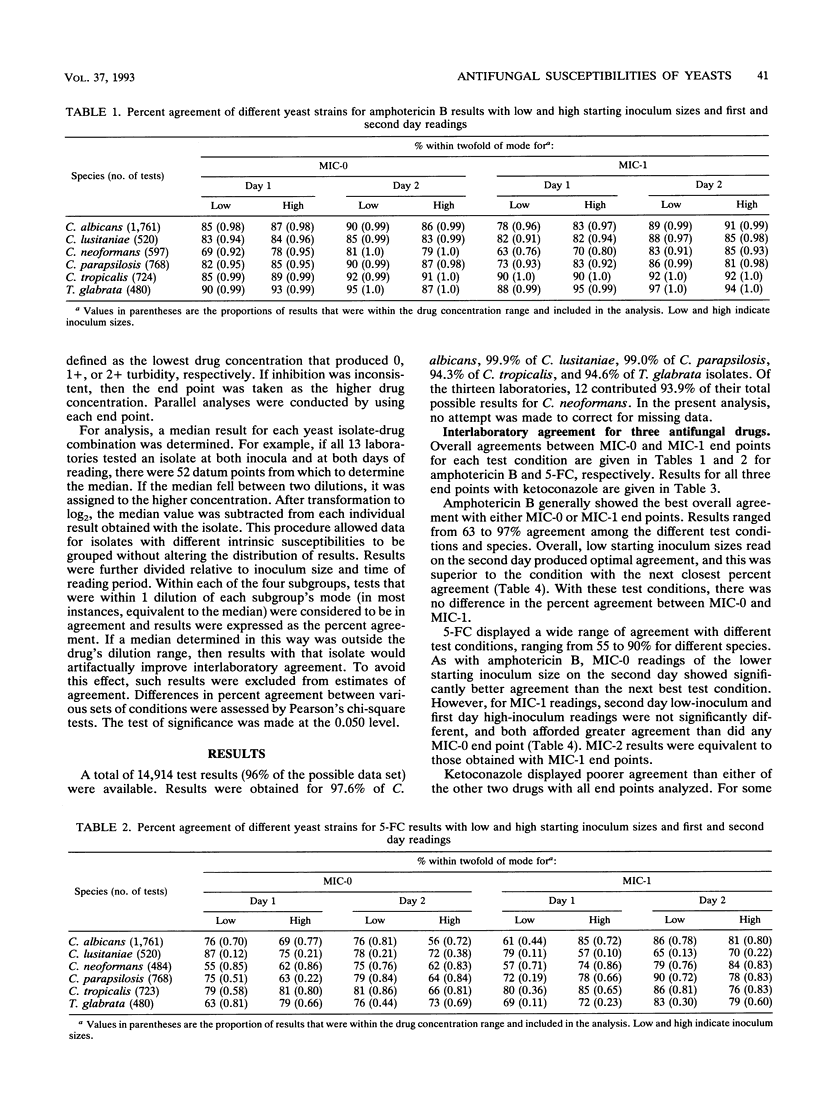
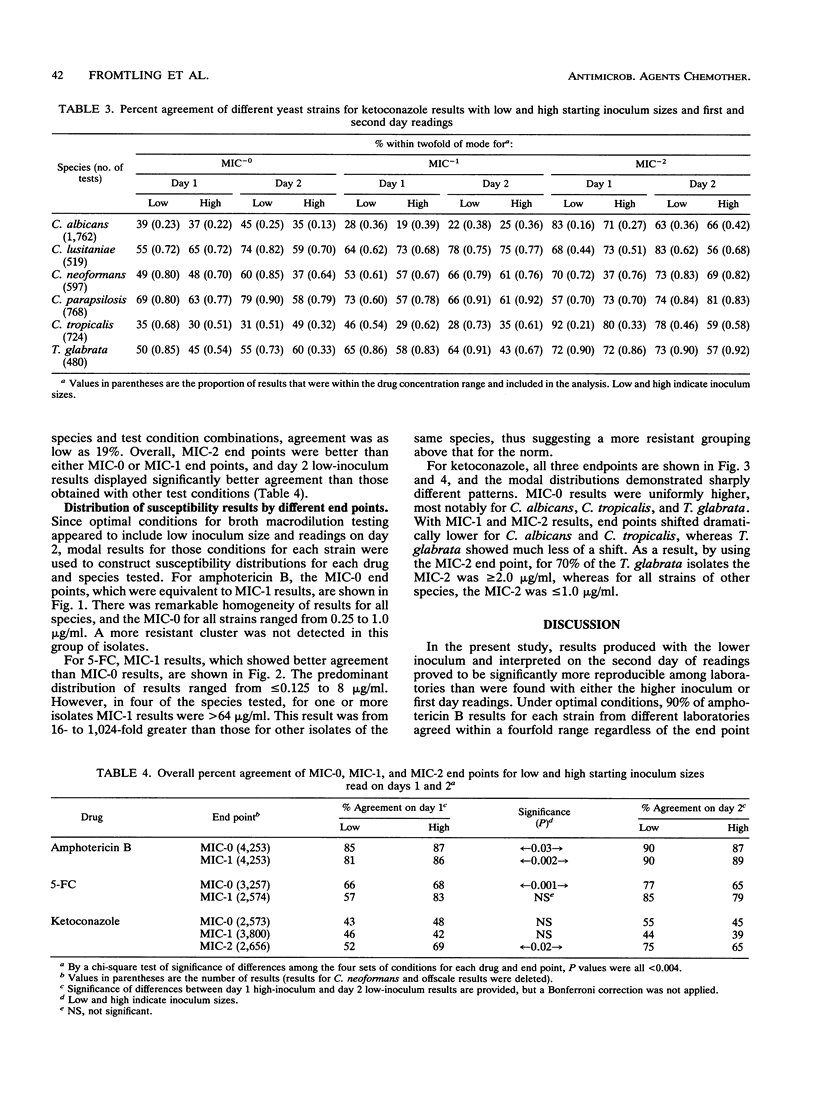
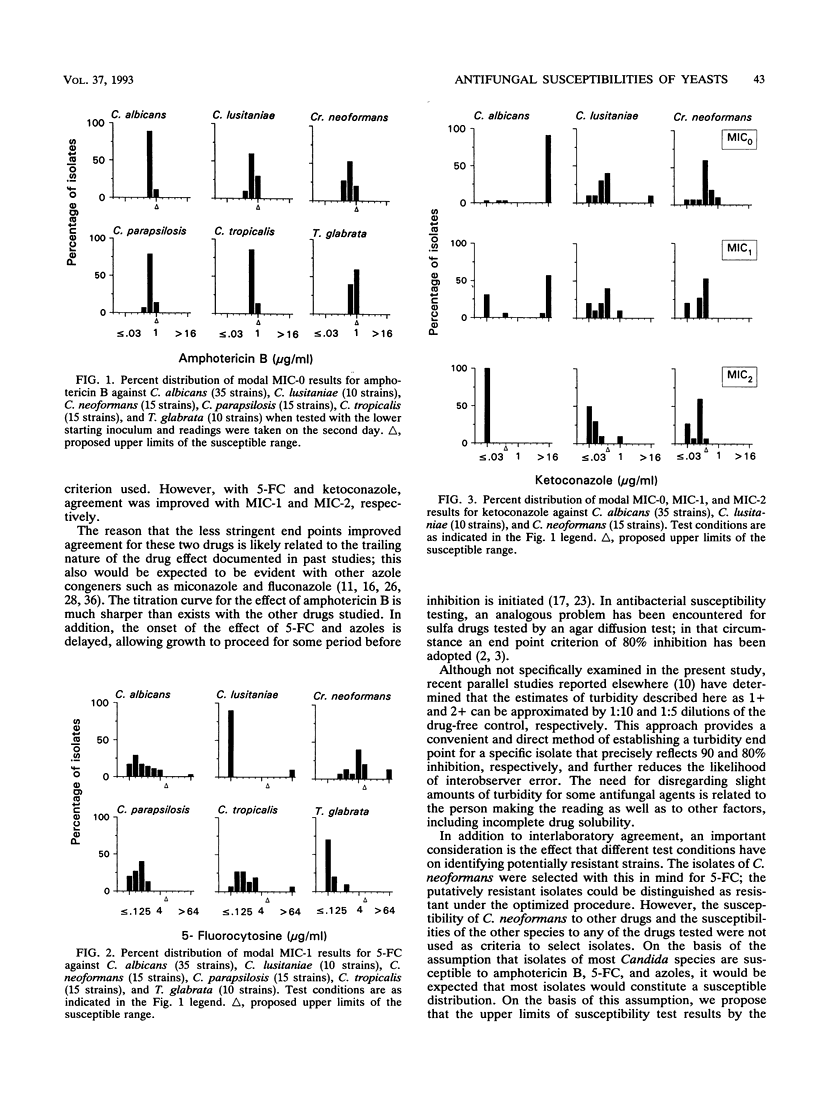
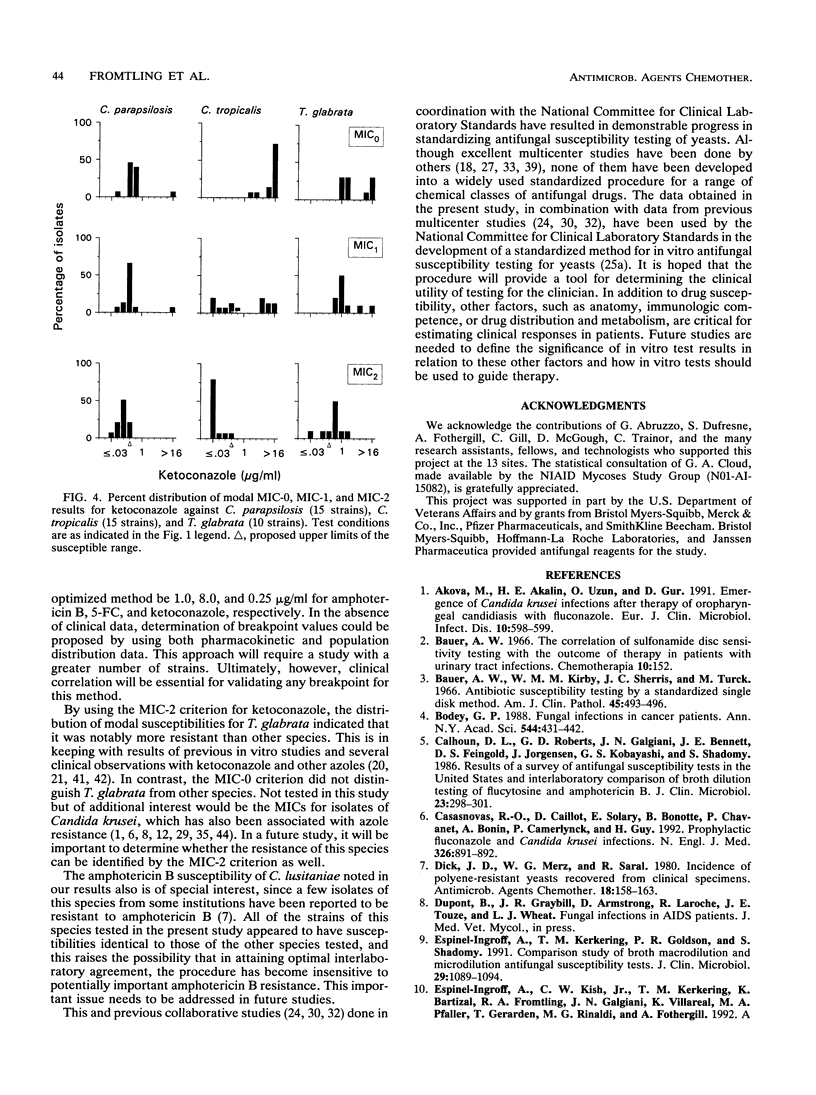
Abstract
Thirteen laboratories collaborated to optimize interlaboratory agreement of results of a broth macrodilution procedure for testing three classes of antifungal drugs against pathogenic yeasts. The activities of amphotericin B, flucytosine, and ketoconazole were tested against 100 coded isolates of Candida albicans, Candida tropicalis, Candida parapsilosis, Candida lusitaniae, Torulopsis (Candida) glabrata, and Cryptococcus neoformans. Two starting yeast inoculum sizes (5 x 10(4) and 2.5 x 10(3) cells per ml) were compared, and readings were taken after 24 and 48 h of incubation. All other test conditions were standardized. The resultant turbidities in all tubes were estimated visually on a scale from 0 to 4+ turbidity, and MIC-0, MIC-1, and MIC-2 were defined as the lowest drug concentrations that reduced growth to 0, 1+, or 2+ turbidity, respectively. For flucytosine, agreement among laboratories varied between 57 and 87% for different inocula, times of incubation, and end point criteria. Agreement was maximized (85%) when the lower inoculum was incubated for 2 days and the MICs were defined as 1+ turbidity or less. For amphotericin B, variations in test conditions produced much smaller differences in interlaboratory agreement. For ketoconazole, interlaboratory agreement was poorer by all end point criteria. However, MIC-2 endpoints distinguished T. glabrata as resistant compared with the other species. Overall, the studies indicated that readings from the lower inoculum obtained on the second day of reading result in the greatest interlaboratory