ABSTRACT
Aim: Hearing preservation is one of the major goals of acoustic neuroma surgery. In NF-2 patients, bilateral hearing loss is frequently caused by the disease or results from its treatment. Several implant devices for electrical stimulation of the cochlear nucleus have been developed to restore serviceable hearing in these patients. We report our experience and results using a high rate continuous interleaved sampling (CIS) auditory brainstem implant (ABI). Methods: Between June 1997 and May 2004, 24 NF-2 patients were managed by our group. In 20 patients an ABI was implanted successfully. The cochlear nucleus was located using anatomical landmarks and E-ABR recordings after resection of the neuroma via a retrosigmoid approach in the semi-sitting position. The 12-channel stimulating electrode array was inserted and fixed in the lateral recess. There were no surgical complications related to implantation apart from pseudomeningo that were managed by lumbar drainage. Results: In one patient the electrode array became dislocated and this necessitated revision surgery which was successful. One patient failed to gain benefit from the implant. Overall, 70% of electrodes were found to be serviceable for auditory stimulation, 5.3% of electrodes were primarily nonauditory, and in 7.8% side effects during stimulation were observed. Lip reading was improved by more than 100% as a result of the additional auditory input. For many patients, comprehension of open speech was restored to a useful level. Almost all patients were able to perceive environmental sounds and tinnitus was masked. Conclusions: Restoration of hearing using ABIs in NF-2 patients is a safe and promising procedure for those who would otherwise be totally deaf. The high rate CIS speech processing strategy has proven to be very useful and effective in direct cochlear nucleus stimulation.
Keywords: Cochlear nucleus stimulation, auditory brainstem implant, neurofibromatosis, acoustic neurinoma surgery, high rate CIS stimulation
The first device for direct electrical stimulation of the cochlear nucleus in a human subject was implanted 20 years ago by Hitselberger and colleagues.1 Since their pioneering work several modifications and new developments have been made. The main driving force for improvement has been the success of cochlear implant surgery. Other stimuli have been refinements in microsurgery, intraoperative monitoring, and neuroanesthesia. There has also been a change in the attitude of surgeons who are no longer satisfied with removal of an acoustic neuroma but now want to preserve function. Initially this desire was restricted to the facial nerve but it now also applies to the cochlear nerve.2
The combined efforts of technicians and physicians have resulted in the development of implantable devices that stimulate the cochlear nerve. These auditory brainstem implants (ABIs) use different encoding and stimulation strategies. Efforts to refine these continue and further improvements will undoubtedly become available. At the Department of Otorhinolaryngology of the University of Würzburg, long-lasting and good results have been achieved with the Combi 40 / 40 + (Med-El Company; Innsbruck, Austria) cochlear implant using the high rate continuous interleaved sampling (CIS) strategy. In their series, 54% of patients recognized more than 50% of monosyllabic words.3,4 An electrode for cochlear nucleus stimulation was developed using the same electronics and coding strategy. This study reports the results with the first high rate CIS ABI. The problems we encountered and our philosophy for hearing rehabilitation surgery are discussed.
MATERIAL AND METHODS
The cooperative Auditory Brain-Stem Implant Study was started in June 1997 after obtaining approval from the Ethics Committee of the University of Würzburg. Up until May 2004, 24 patients with NF-2 had been recruited into the program. Some of these patients were treated by ABI teams at different institutions abroad.
Four patients did not receive an ABI. In one patient, implantation was unnecessary because hearing was preserved and the patient was provided after surgery with a conventional hearing aid. In another patient implantation could not be performed safely because of a large vein inside the lateral recess. In one patient the acoustic nerve was preserved anatomically but postoperative testing revealed a loss of hearing with preserved electrical integrity. This patient was successfully provided with a conventional cochlear implant. The surgery for one patient with a severe form of NF-2, Wishard type, was postponed and he refused implantation later. In total, 21 ABI probes were implanted in 20 patients (one patient was implanted twice on the same side because of dislocation of the first electrode). Patients numbered 1, 7, and 17 have received an ABI but still have serviceable hearing on the contralateral side. The mean age of the patients was 32.75 years (range, 18 to 56 years; SD, 11.6 years). The mean duration of deafness was 5.7 years (range, 0.5 to 24 years; SD, 7.6 years). The data of the implanted patients are summarized in Table 1.
Table 1.
The Data of the Implanted Patients Are Summarized
| Patient/Sex | Born Month/Year | Date of Implantation | Hearing (Ipsilateral) | Hearing (Contralateral) | Procedure | Comment |
|---|---|---|---|---|---|---|
| ABI, auditory brainstem implant; GR, Gardner-Robertson classification of hearing. | ||||||
| 1/Male | 3/1958 | 6/1997 | Deaf 1.5 y | Hearing | ABI | Revision, tumor recurrence |
| 2/Male | 9/1975 | 10/1997 | Deaf 5 y | Deaf | ABI | |
| 3/Female | 7/1970 | 1/1998 | Deaf 4 y | Deaf | ABI | Operation abroad |
| 4/Female | 7/1951 | 1/1998 | Deaf 20 y | Deaf | ABI | |
| 5/Male | 12/1965 | 5/1998 | Residual hearing GR IV | Deaf | ABI | |
| 6/Male | 5/1967 | 6/1998 | Deaf 24 y | Residual hearing | ABI | |
| 7/Male | 12/1965 | 6/1998 | Deaf 1.5 y | Hearing | ABI | One revision due to dislocation |
| 8/Female | 8/1968 | 7/1999 | Residual hearing GR IV | Deaf | ABI | |
| 9/Female | 11/1981 | 11/1999 | Deaf 3 y | Deaf | ABI | Revision residual tumor, operation abroad |
| 10/Female | 2/1981 | 2/2000 | Residual hearing GR IV | Deaf | ABI | Revision residual tumor, operation abroad |
| 11/Male | 12/1982 | 5/2000 | Deaf 0.5 y | Deaf | ABI | Revision residual tumor |
| 12/Male | 3/1981 | 8/2000 | Deaf 2 y | Deaf | ABI | Operation abroad |
| 13/Female | 7/1978 | 11/2000 | Deaf 1 y | Deaf | ABI | Operation abroad Wishard type |
| 14/Female | 10/1954 | 2/2001 | Deaf 2 y | Deaf | ABI | |
| 15/Female | 1/1938 | 4/2001 | Residual hearing GR IV | Deaf | ABI | Operation abroad |
| 16/Male | 11/1970 | 5/2001 | Deaf 13 y | Deaf | ABI | |
| 17/Male | 12/1946 | 4/2002 | Deaf 1.5 y | Hearing | ABI | Operation abroad |
| 18/Male | 8/1960 | 4/2002 | Residual hearing GR V | Deaf | ABI | Operation abroad |
| 19/Male | 4/1974 | 4/2004 | Deaf 1 y | Deaf | ABI | Operation abroad |
| 20/Female | 5/2004 | Residual hearing GR IV | Deaf | ABI | Pulsar ABI | |
ABI Device
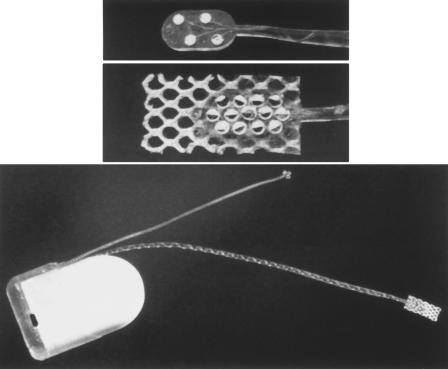
