Abstract
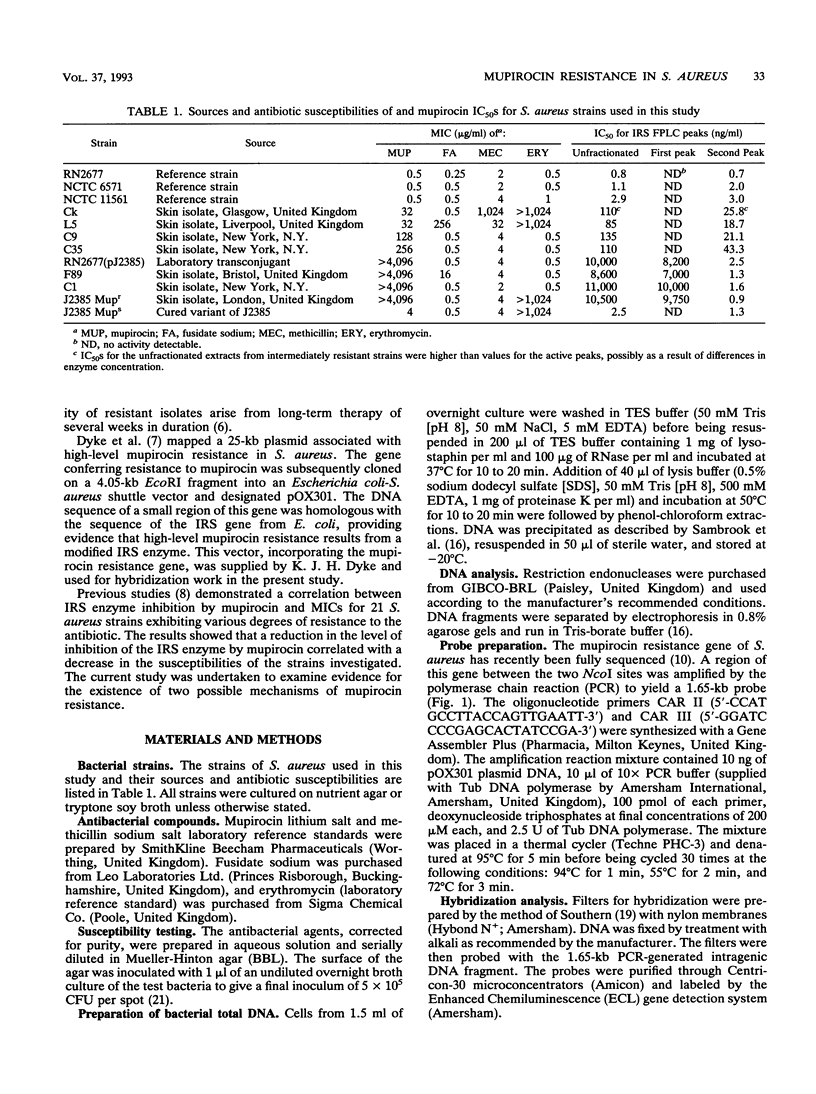
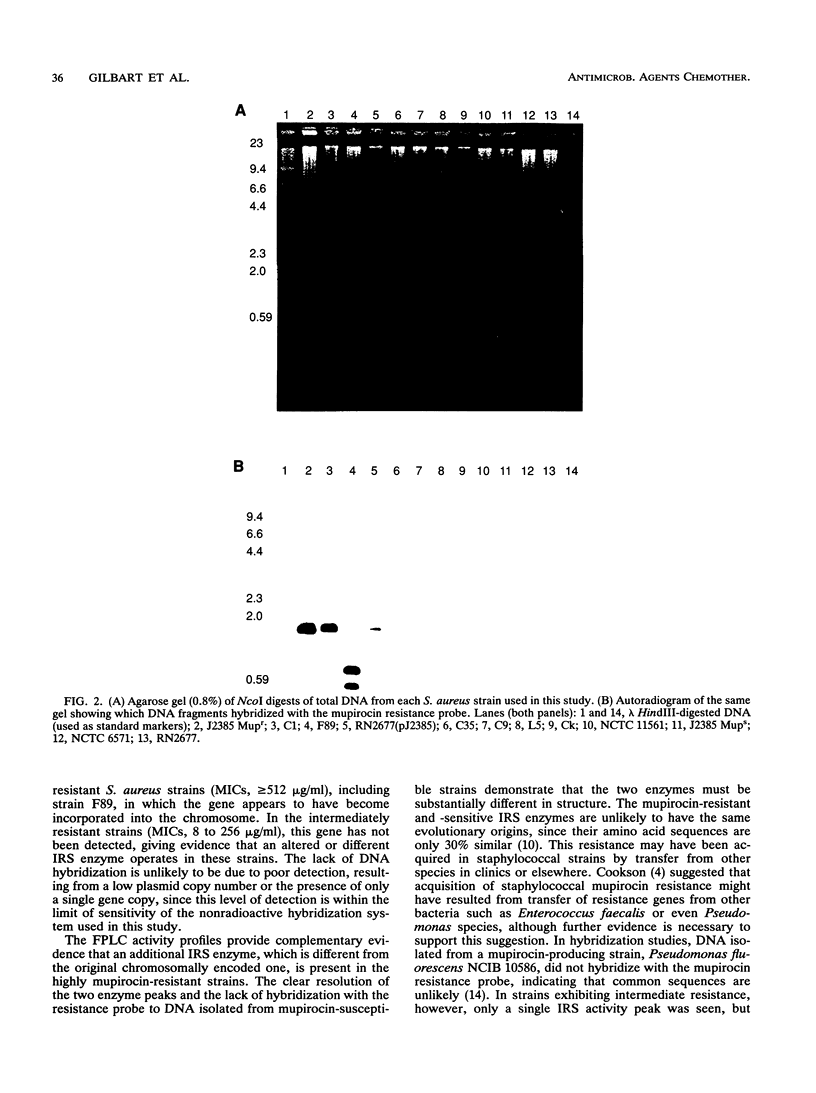
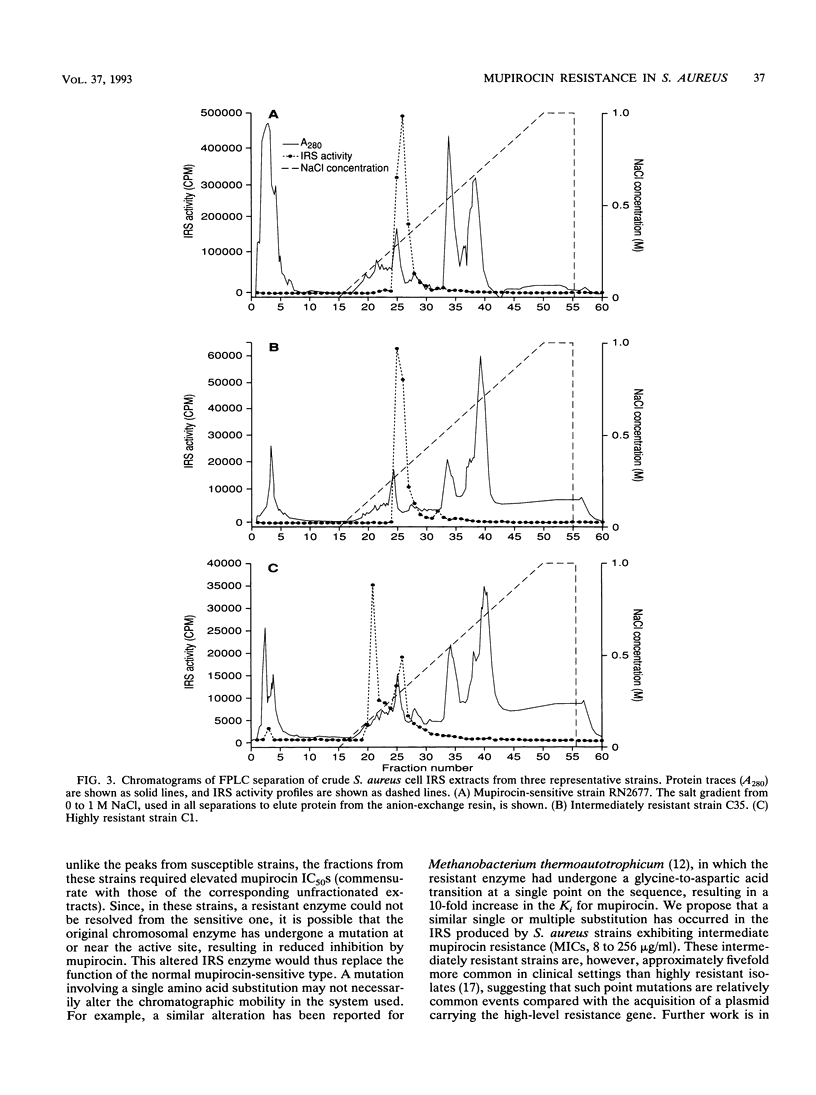
Mupirocin resistance in Staphylococcus aureus results from changes in the target enzyme, isoleucyl-tRNA synthetase (IRS). Twelve strains of S. aureus comprising four susceptible (MICs < or = 4 micrograms/ml), four intermediate level-resistant (MICs between 8 and 256 micrograms/ml), and four highly resistant (MICs > or = 512 micrograms/ml) isolates were examined for their IRS content and the presence of a gene known to encode high-level mupirocin resistance. Ion-exchange chromatography of cell extracts showed a single IRS active peak in mupirocin-susceptible strains, with 50% inhibitory concentrations (IC50s) of 0.7 to 3.0 ng of mupirocin per ml. In strains showing intermediate mupirocin resistance, similar single IRS activity peaks were observed, but these were less sensitive to inhibition, and the mupirocin IC50s for them were 19 to 43 ng/ml. Strains that were highly resistant to mupirocin displayed two distinct peaks; one was similar to that found with susceptible strains (IC50, 0.9 to 2.5 ng/ml), but