agreement. In combination with data from previous multicenter studies (National Committee for Clinical Laboratory Standards, Antifungal Susceptibility Testing: Committee Report, Vol. 5, No. 17, 1988; M. A. Pfaller, L. Burmeister, M. S. Bartlett, and M. G. Rinaldi, J. Clin. Microbiol. 26:1437-1441, 1988; M. A. Pfaller, M. G. Rinaldi, J. N. Galgiani, M. S. Bartlett, B.A. Body, A. Espinel-Ingroff, R.A. Fromtling, G.S. Hall, C.E. Hughes, F. C. Odds, and A. M. SUgar, J. Clin. Microbiol. 34:1648-1654, 1990), these findings will be used by the National Committee for Clinical Laboratory Standards to develop a standardized method for in vitro antifungal susceptibility testing for yeasts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akova M., Akalin H. E., Uzun O., Gür D. Emergence of Candida krusei infections after therapy of oropharyngeal candidiasis with fluconazole. Eur J Clin Microbiol Infect Dis. 1991 Jul;10(7):598–599. doi: 10.1007/BF01967286. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bodey G. P. Fungal infections in cancer patients. Ann N Y Acad Sci. 1988;544:431–442. doi: 10.1111/j.1749-6632.1988.tb40441.x. [DOI] [PubMed] [Google Scholar]
- Calhoun D. L., Roberts G. D., Galgiani J. N., Bennett J. E., Feingold D. S., Jorgensen J., Kobayashi G. S., Shadomy S. Results of a survey of antifungal susceptibility tests in the United States and interlaboratory comparison of broth dilution testing of flucytosine and amphotericin B. J Clin Microbiol. 1986 Feb;23(2):298–301. doi: 10.1128/jcm.23.2.298-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casasnovas R. O., Caillot D., Solary E., Bonotte B., Chavanet P., Bonin A., Camerlynck P., Guy H. Prophylactic fluconazole and Candida krusei infection. N Engl J Med. 1992 Mar 26;326(13):891–893. [PubMed] [Google Scholar]