The ABI was developed from the Combi 40 + cochlear implant (Med-El Company, Innsbruck, Austria) that was evaluated in an European multicenter clinical study.4 The housing of the implanted receiver/stimulator consists of a hermetically sealed Al2O3-ceramic that measures 33.5 mm × 23.4 mm × 3.95 mm. It has an array with 12 platinum electrodes with a diameter of 0.6 mm, which is embedded in a silicone carrier 5.5 mm × 3.0 mm × 0.6 mm (Fig. 1, bottom). On the reverse side of the silicone carrier is a Dacron mesh that facilitates fixation in the lateral recess. The electrode array is preshaped by cross-running platinum wires. This allows an element of individual shaping so that it adapts to the contour of the cochlear nucleus.5 The receiver/transmitter is driven by a body-worn or behind-the-ear speech processor. The CIS speech processing strategy developed by Wilson and associates6 is used for encoding. With this device, a high rate stimulation to a maximum 18.180 pulses/sec is possible. The maximum stimulation rate per channel is 1.515/sec with 12 active channels. The stimulation rate/channel can be increased when the number of usable channels is decreased. The stimulation mode is monopolar against a reference electrode which is implanted under the temporalis fascia.
Figure 1.
Auditory brainstem implant (Med-EL, Combi 40 + R), housing and electrodes. (Top) Four-channel test electrode for localization of the cochlear nucleus. (Middle) Twelve-channel stimulating electrode to be implanted. (Bottom) Ceramic housing with stimulating electrode and reference electrode.
Intraoperative Monitoring
Intraoperative neurophysiologic monitoring of auditory evoked potentials and cranial motor nerve activity was performed throughout all surgical interventions. Cranial nerve function was monitored by continuous electromyogram (EMG) recording and by direct electrical stimulation and detection of compound muscle action potentials. Square wave pulses of 30 Hz, 100-μs duration, and 0.05-mA intensity, delivered through a Neurosign™ 100 device, were utilized for electrical stimulation of motor nerves. Constant-current stimuli were applied by coaxial bipolar probes. EMG recordings of the involved muscles were acquired using a Pentium PC-based system that enabled online registration of eight EMG channels. The fifth, seventh, and ninth through twelfth cranial nerves were monitored using this system.
Auditory brainstem responses (ABR) monitored hearing if still present. Rarefaction clicks, 150 μs in duration, were delivered through insert earphones at a rate of 20 Hz . Changes in ABR wave V latency and/or amplitude were used as warning signs of endangered hearing. A Pentium PC-based system and the EWACS™ software were used for recording data. More details and technical notes of the whole monitoring program are listed elsewhere.7
Anatomical landmarks were used to identify the site of the cochlear nucleus and determine the optimal placement of the 12-channel ABI stimulation probe. Intraoperative recording of electrically evoked auditory brainstem response (E-ABR) ensured that the electrode activated the auditory system and did not stimulate nearby nonauditory structures.
E-ABRs were elicited by rectangular biphasic current pulses of 100-μs duration alternating in polarity. The stimulus amplitude was gradually increased up to a maximum of 600 μA. The patients' cardiovascular status and cranial nerve EMG were continuously monitored while the stimulus current level was gradually increased. The current pulses were delivered with an interstimulus interval of 70 ms/s through a specially designed stimulating electrode.5 The stimulating electrode had four contact sites. A bipolar mode of stimulation was used. The responses were picked up using subdermal needle electrodes inserted at the vertex (Cz), contralateral mastoid, or C7 on the neck, with a ground electrode placed either at the ipsilateral mastoid or in the neck. The recorded responses were amplified (gain: 100,000) and filtered with a low cut-off frequency of 10 Hz. Up to 1000 sweeps were averaged using a clinical ABR recording instrument (Westra Q/s 2). The averager and electrical stimulus source were triggered simultaneously.
Surgical Procedure
The patients were placed in the semi-sitting position with the head inclined and turned 30 degrees toward the side of the tumor and then fixed in a Mayfield clamp. A precordial Doppler probe or transesophageal Doppler and end-tidal CO2 measurement were used to warn of air embolism.
A question mark-shaped retroauricular skin incision was made and the skin flap elevated. This incision ensured sufficient cover for the receiver/transmitter of the ABI device. The periosteum and adjacent fascia of the temporalis muscle were elevated and retracted anteriorly to create a second layer which later covered the implant. A lateral retromastoid osteoplastic flap was raised and the dura opened behind the sigmoid and below the transverse sinuses. The cerebellar hemisphere was retracted after drainage of cerebrospinal fluid (CSF) by opening the basal cisterns. The arachnoid membrane covering the tumor was dissected, the tumor debulked and resected in a piecemeal fashion. Depending on the tumor size and intraoperative findings, the internal auditory canal was drilled before, during, or after resection of the extrameatal portion of the tumor. After complete tumor removal the caudal cranial nerves were separated from the arachnoid membrane. The cerebellar flocculus and chorioid plexus of the lateral recess of the fourth ventricle were exposed. Access to the foramen of Luschka was facilitated by rotation of the head toward the tumor side. The four-polar test stimulation probe (Fig. 1, top) was inserted into the foramen of Luschka. The best position for the ABI probe was determined by using bipolar stimulation of two of the four electrodes in longitudinal, transverse, and oblique directions while recording the E-ABR. The bony bed for the transceiver was then drilled in the temporo-occipital region above the transverse sinus. The depth of the bed was adjusted so that approximately one half of the height of the transmitter/receiver, ~2 mm, was inserted. Care was taken to ensure that the internal cortical layer of bone was not removed. The device was fixed with a star-shaped suture and later covered with fascia and periosteum, after which the skin flap was returned and closed in two layers. After repeated E-ABR stimulation, the test stimulation array was then replaced by the 12-channel electrode array of the ABI (Fig. 1, middle). This was secured at the entrance of the foramen with some drops of fibrin glue. The wire itself was fixed at the rostral surface of the cerebellar hemisphere with a collagen sponge impregnated with instant fibrin glue. The internal auditory canal was sealed with a piece of muscle and tissue glue. After closing the dura with a running suture, the perforation where the wire passed through the dura was sealed with fibrin glue. Before closure, the reference electrode was placed beneath the temporalis fascia.