an additional peak with an IC50 of 7,000 to 10,000 ng/ml was also observed. A strain cured of the plasmid encoding high-level mupirocin resistance lacked the resistant IRS peak. Restriction digests, produced by endonuclease NcoI, of total bacterial DNA isolated from the highly resistant strains hybridized with a mupirocin resistance gene probe, whereas DNA isolated from the intermediate level-resistant and susceptible strains did not. These results demonstrate that two different IRS enzymes were present in highly mupirocin-resistant S. aureus strains. In strains expressing intermediate levels of resistance, only a chromosomally encoded IRS which was inhibited less by mupirocin than IRS from fully susceptible strains was detected.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Capobianco J. O., Doran C. C., Goldman R. C. Mechanism of mupirocin transport into sensitive and resistant bacteria. Antimicrob Agents Chemother. 1989 Feb;33(2):156–163. doi: 10.1128/aac.33.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casewell M. W., Hill R. L. In-vitro activity of mupirocin ('pseudomonic acid') against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1985 May;15(5):523–531. doi: 10.1093/jac/15.5.523. [DOI] [PubMed] [Google Scholar]
- Cookson B. D., Farrelly H., Stapleton P., Garvey R. P., Price M. R. Transferable resistance to triclosan in MRSA. Lancet. 1991 Jun 22;337(8756):1548–1549. doi: 10.1016/0140-6736(91)93242-2. [DOI] [PubMed] [Google Scholar]
- Cookson B. D., Lacey R. W., Noble W. C., Reeves D. S., Wise R., Redhead R. J. Mupirocin-resistant Staphylococcus aureus. Lancet. 1990 May 5;335(8697):1095–1096. doi: 10.1016/0140-6736(90)92667-7. [DOI] [PubMed] [Google Scholar]
- Cookson B. D. Mupirocin resistance in staphylococci. J Antimicrob Chemother. 1990 Apr;25(4):497–501. doi: 10.1093/jac/25.4.497. [DOI] [PubMed] [Google Scholar]
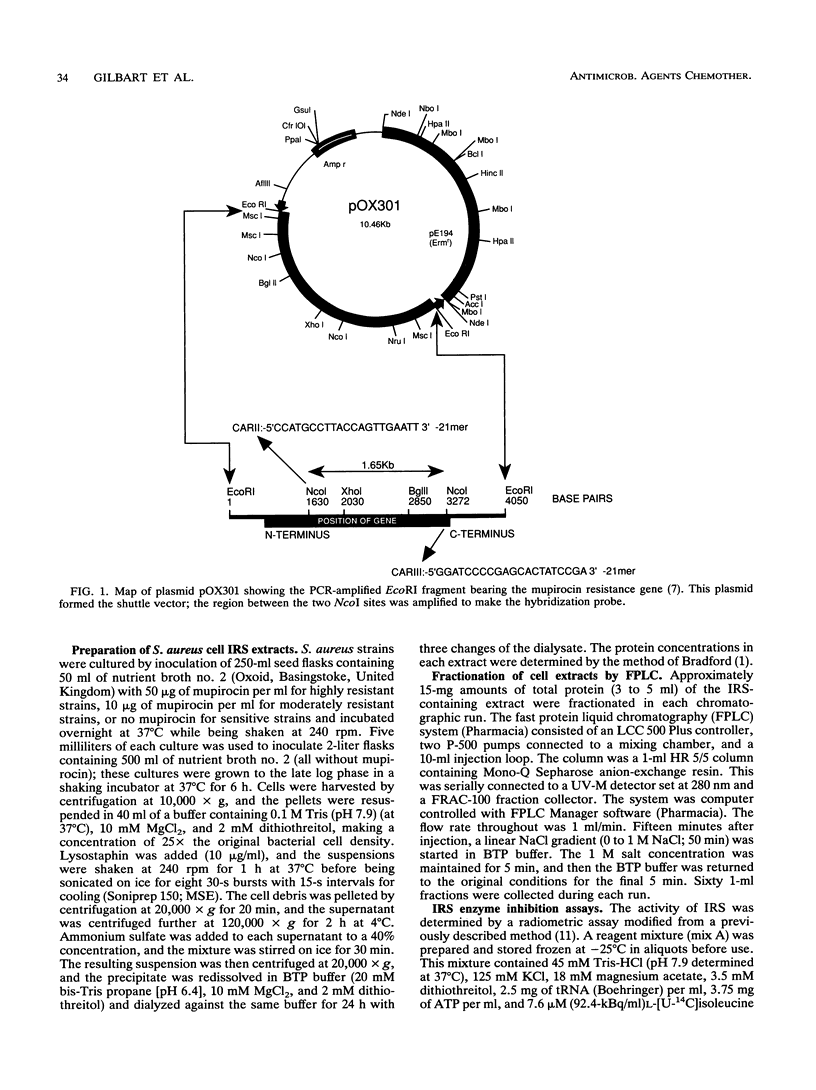
- Dyke K. G., Curnock S. P., Golding M., Noble W. C. Cloning of the gene conferring resistance to mupirocin in Staphylococcus aureus. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):195–198. doi: 10.1016/0378-1097(91)90550-t. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hughes J., Mellows G. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem J. 1980 Oct 1;191(1):209–219. doi: 10.1042/bj1910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U., Rechsteiner T., Tan P. Y., Bühlmann E., Meile L., Leisinger T. Isoleucyl-tRNA synthetase of Methanobacterium thermoautotrophicum Marburg. Cloning of the gene, nucleotide sequence, and localization of a base change conferring resistance to pseudomonic acid. J Biol Chem. 1991 Jun 5;266(16):10570–10577. [PubMed] [Google Scholar]
- Mupirocin-resistant Staphylococcus aureus. Lancet. 1987 Aug 15;2(8555):387–388. [PubMed] [Google Scholar]
- Slocombe B., Perry C. The antimicrobial activity of mupirocin--an update on resistance. J Hosp Infect. 1991 Sep;19 (Suppl B):19–25. doi: 10.1016/0195-6701(91)90198-h. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Kennedy C. T. Staphylococcus aureus resistant to mupirocin. J Antimicrob Chemother. 1988 Jan;21(1):141–142. doi: 10.1093/jac/21.1.141. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Boon R. J., Griffin K. E., Masters P. J., Slocombe B., White A. R. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985 Apr;27(4):495–498. doi: 10.1128/aac.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]