- Dick J. D., Merz W. G., Saral R. Incidence of polyene-resistant yeasts recovered from clinical specimens. Antimicrob Agents Chemother. 1980 Jul;18(1):158–163. doi: 10.1128/aac.18.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kerkering T. M., Goldson P. R., Shadomy S. Comparison study of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1991 Jun;29(6):1089–1094. doi: 10.1128/jcm.29.6.1089-1094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Kish C. W., Jr, Kerkering T. M., Fromtling R. A., Bartizal K., Galgiani J. N., Villareal K., Pfaller M. A., Gerarden T., Rinaldi M. G. Collaborative comparison of broth macrodilution and microdilution antifungal susceptibility tests. J Clin Microbiol. 1992 Dec;30(12):3138–3145. doi: 10.1128/jcm.30.12.3138-3145.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Shadomy S. In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol Infect Dis. 1989 Apr;8(4):352–361. doi: 10.1007/BF01963469. [DOI] [PubMed] [Google Scholar]
- Fisher M. A., Shen S. H., Haddad J., Tarry W. F. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 1989 Sep;33(9):1443–1446. doi: 10.1128/aac.33.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling R. A. Overview of medically important antifungal azole derivatives. Clin Microbiol Rev. 1988 Apr;1(2):187–217. doi: 10.1128/cmr.1.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Stevens D. A. Antimicrobial susceptibility testing of yeasts: a turbidimetric technique independent of inoculum size. Antimicrob Agents Chemother. 1976 Oct;10(4):721–728. doi: 10.1128/aac.10.