RESULTS
Surgical Results
One patient had useful hearing in his only hearing ear before surgery and an ABI had been planned as it seemed unlikely that function could be preserved. However, the cochlear nerve and intraoperative ABRs were preserved. As a result, this patient did not have an ABI but instead was provided with a conventional hearing aid.
In another patient, the acoustic nerve was preserved anatomically but ABRs were lost during surgery. After surgery the patient was completely deaf but it was still possible to stimulate the cochlear nerve. In a second procedure the patient was given a cochlear implant with a successful outcome.
A similar situation happened in another patient. The intraoperative ABRs were lost, but the cochlear nerve was preserved anatomically and an E-ABR seemed to be present on electrical stimulation. Postoperative testing, however, showed no responses to electrical stimuli. A decision was taken to implant an ABI at a second stage 5 weeks after the first operation. At operation there was very little scar formation and access to the lateral recess was straightforward. However, in the depth of the recess there was a large vein that ran in a craniocaudal direction. This prevented access to the area of the cochlear nucleus and implantation of an ABI probe was not possible. It is likely that there will be a second chance to consider an ABI for this patient when the contralateral tumor is resected. One other patient was postponed due to his clinical condition and later refused implantation.
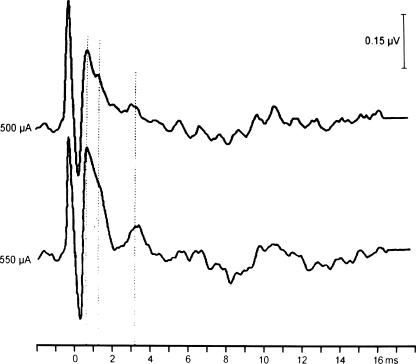
All of the other 20 patients who were deaf preoperatively or who had little residual hearing on the side of the resection have received an ABI. In all, intraoperative electrical stimulation of the cochlear nucleus was successful and unequivocal E-ABR waves were derived that increased in amplitude with increasing stimulation current (Fig. 2). In most patients total tumor removal was achieved. In two patients, tiny tumor remnants were left in situations where removal would have damaged the facial nerve. The 12-electrode array was successfully implanted in the lateral recess of the fourth ventricle in every patient.
Figure 2.
E-ABR recordings using the test electrode demonstrate a reproducible stimulability of the cochlear nucleus with increasing amplitude by increasing the stimulating current. E-ABR, electrically evoked auditory brainstem response.
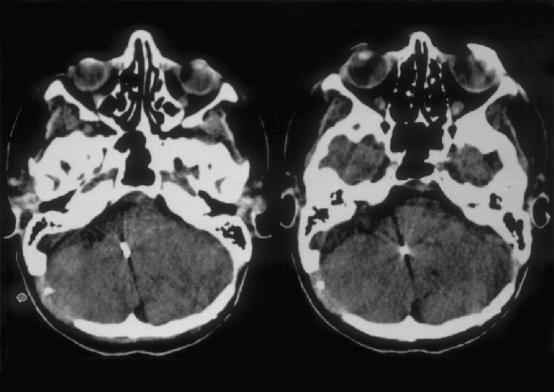
There were no complications directly related to implantation, particularly no surgically induced lesions of the lower cranial nerves, no vascular damage or brainstem hemorrhage even in the revision cases. The postoperative course was uneventful in every patient. After surgery all patients spent a period of time in the intensive care unit until they were sufficiently conscious to return to the ward. A computed tomography (CT) scan was acquired on the first postoperative day. No problems were encountered with the electrode array which was always in the correct position (Fig. 3).
Figure 3.
CT scans 1 day after implantation show the correct position of the stimulating electrode in the lateral recess of the fourth ventricle.
A pseudomeningocele developed in five patients 7 to 20 days after surgery. Because of the importance of the distance between the transmitter coil on the skin surface and the implanted receiver, this complication must be treated even if it would not be necessary in nonimplanted patients. In four patients there was successful resolution with lumbar drainage. In the very first patient lumbar drainage was not successful so that an open procedure was required. At revision, a CSF leak was found at the lower part of the dural closure but the dural entry point of the lead was watertight.
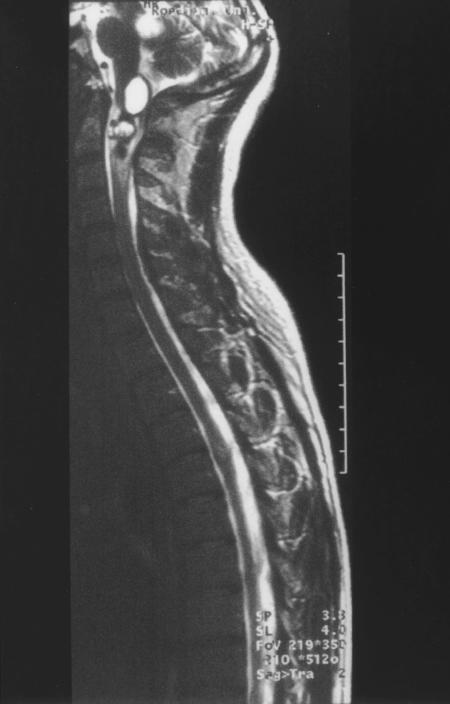
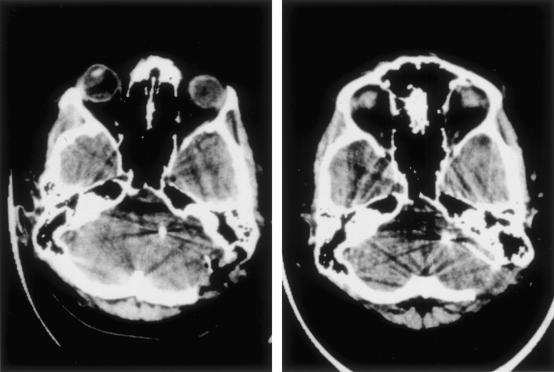
One patient developed hydrocephalus and had to have a ventriculoperitoneal shunt 2 months after the initial operation. In addition, this patient had a glioma in the region of the brachium pontis of the cerebellum contralateral to the implanted side and an asymptomatic solid and cystic glioma of the lower brainstem (Fig. 4). The cystic compartment was fenestrated during the ABI operation. Both lesions might have interfered with CSF circulation.
