infrared-sensitive goggles to orient in the test room, place the animal on the apparatus, and monitor its behavior in the dark.
Procedure
Testing
The mice were divided into two groups of seven mice each. All mice were tested in the light and dark, assignment to test condition was random. At the beginning of the trial, a mouse was brought into the test room in its own home cage. A small amount of the animal's bedding (torn paper towel) was then removed from the mouse's home cage and placed onto a square of linoleum (30 × 30 cm) that was positioned in the center of one of the arbitrarily defined quadrants of the table. The table had been thoroughly washed prior to the test. The mouse was then placed in the bedding and allowed to explore the novel environment for a period of 10 min. At the end of the trial, the mouse and its bedding were removed from the apparatus and returned to the home cage.
Behavioral Analysis
The exploratory outward paths and homeward or return paths of control and FF mice in light and dark conditions were reconstructed from video records using a computer graphics program. The following measures were obtained from the video record: (1) Stops. A stop was defined as an arrest in walking in which the animal's hind feet came to a complete stop. In addition, stop duration, obtained for each stop from the time bar displayed on a Hi8 videotape player (Sony EV-S900), consisted of the time during which the animal remained at the location where it had stopped. (2) Number of trips and trip duration. An exploratory trip, or excursion, was defined as a trip away from and back to the home location. The time required to complete each exploratory trip and return to the refuge was obtained from a time bar displayed on a Hi8 videotape player. (3) Outward and homeward trips. Exploratory trips were divided into outward and homeward components. Furthermore, the homeward portion of the trip was specified as that portion of a trip that followed the last stop that the mouse made before reaching its home base. The homeward segment of an exploratory trip was classified as long or short on the basis of where the segment began with respect to the oval drawn in the diagram of the table in Figure 7. Homeward segments that originated outside of the oval are classified as long; whereas segments that originated inside of the oval are classified as short. Finally, the velocity associated with the homeward and outward segments of each excursion was calculated by dividing the travel distance by trip duration for 10 random exploratory trips.
Histological Analysis
At the completion of the experiment, the mice were deeply anesthetized, perfused with saline and unbuffered 4% paraformaldehyde, the brains were removed, and stored in a 30% sucrose-unbuffered 4% paraformaldehyde solution. The brains were sliced at 40-mm sections on a cryostat, and alternate sections were stained with Cresyl violet and acetylcholinesterase.
Acknowledgments
This research was supported by grants from the Canadian Institute of Health and Research (CIHR) and the Natural Science and Engineering Research Council of Canada (NSERC).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL whishaw@uleth.ca; FAX 1-403-329-2775.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.53002.
REFERENCES
- Alyan SA. Evidence for resetting the directional component of pat integration in the house mouse (Mus musculus) Ethology. 1996;102:629–638. [Google Scholar]
- Bach ME, Bard M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatiam memory are correlated with defects in the late phase of hippocampal ling-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci. 1999;96:5280–85. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: A neurophysiological and behavioural study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Drai D, Golani I. SEE: A tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev. 2001;25:409–426. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Drai D, Benjamin Y, Golani I. Statistical discrimination of natural modes of motion in rat exploratory behavior. J Neurosci Methods. 2000;96(2):119–131. doi: 10.1016/s0165-0270(99)00194-6. [DOI] [PubMed] [Google Scholar]
- Drai D, Kafkafi N, Benjamini Y, Elmer G, Golani I. Rats and mice share common ethologically relevant parameters of exploratory behaviour. Behav Brain Res. 2001;125:133–140. doi: 10.1016/s0166-4328(01)00290-x. [DOI] [PubMed] [Google Scholar]
- Eilam G, Golani I. Home base behaviour of rats (Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34:199–211. doi: 10.1016/s0166-4328(89)80102-0. [DOI] [PubMed] [Google Scholar]
- Etienne AS. The orientation of the golden hamster to its nest-site after the elimination of various sensory cues. Experientia. 1980;36:1048–1050. doi: 10.1007/BF01965961. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res Rev. 1982;4:327–388. doi: 10.1016/0165-0173(82)90011-x. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Golani I, Benjamini Y, Eilam D. Stopping behaviour: Constraints on exploration in rats (Rattus norvegicus) Behav Brain Res. 1993;53:21–33. doi: 10.1016/s0166-4328(05)80263-3. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Behavior of hippocampal lesioned rats in home cage and novel situations. Physiol Behav. 1968;3:65–69. [Google Scholar]
- Jeltsch H, Cassel JC, Jackisch R, Neufang B, Green PL, Kelche C, Hertting G, Will B. Lesions of supracallosal or infracallosal hippocampal pathways in the rat: Behavioural, neurochemical, and histochemical effects. Behav Neural Biol. 1994;62:121–133. doi: 10.1016/s0163-1047(05)80033-0. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: Memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford, UK: Oxford University Press; 1978. [Google Scholar]
- Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: Use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. J Neurosci Met. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- Shute CCD, Lewis PR. The ascending cholinergic reticular system: Neocortical, olfactory and subcortical projections. Brain. 1963;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ. The navigating hippocampus: An individual medley of movement, space and memory. In: Buzsaki G, Vanderwolf CH, editors. Electrical activity of the archicortex. Budapest: Ajadenuau Juadi; 1985. pp. 255–280. [Google Scholar]
- Sutherland RJ, Rodriguez AJ. The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behav Brain Res. 1989;32:265–277. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Benjamini Y. The dynamics of long term exploration in the rat. Part I. A phase place analysis of the relationship between location and velocity. Biol Cybernetics. 1998;78:433–440. doi: 10.1007/s004220050446. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Benjamini Y, Golani I. Constraints and the emergence of freedom in the ontogeny of rat exploratory behaviour. Behaviour. 1996;133:519–539. [Google Scholar]
- Wallace DG, Hines DJ, Whishaw IQ. Quantification of a single exploratory trip reveals hippocampal formation mediated dead reckoning. J Neurosci Meth. 2002a;113:131–145. doi: 10.1016/s0165-0270(01)00489-7. [DOI] [PubMed] [Google Scholar]
- Wallace DG, Hines DJ, Gorny JH, Whishaw IQ. A role for the hippocampus in dead reckoning: An ethological analysis using natural exploratory and food carrying tasks. In: In: Jeffery K, editor. Biological basis of navigation. New York, NY: Oxford University Press; 2002b. [Google Scholar]
- Whishaw IQ, Jarrard LE. Similarities vs. differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5:595–604. doi: 10.1002/hipo.450050610. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Gorny B. Path integration absent in scent-tracking FF rats: Evidence for hippocampal involvement in “sense of direction” and “sense of distance” using self-movement cues. J Neurosci. 1999;19:4662–4673. doi: 10.1523/JNEUROSCI.19-11-04662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B, Sutherland RJ. Analysis of behavior in the laboratory rat. In: Robinson TE, editor. Behavioral contribution to brain research. New York: Oxford University Press; 1982. [Google Scholar]
- Whishaw IQ, Cassel J-C, Majchrzak M, Cassel S, Will B. “Short-stops” in rats with fimbria-fornix lesions: Evidence for change in the mobility gradient. Hippocampus. 1994;4:577–582. doi: 10.1002/hipo.450040507. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: Evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic tests) Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]