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiant J. N., Stevens D. A. Turbidimetric studies of growth inhibition of yeasts with three drugs: inquiry into inoculum-dependent susceptibility testing, time of onset of drug effect, and implications for current and newer methods. Antimicrob Agents Chemother. 1978 Feb;13(2):249–254. doi: 10.1128/aac.13.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinet R., Nerson D., de Closets F., Dupouy-Camet J., Kures L., Marjollet M., Poirot J. L., Ros A., Texier-Maugein J., Volle P. J. Collaborative evaluation in seven laboratories of a standardized micromethod for yeast susceptibility testing. J Clin Microbiol. 1988 Nov;26(11):2307–2312. doi: 10.1128/jcm.26.11.2307-2312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. M., Richardson M. D., Warnock D. W. In-vitro resistance to imidazole antifungals in Candida albicans. J Antimicrob Chemother. 1984 Jun;13(6):547–558. doi: 10.1093/jac/13.6.547. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Fasoli M., Wayman F. J. Drug resistance in Candida albicans and Candida glabrata. Ann N Y Acad Sci. 1988;544:245–259. doi: 10.1111/j.1749-6632.1988.tb40410.x. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Nicholas R. O. Drug resistance in the opportunistic pathogens Candida albicans and Candida glabrata. J Antimicrob Chemother. 1986 Oct;18 (Suppl B):39–49. doi: 10.1093/jac/18.supplement_b.39. [DOI] [PubMed] [Google Scholar]
- McIlroy M. A. Failure of fluconazole to suppress fungemia in a patient with fever, neutropenia, and typhlitis. J Infect Dis. 1991 Feb;163(2):420–421. doi: 10.1093/infdis/163.2.420. [DOI] [PubMed] [Google Scholar]