Figure 4.
MRI scan of Patient 6 (nonuser) prior to implantation. In the lower brainstem there is a cystic lesion with a mixed solid and cystic lesion immediately below and a low-grade glioma in the cerebellar peduncle.
Removal of the tumor was associated with a facial paresis in six patients despite preservation of the facial nerve. All of them have recovered well (House-Brackmann I and II). A permanent H-B Grade IV paresis was acquired by one patient who had revision surgery and had been irradiated previously. Transient swallowing problems were encountered in two patients with large tumors.
Dislocation of the electrode array happened in one patient even though the postoperative CT scan suggested that it was in the correct position. When the transmitter coil was fitted no auditory sensation was perceived, no side effects sustained, and there were normal electrode impedance measurements. A second CT scan showed a small lateral displacement of the array (Fig. 5) when compared with the first scan. At revision, 8 months after the first operation, the CT finding was confirmed. The electrode array was located ~4 mm lateral to the correct position. After repositioning, as in the first operation, E-ABRs were recorded by stimulation of each electrode of the test array. Postoperatively there were no complications and no neurological deterioration. At the first trial fitting, 10 of 12 electrodes elicited auditory sensations and tonotopy was present. Interestingly, that patient had a very large lateral recess and a reduced amount of choroidal plexus which might have facilitated dislocation.
Figure 5.
CT scans of the revision case due to dislocation. (Left) CT scan 1 day after implantation depicts a correct placement of the electrode array in the lateral recess. After first fitting, which failed, the CT scan shows the artifact of the array a little more lateral and closer to the petrus bone, indicating a lateral displacement out of the recess of the fourth ventricle. CT, computed tomography.
Audiological Results
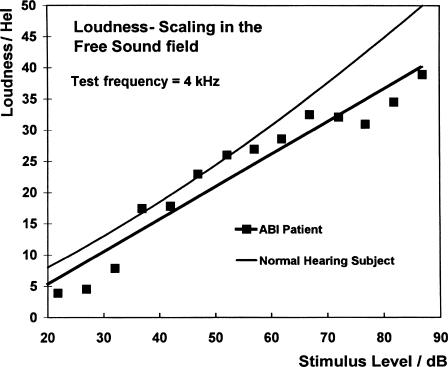
The first processor fitting was performed about 6 weeks following surgery. It was undertaken in the ICU with an anesthetist in attendance lest there be any cardiovascular side effects. However, no such complication was encountered. Fittings and test recordings were undertaken as an inpatient for 3 days, after which the patient was discharged home. During the initial phase, each of the 12 electrodes of the array was tested for auditory sensations, side effects, mean comfortable level of loudness (MCL), threshold, tonotopy, and pulse duration. Electrodes eliciting side effects were switched off. In all but one of our patients a tonotopic pattern was obtained by stimulating the various electrodes. In most cases, the lower frequencies were located laterally, the intermediate frequencies were relatively medial and the high tones were situated in between. However, this pattern was not observed in all patients, and there were some minor variations among adjacent electrodes from fitting session to fitting session. In almost all patients a loudness scaling was possible (Fig. 6).7a,7b
Figure 6.
Loudness scaling of a normal hearing subject and an ABI patient. The regression line of the ABI patient is very similar to the curve of a normal hearing subject. ABI, auditory brainstem implant.
Refitting and adjustment were scheduled at 1, 3, 6, 12, and 24 months after surgery. For various reasons it proved impossible to achieve this protocol in every patient. The audiological data presented focused on patient outcomes in terms of improvement of daily life at specific time intervals after surgery, that is, at 0 to 6 months, 6 to 12 months, and after 1 year. In 12 of the 19 patients all the electrodes elicited auditory sensation. In 3 patients three electrodes were functional, in 2 patients two electrodes, and in another patient just one electrode. Only 1 patient failed to have any functioning electrodes (Table 2). One young female (Number 13) with a Wishard type of NF-2 was not assessed again after the initial fitting, at which good pitch discrimination and sound recognition were documented, because of severe general deterioration 3 months after surgery. One patient did not use the implant. This patient had some auditory sensation but only with very high stimulating current. Although CT data showed that the electrode was correctly positioned in the lateral recess and good E-ABRs were recorded during surgery, the patient experienced many adverse effects. In this particular patient there was additional pathology in the adjacent brainstem. The patient had a solid and cystic tumor inside the lower brainstem and a glioma in the contralateral cerebellar hemisphere as well as a second acoustic neuroma, both displacing and compressing regions of the auditory pathway (Fig. 4), which may have interfered with hearing sensation.
Table 2.
Number of Patients in Relation to Auditory Channels after First Fitting and Actually Used Channels
| Number of Patients | Auditory Channels | Number of Patients | Used Channels |
|---|---|---|---|
| 13 | 12 | 3 | 12 |
| 2 | 10 | 3 | 10 |
| 1 | 11 | 1 | 9 |
| 3 | 9 | 3 | 8 |
| 1 | 0 | 3 | 6 |
| 4 | 5 | ||
| 1 | 4 | ||
| 1 | 3 | ||
| 1 | 0 |
In the most recent patient a new generation of ABI (Med-El Pulsar) was implanted for the first time. First assessments suggest that this patient has a good result with all electrodes active. The data relating to this patient will be presented elsewhere.
Nonauditory Side Effects
Minor side effects were observed in nine patients. When the incriminated electrode was activated, sensations such as twitching in the arm and belly, pressure in the ear, and diplopia developed in one patient. These symptoms and signs resolved on deactivating the electrode. In total, 11.4% of the implanted electrodes produced unwanted side effects. If patients 6, 13, and 20 were excluded because they were either nonusers or had been implanted with a new device, the percentage of electrodes causing unwanted side effects was 7.8%. Electrodes that required high current intensity were switched off to improve the acoustic sensation. The mean number of electrodes used for auditory stimulation in all our patients was 7 (range, 3 to 12; SD, 3.1; Table 2). Excluding patients 6 and 13 (nonusers), the mean number of electrodes used was 7.7 (SD, 2.57). Table 3 gives an overview of total numbers and percentages.
Table 3.
Comparison of Implanted, Auditory Active, Used, and Side-Effect–Producing Electrode Contacts
| User (n = 18) | Patients 1 to 20 | |
|---|---|---|
| The user group excludes Patients 6 and 13. | ||
| Electrodes total | 216 | 240 |
| Auditory electrodes | 196 = 90.7% | 205 = 85.4% |
| Used electrodes | 141 = 71.9% | 144 = 70.2% |
| Side effects | 7.8% | 11.4% |
| Primarily not auditive | 11 = 5.0% | 26 = 10.8% |
Auditory Performance
Seventeen patients regained basic audiological functions and displayed a tonotopic pattern between the electrodes. Patients were able to recognize and discriminate sounds and many could identify environmental sounds such as a doorbell and telephone ring. They distinguished different loudness categories tested with loudness scaling techniques. Patients were able to monitor and achieve better voice control and this made them more confident about interpersonal communication. Tinnitus, a severe problem in many patients, was masked very well by ABI stimulation.
Test Results
Speech discrimination tests were performed in completely deaf patients. Patients numbered 1, 7, and 17 had residual hearing on the contralateral side and were not included in these tests even though they acquired significant auditory information from ABI. Patients numbered 6 and 13 were not tested because they either failed to acquire auditory sensation (number 6) or were not fit to test. Patient 20 received a new generation ABI and is reported separately.
In the first time period, 6 months after implantation, auditory improvement was characterized by increased lip reading ability (LR). Patient 3 was not assessed for LR skill as it was inappropriate because of excellent ABI performance. Using Innsbruck sentences, LR was improved in 9 patients from 19.4% (SD, 13.6) to 59.6% (SD, 23.7) with the ABI, the mean score for numbers was 62.9% (SD, 24.7). ABI-only performers correctly recognized sentences in 46% (SD, 37.2; 4 patients tested) and numbers in 40.1% (SD, 35.6; 8 patients tested). Since numbers are easier to understand than sentences, number tests were performed in more patients. In 4 patients it was possible to undertake both tests. For this group of patients, their number score was better, 53.25% (SD, 44.6), which emphasized the point that patients capable of both tests perform better. In the 12 patients who were able to communicate with ABI only or ABI + LR, the mean score for sentences was 58.5% (SD, 26.3) and for numbers 53.3% (SD, 36.2; n = 9).
In the second time period, up to 1 year after implantation, two additional patients were able to use their ABI only. They scored 65% and 94% correct sentences and 95% and 100% correct numbers, respectively. In five additional patients, LR scores were 15.2% for sentences (SD, 2.87) and LR + ABI 79.3% (SD, 17.2). Numbers were correctly understood by LR in 63.6% (SD, 12.1) and 87.5% (SD, 8.6) with LR + ABI. The ABI only performance for sentences was 48.4% (SD, 37.4; n = 6) and for numbers 69.5% (SD, 40.0; n = 5).
In the combined group of ABI-only performers and those using LR + ABI (n = 7), the mean score for sentences was 81.3% (SD, 13.6) and for numbers 84.3% (SD, 23.3).
In the third time period, after 1 year, there were two more ABI-only patients who scored 84% and 62% sentences and 100% and 55% numbers, respectively. In Table 4 the cumulative average results over the three time periods are summarized.
Table 4.
Audiological Results during Three Time Periods in Terms of LR Improvement with the ABI Device
| 6 Months | 12 Months | 2 Years | |
|---|---|---|---|
| LR, lip reading; ABI, auditory brainstem implant. | |||
| ABI-only performance and both patient groups combined depicting the overall benefit. | |||
| LR + ABI | |||
| Sentences % | 59.6 (n = 9) | 79.3 (n = 5) | 67.5 (n = 9) |
| Numbers % | 62.9 (n = 9) | 87.5 (n = 5) | 81.3 (n = 5) |
| ABI Only | |||
| Sentences % | 46.0 (n = 4) | 48.4 (n = 6) | 42.7 (n = 8) |
| Numbers % | 40.1 (n = 8) | 69.5 (n = 5) | 51.7 (n = 9) |
| Both Groups | |||
| Sentences % | 58.5 (n = 12) | 81.3 (n = 7) | 71.1 (n = 12) |
| Numbers % | 53.3 (n = 9) | 84.3 (n = 7) | 78.5 (n = 11) |
There was a lot of individual variability which could not be expressed adequately by means and standard deviations. Therefore some patients are described in more detail:
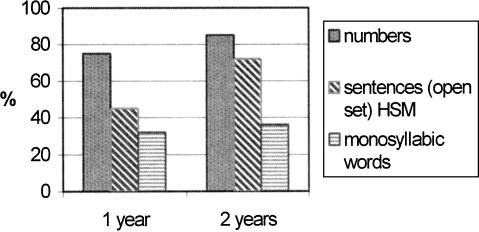
Patient number 3 achieved very good scores on different speech tests: 95% Freiburg numbers, 41% Freiburg monosyllables, 67% vowels and 40% consonants, 65% sentences (all open set without LR) 1 year after the first fitting (Fig. 7). She learned a foreign language, Italian, by using audiocassettes and a text book and can use the telephone with familiar people. Figure 7 shows the audiological results (open set testing) for this patient 1 and 2 years after implantation.
Figure 7.
Audiological results, open set testing, of Patient 3, at 1 and 2 years after implantation.
Patient 4 had only limited speech discrimination and had been deaf for a very long time. Despite a lack of ABI-only open set speech understanding, she was very satisfied with her implant. Before implantation she had felt very isolated, did not leave her house, had very limited social contacts, and had distressing tinnitus. After tumor resection and ABI implantation her life changed. The tinnitus was almost completely masked, she was able to control her own voice, and she was no longer afraid to leave her house as she was now able to identify traffic noise. Most important, she is now confident to walk with her grandson and to play with him outside. This case shows very clearly that the results of hearing rehabilitation cannot be described only in terms of open set speech discrimination, although this should be our goal. Individual expectations and the individual degree of satisfaction must be taken into consideration when evaluating the benefits from ABI.