- McIntyre K. A., Galgiani J. N. In vitro susceptibilities of yeasts to a new antifungal triazole, SCH 39304: effects of test conditions and relation to in vivo efficacy. Antimicrob Agents Chemother. 1989 Jul;33(7):1095–1100. doi: 10.1128/aac.33.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B., Pye G., Troke P. F. Improved method for estimation of azole antifungal inhibitory concentrations against Candida species, based on azole/antibiotic interactions. J Med Vet Mycol. 1986 Aug;24(4):305–311. doi: 10.1080/02681218680000461. [DOI] [PubMed] [Google Scholar]
- Persons D. A., Laughlin M., Tanner D., Perfect J., Gockerman J. P., Hathorn J. W. Fluconazole and Candida krusei fungemia. N Engl J Med. 1991 Oct 31;325(18):1315–1315. [PubMed] [Google Scholar]
- Pfaller M. A., Burmeister L., Bartlett M. S., Rinaldi M. G. Multicenter evaluation of four methods of yeast inoculum preparation. J Clin Microbiol. 1988 Aug;26(8):1437–1441. doi: 10.1128/jcm.26.8.1437-1441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Cabezudo I., Hollis R., Huston B., Wenzel R. P. The use of biotyping and DNA fingerprinting in typing Candida albicans from hospitalized patients. Diagn Microbiol Infect Dis. 1990 Nov-Dec;13(6):481–489. doi: 10.1016/0732-8893(90)90080-f. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak A. Multicenter-Studie zur Standardisierung der Empfindlichkeitsbestimmung von Pilzen gegen 5-Fluorcytosin und Amphotericin B. Mykosen. 1987 Jul;30(7):306-8, 311-4. [PubMed] [Google Scholar]
- Reagan D. R., Pfaller M. A., Hollis R. J., Wenzel R. P. Characterization of the sequence of colonization and nosocomial candidemia using DNA fingerprinting and a DNA probe. J Clin Microbiol. 1990 Dec;28(12):2733–2738. doi: 10.1128/jcm.28.12.2733-2738.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. E., Galgiani J. N. Activity of fluconazole (UK 49,858) and ketoconazole against Candida albicans in vitro and in vivo. Antimicrob Agents Chemother. 1986 Sep;30(3):418–422. doi: 10.1128/aac.30.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryley J. F., Wilson R. G., Barrett-Bee K. J. Azole resistance in Candida albicans. Sabouraudia. 1984;22(1):53–63. [PubMed] [Google Scholar]