Patient 5 has reached a score of 65% Freiburg numbers, 10% monosyllables, 13% sentences (open set, without LR, 4 months after first fitting).
Patient 8 could discriminate 65% of presented Freiburg numbers with the ABI only, and 100% ABI + LR. The discrimination scores for sentences (ABI + LR) were 76%. Her tinnitus was almost completely masked and this had a significant impact on the quality of her life.
Patient 9, at the second fitting, achieved 95% correct results in a four-choice word identification test (closed set) and in an open set sentence test 25% (ABI + LR) correct answers. She scored 85% in a vowel identification test and 25% in a consonant identification test. In a sound recognition test (five different sounds), 100% were correct. The spondee identification word test scores were 10%.
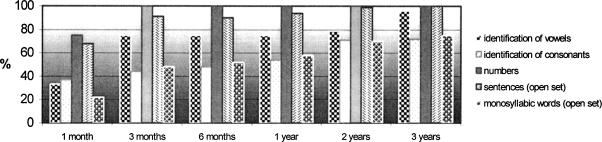
Patient 12, 3 months after fitting, scored with ABI only (open set): 75% vowels, 44% consonants, 100% Freiburg numbers, 48 to 58% monosyllables, and 91% sentences. This patient uses a mobile telephone for basic communication. Figure 8 shows that this patient has performed to a very high level over a long period of time.
Figure 8.
Long-time results of Patient 12. He is performing on a very high level over a long period.
DISCUSSION
Considerable advances have been made since Hitselberger and associates1 implanted the first single-channel ABI in 1979 following the resection of a vestibular schwannoma. The first ABI device consisted of a pair of ball electrodes which were inserted into the substance of the cochlear nucleus. The electrodes were driven by a modified Bosch hearing aid. However, this electrode pair migrated and produced nonauditory side effects. At a revision operation in 1981 a paddle-shaped electrode array with two electrodes was implanted into the lateral recess of the fourth ventricle at the surface of the cochlear nucleus. Up until 1991 this type of electrode array was used and all newly developed arrays have followed this prototype and been implanted in the lateral recess.
The number of electrodes has increased and now the available systems boast anything from 8 to 21 channels.7c,7d,7e,7f It might be argued that a large number of electrodes should increase auditory performance but this is not the case. In a comparison of two ABI-implanted patients, Otto and Staller8 in 1994 described comparable perceptual performance between a patient with six active electrodes and a much longer ABI experience with a patient with three electrodes and only minimal ABI training. In our experience, we have been unable to detect a correlation between functional performance and the number of active electrodes. However, from experience with cochlear implants we know that in general a minimum number of electrodes, four or more, is necessary to achieve adequate auditory perception. The number of active electrodes for useful hearing sensation in our series ranged from 4 to 12. Of more importance than the absolute number of electrodes is the ability of the patient to discriminate different tone pitches and rank them in order reproducibly. This ability was the main difference in the patients investigated by Otto and Staller.8
Improvements in speech processors are another important aspect. Technical developments during the past 20 years were inspired by the success of cochlear implants. A large number of speech coding strategies have been developed and tested in both laboratory and acoustic free field conditions. The high rate CIS strategy elaborated by Wilson and coworkers6 has been one of the most effective. Sixty patients were evaluated in a European multicenter clinical study4 and achieved mean monosyllabic word discrimination scores of 48% 6 months after the first fitting and 54% after 1 year. These results were better than those achieved with the multipeak (24.6%), spectral peak (33.8%), and low rate CIS (28%) strategies.9,10,11 Of further importance is that patients with the high rate CIS strategy implants scored significantly better in sentence recognition tests in a noisy environment (10- to 15-dB signal/noise ratio) compared with multipeak and spectral peak strategies.12 On the basis of these results it was reasonable for us to implement the high rate CIS strategy in an ABI device.
At present, patients who are selected for an ABI are those with NF-2 who have bilateral vestibular schwannomas. In the future, the indication may also include individuals after trauma and patients suffering from neuropathy of the eighth nerve. It may also include those with structural abnormalities that preclude conventional cochlea implantation, for example, ossification of the cochlear duct.13,14 Recently, Colletti and coworkers15 published a report on six patients who were implanted with an ABI after head injury. All recovered well and regained open set speech understanding.
Selection criteria should consider the individual's motivation for repeated postoperative fitting procedures and his or her willingness to undergo daily training with the device. The patients must be completely aware of their likely outcome to avoid any disappointment that might affect their general motivation and cooperation. Candidates should have a normal level of intelligence. Signs and symptoms of the underlying disease should not interfere with the fitting process and rehabilitation. In our opinion, the Wishard type of NF-2, in which there are severe signs and symptoms and a reduced life expectancy, should not be considered an exclusion. The pros and cons of implantation in this NF-2 subtype should be discussed thoroughly on an individual basis. If a patient is physically and psychologically able and willing to go through all the necessary postoperative rehabilitation and fitting procedures, and has good family support, there is no reason to withhold the chance of improving his or her hearing.
The patient's family must be involved from the outset. In our series, there was one patient who had a history of paraplegia after removal of a spinal neuroma at another institution. He had developed gliomas in the lower brainstem and in the contralateral cerebellar peduncle. His paraplegia resolved and he wanted to have an ABI when the vestibular schwannoma was resected on the side of his only hearing ear. During surgery good E-ABRs were recorded but postoperatively he had only minor initial auditory sensations. He refused any further fitting procedures and was reluctant to go to any hospital because of previous experiences. In his case, the additional pathology and the psychological problems worked together and illustrate quite well the many facets which have to be considered prior to implantation.
The question of whether to implant the first or second side is still a matter of debate. For several reasons we recommend implanting the first tumor side. If there is any functional hearing and the indication for surgery is tumor growth and/or compression of the brainstem, every effort should be made to preserve hearing or at least the anatomical integrity of the eighth nerve. In this way there might be the chance to restore hearing with a conventional cochlear implant. According to the data collected in Würzburg, the incidence of persistent electrical stimulability despite postoperative deafness in non–NF-2 schwannomas was 12.5% (personal communication). An ABI should be implanted if the eighth nerve is destroyed, especially in those who have a large tumor on the contralateral side and/or have poor hearing, as the chance of hearing preservation in the contralateral ear is very low. If the contralateral ear has been deaf for a long period (more than 8 to 10 years), or has developed a recurrence, the ipsilateral side should be implanted. More problematic cases are those who have a small or middle-sized contralateral tumor with good functional hearing. In this situation the patient must be very well informed about the surgical and technical problems that might impair implantation in the future. If the first side is not implanted, these problems might prejudice implantation when surgery is necessary on the second side. In that situation, the first side would have to be reopened and scarring might then make implantation extremely difficult, notwithstanding all the additional psychological stress for the patient and increased costs. Our patients who have serviceable hearing in the second ear use their implant for training purposes. This gives them confidence and reassurance for the future. This view is shared by others.16 The alternative argument that this policy might deprive the recipient of potential future improvements in implant technology does not hold as revision surgery is possible.
It is worth considering the functional problems that have been encountered when placing electrodes. In some cases we have experienced difficulties in deriving reproducible E-ABRs. If the patient was already deaf before surgery, it is impossible to know whether the cochlear nucleus can be stimulated at all or if the lack of response has been caused by a technical failure. Optimal placement of the array is also much more difficult and not possible without E-ABR feedback. The precise site of implantation is then determined by anatomical landmarks only. Fortunately, a reproducible E-ABR was derived every time in our series of patients. The position of the array did have to be modified to obtain the best responses by alternating bipolar stimulation of the four electrodes on the array. We found that in some individuals the lateral recess may be partially or completely occluded. In a study by Lang and Schäfer,17 complete occlusion was found in 3%, partial occlusion in 45%, and in 52% the recess was entirely open. Indeed this has been known for a very long time as Alexander18 (in 1926) reported occlusion of the recess in 20%. Other investigators have reported similar rates.17,19,20,21 In one of our cases, we observed another obstacle for implantation. The recess itself was open, but a large vein was running in a craniocaudal direction inside the recess. Some small veins coming from the floor of the recess drained into this vein. To implant, the vein would have had to be coagulated. This could have had a serious effect on the underlying brainstem and cochlear nucleus and therefore implantation was aborted.
We also have views about the three main approaches that are used for the resection of vestibular schwannomas. It is our opinion that the middle fossa approach is suitable only for small intracanalicular tumors and plays no role in ABI implantation. Both the translabyrinthine and retrosigmoid approach are employed for larger schwannomas and can be used for ABI placement. Rarely, some tumors invade the vestibule and resection of these by the retrosigmoid approach is more difficult. This situation can be predicted from preoperative magnetic resonance (MR) scans. It is still possible to achieve a complete resection through the retrosigmoid approach by extending the opening of the internal auditory canal down to the fundus. An additional important argument in favor of the retrosigmoid approach is that it preserves the cochlea and allows the subsequent use of a conventional hearing aid or cochlear implant.
Placement of the electrode array is feasible by both approaches. The route to the lateral recess is a little bit more straightforward in the translabyrinthine approach because the opening of the skull is more lateral than with the retrosigmoid approach. However, the lateral recess may be very deep and caudal in respect to the surgical opening. It may also be obscured by blood or CSF, which may interfere with safe placement without damage to the caudal cranial nerves by suction or manipulation. Fixation of the array by fibrin glue is more difficult in a wet environment, better achieved in almost dry surroundings. The retrosigmoid approach in the semi-sitting position achieves this albeit that the skull opening is more medial the resultant angle of access to the recess is more acute. This anatomical disadvantage can be reduced easily by rotating the head by 30 degrees toward the tumor side.
The tonotopy of the auditory nucleus is organized in a three-dimensional structure. High frequencies are represented in a deeper layer of the cochlear nucleus complex than lower ones.22,23,24 With surface electrodes it seems to be more difficult to stimulate more deeply located neurons of the ventral cochlear nucleus and thus it is more problematic to generate pitch differences by surface stimulation. This may interfere with speech recognition performance. To overcome this problem, penetrating ABI electrodes (PABI)25,26,27,28 have been developed and implanted in humans. Potential problems with this type of electrode are that the location and dimension of the cochlear nucleus has considerable variability. The initial results with this new type of electrode are comparable with those achieved by surface stimulation.26 Long-term results have yet to be published. Most patients achieve pitch discrimination with surface stimulation and this would suggest that a tonotopic pattern of stimulation is achievable.
Although CT scans are sufficient for follow-up, most patients require MR to assess their underlying disease process. Teissl and coworkers29 showed no adverse effects on implants by MR in 0.2 and 1.5 T machines. This has also been our experience with the Med-El system using a 1.5 T machine.
REFERENCES
- Hitselberger W E, House W F, Edgerton B J, et al. Cochlear nucleus implant. Otolaryngol Head Neck Surg. 1984;92:52–54. doi: 10.1177/019459988409200111. [DOI] [PubMed] [Google Scholar]
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery. 1997;40:1–9. doi: 10.1097/00006123-199701000-00001. [DOI] [PubMed] [Google Scholar]
- Helms J, Müller J. Die Auswahl eines Cochlea-Inplants und die Ergebnisse der Implantation [Selection of a cochlear implant and results of implantation] Laryngorhinootologie. 1999;78:12–13. doi: 10.1055/s-2007-996820. [DOI] [PubMed] [Google Scholar]
- Helms J, Müller J, Schön F, et al. Evaluation of performance with the Combi 40 cochlear implant in adults: a multicentric clinical study. ORL J Otorhinolaryngol Relat Spec. 1997;59:23–35. doi: 10.1159/000276901. [DOI] [PubMed] [Google Scholar]
- Kovacs R, Janka M, Hochmair E, et al. A new electrode design for the stable placing of a brainstem electrode. New York, NY: Paper presented at: Proc. ICI 97, NYU–Medical Center; 1997.
- Wilson B S, Finley C C, Lawson D T, Wolford R D, Eddington D K, Rabinowitz W M. Better speech recognition with cochlear implants. Nature. 1991;352:236–238. doi: 10.1038/352236a0. [DOI] [PubMed] [Google Scholar]
- Schlake H-P, Goldbrunner R, Milewski C, et al. Technical developments in intra-operative monitoring for the preservation of cranial motor nerves and hearing in skull base surgery. Neurol Res. 1999;21:11–24. doi: 10.1080/01616412.1999.11740885. [DOI] [PubMed] [Google Scholar]
- Heller O. Hörfeldaudiometrie mit dem verfahren der kategorienunterteilung (KU) Psychol Beitr. 1985;27:478–493. [Google Scholar]
- Moser L M, Hellbrück J. The functional gain of hearing aids measured with a psycho-acoustical scaling test. Santa Barbara, CA: Paper presented at: XVII International Congress of Audiology; 1984.