- Røder B. L., Sonnenschein C., Hartzen S. H. Failure of fluconazole therapy in Candida krusei fungemia. Eur J Clin Microbiol Infect Dis. 1991 Mar;10(3):173–173. doi: 10.1007/BF01964453. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Warnock D. W., Kennedy C. T., Johnson E. M., Hopwood V., Van Cutsem J., Vanden Bossche H. Azole resistance in Candida albicans. J Med Vet Mycol. 1986 Apr;24(2):133–144. [PubMed] [Google Scholar]
- Walsh T. J., Rubin M., Hathorn J., Gress J., Thaler M., Skelton J., McKnight J., Browne M., Marshall D., Cotton D. Amphotericin B vs high-dose ketoconazole for empirical antifungal therapy among febrile, granulocytopenic cancer patients. A prospective, randomized study. Arch Intern Med. 1991 Apr;151(4):765–770. [PubMed] [Google Scholar]
- Warnock D. W., Burke J., Cope N. J., Johnson E. M., von Fraunhofer N. A., Williams E. W. Fluconazole resistance in Candida glabrata. Lancet. 1988 Dec 3;2(8623):1310–1310. doi: 10.1016/s0140-6736(88)92919-4. [DOI] [PubMed] [Google Scholar]
- Willocks L., Leen C. L., Brettle R. P., Urquhart D., Russell T. B., Milne L. J. Fluconazole resistance in AIDS patients. J Antimicrob Chemother. 1991 Dec;28(6):937–939. doi: 10.1093/jac/28.6.937. [DOI] [PubMed] [Google Scholar]
- Wingard J. R., Merz W. G., Rinaldi M. G., Johnson T. R., Karp J. E., Saral R. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N Engl J Med. 1991 Oct 31;325(18):1274–1277. doi: 10.1056/NEJM199110313251803. [DOI] [PubMed] [Google Scholar]