- Laszig R, Sollmann W P, Marangos N, et al. Nucleus 20-channel and 21-channel auditory brain stem implants: first European experiences. Ann Otol Rhinol Laryngol Suppl. 1995;166:28–30. [PubMed] [Google Scholar]
- Laszig R, Marangos N, Sollmann W P, et al. Initial results from the clinical trial of the nucleus 21-channel auditory brain stem implant. Am J Otol. 1997;18:160. [PubMed] [Google Scholar]
- Otto S R, Brackmann D E, Hitselberger W E, et al. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96:1063–1071. doi: 10.3171/jns.2002.96.6.1063. [DOI] [PubMed] [Google Scholar]
- Nevinson B, Laszig R, Sollmann W P, et al. Results from a European clinical investigation of the nucleus multichannel auditory brainstem implant. Ear Hear. 2002;23:170–183. doi: 10.1097/00003446-200206000-00002. [DOI] [PubMed] [Google Scholar]
- Otto S, Staller S. Multichannel auditory brainstem implant: case studies comparing fitting strategies and results. Ann Otol Rhinol Laryngol Suppl. 1995;166:36–39. [PubMed] [Google Scholar]
- Kessler D K. The Clarion multi-strategy cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1999;177:8–16. [PubMed] [Google Scholar]
- Kessler D K, Loeb G E, Barlo M J. Distribution of speech recognition results with the Clarion cochlear prosthesis. Otol Rhinol Laryngol Suppl. 1995;166:283–285. [PubMed] [Google Scholar]
- Skinner M W, Clark G M, Whitford L A, et al. Evaluation of a new spectral peak coding strategy for the Nucleus 22 Channel Cochlear Implant System. Am J Otol. 1994;15(suppl 2):15–27. [PubMed] [Google Scholar]
- Kiefer J, Müller J, Pfennigdorf T, et al. Speech understanding in quiet and in noise with the CIS speech coding strategy (MED-EL Combi 40) compared to the multipeak and spectral peak strategies (Nucleus) ORL J Otorhinolaryngol Relat Spec. 1996;58:127–135. doi: 10.1159/000276812. [DOI] [PubMed] [Google Scholar]
- Colletti V, Fiorino F, Miorelli V, et al. Auditory brainstem implant as a salvage treatment after unsuccessful cochlear implantation. Otol Neurotol. 2004;25:485–496. doi: 10.1097/00129492-200407000-00016. [DOI] [PubMed] [Google Scholar]
- Colletti V, Carner M, Fiorino F, et al. Hearing restoration with auditory brainstem implant in three children with cochlear nerve aplasia. Otol Neurotol. 2002;23:682–693. doi: 10.1097/00129492-200209000-00014. [DOI] [PubMed] [Google Scholar]
- Colletti V, Carner M, Miorelli V, et al. Auditory brainstem implant in post traumatic cochlear nerve avulsion. Audiol Neurootol. 2004;9:247–255. doi: 10.1159/000078394. [DOI] [PubMed] [Google Scholar]
- Brackmann D E, Hitselberger W E, Nelson R A, et al. Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg. 1993;108:624–633. doi: 10.1177/019459989310800602. [DOI] [PubMed] [Google Scholar]
- Lang J, Schäfer K. Ueber Form, Gröesse und Variabilität des Plexus chorioideus ventriculi IV [Form, size and variations of the plexus chorioideus ventriculi IV] Gegenhaurs Morphol Jahrb. 1977;123:727–741. [PubMed] [Google Scholar]
- Alexander L. Hyperplasien des Recessus lateralis ventriculi 4. Anat Anz. 1926;61:479–487. [Google Scholar]
- Hess C. Das Foramen Magendi und die Oeffnungen an den Recessus lateralis des IV. Ventrikels. Gegenbaurs Morphol Jahrb. 1885;10:578–602. [Google Scholar]
- Retzius G M. Das Menschenhirn: Studien in der makroskopischen Morphologie. Stockholm, Sweden: Kgl Buchdr PA Norstedt & Söner; 1896.
- Zuzuki T. Der Rezessus lateralis und das Foramen Luschkae des vierten Ventrikels bei Japanern. Anat Anz. 1939;88:145–160. [Google Scholar]
- Bourk T R, Mielcarz J P, Norris B E. Tonotopic organization of the anteroventral cochlear nucleus of the cat. Hear Res. 1981;4:215–241. doi: 10.1016/0378-5955(81)90008-3. [DOI] [PubMed] [Google Scholar]
- Dublin W B. The cochlear nucleus revisited. Otolaryngol Head Neck Surg. 1982;90:744–760. doi: 10.1177/019459988209000613. [DOI] [PubMed] [Google Scholar]
- Rauschecker J P, Shannon R V. Sending sound to the brain. Science. 2002;295:1025–1029. doi: 10.1126/science.1067796. [DOI] [PubMed] [Google Scholar]
- McCreery D B, Shannon R V, Moore J K, et al. Accessing the tonotopic organization of the ventral cochlear nucleus by intranuclear microstimulation. IEEE Trans Rehabil Eng. 1998;6:391–399. doi: 10.1109/86.736153. [DOI] [PubMed] [Google Scholar]
- Spieth Nuber C for House Ear Institute First successful use of penetrating microelectrodes in human brainstem restores some hearing to deaf patient. Available at: http://www.hei.org/news/pabi/040115pabi.htm. Accessed June 16, 2004. Available at: http://www.hei.org/news/pabi/040115pabi.htm
- Lenarz T, Moshrefi M, Matthies C, et al. Auditory brainstem implant: part I. Auditory performance and its evolution over time. Otol Neurotol. 2001;22:823–833. doi: 10.1097/00129492-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Lenarz T, Matthies C, Lesinski-Schiedat , et al. Auditory brainstem implant: part II. Subjective assessment of functional outcome. Otol Neurotol. 2002;23:694–697. doi: 10.1097/00129492-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Teissl C, Kremser C, Hochmair E, et al. Cochlear implants: in vitro investigation of electromagnetic interference at MR imaging—compatibility and safety aspects. Radiology. 1998;208:700–708. doi: 10.1148/radiology.208.3.9722849. [DOI] [PubMed] [Google Scholar]