ABSTRACT
Cerebrospinal fluid (CSF) leaks result from a communication between the subarachnoid space and the upper aerodigestive tract. Because of the risk of complications such as meningitis, brain abscess, and pneumocephalus, all persistent CSF leaks should be repaired. Surgical repair may be achieved transcranially or extracranially using a wide variety of autogenous, allogenic, and synthetic patching materials. We report our results with a transnasal transsphenoidal endoscopic approach for the repair of CSF leaks coupled with a multilayer closure using acellular dermis (Alloderm™). We conducted a retrospective review of all patients presenting to our institution over the past 5 years with isolated sphenoid sinus CSF fistulas. Results: Twenty-one patients were included in the study. Nineteen patients (90.5%) had their sphenoid sinus CSF fistula repaired during the first attempt; 2 patients (9.5%) needed a second attempt. The multilayer repair of the CSF leak using acellular dermis via a transsphenoidal endoscopic approach is an effective and successful method of surgical repair of the fistula site. Neither the number, size, nor cause of the CSF fistula affected surgical outcomes. However, the presence of hydrocephalus was a significant negative variable, altering the surgical outcomes of our patients. The acellular dermis offers the advantage of not requiring autogenous tissue for the effective repair of CSF leaks in the sphenoid sinus.
Keywords: Transnasal approach, multilayer repair, sphenoid sinus, CSF leak
A cerebrospinal fluid (CSF) fistula results from a communication between the subarachnoid space and the upper aerodigestive tract. The most frequent causes of traumatic sphenoid sinus CSF leaks are iatrogenic injury during sinus surgery or basilar skull fractures.1
Nontraumatic sphenoid sinus leaks occur as a result of postinfectious sequelae, tumor growth at the skull base, or hydrocephalus (high pressure). Occasionally, a cause cannot be determined, and the CSF leak is classified as truly idiopathic.2 Patients with CSF leaks may have a spectrum of presenting symptoms, ranging from clear nasal discharge and headaches to changes in mental status. Because of the attendant risk for life-threatening complications such as brain abscess, meningitis, or pneumocephalus, all persistent CSF leaks should be repaired. We reviewed the outcomes associated with a multilayer closure technique using acellular dermis performed through a transnasal endoscopic approach.
PATIENTS AND METHODS
Twenty-one patients (6 men, 15 women; age range, 33 to 68 yrs) with CSF fistula into the sphenoid sinus who underwent a transnasal endoscopic repair at The Center for Cranial Base Surgery, St. Luke's Roosevelt Hospital, Manhattan, NY, from 2000 to 2004 were included in the study. All patients were operated upon by a single surgeon (PDC). Patients whose CSF rhinorrhea originated elsewhere than the sphenoid sinus were excluded from this study. Patients' records were reviewed retrospectively for characteristics of the patients, characteristics of the CSF fistula, technique of surgical repair, adjunctive therapies, and outcome. Follow-up data were obtained through clinical examinations and review of hospital records.
Four patients presented with a bilateral CSF leak. Of the 21 patients, 12 (57.2%) leaks were idiopathic and 3 (14.3%) followed a previous brain surgery. Four patients (19%) presented with a sphenoid sinus meningeocele or encephalocele, and 2 (9.5%) had postmeningitis CSF rhinorrhea (Table 1).
Table 1.
Clinical Summary of 21 Patients Who Underwent Transsphenoidal Endoscopic Repair of Sphenoid Sinus CSF Leak
| Patient Number | Age/Sex | U/B | Cause of Fistula | β2-Transferrin | CTC | HRCT | MRC | Fistula Size | POLD | Second Surgery |
|---|---|---|---|---|---|---|---|---|---|---|
| U/B, unilateral/bilateral; CTC, computed tomography cisternography; HRCT, high-resolution computed tomography; MRC, magnetic resonance cisternography; POLD, postoperative lumbar drainage; F/M, female/male; NA, not applicable; SSE, sphenoid sinus encephalocele; SSM, sphenoid sinus meningeocele; + ve, positive; − ve, negative. | ||||||||||
| 1 | 50/F | U | post surgical | + ve | + ve | + ve | NA | 1.7 | 3 | no |
| 2 | 44/F | U | SSE | − ve | + ve | NA | + ve | 0.5 | 4 | no |
| 3 | 42/F | U | idiopathic | + ve | + ve | + ve | NA | 1.3 | 5 | no |
| 4 | 38/F | B | idiopathic | + ve | − ve | NA | + ve | 1 | 7 | yes/VP shunt |
| 5 | 33/F | U | idiopathic | + ve | + ve | + ve | NA | 0.5 | 4 | no |
| 6 | 35/F | U | SSM | NA | + ve | + ve | NA | 1.1 | 4 | no |
| 7 | 56/F | U | idiopathic | + ve | + ve | NA | NA | 1.5 | 6 | yes/VP shunt |
| 8 | 60/F | U | post surgical | + ve | + ve | + ve | NA | 1.6 | 3 | no |
| 9 | 35/F | B | idiopathic | − ve | + ve | NA | + ve | 0.8 | 4 | no |
| 10 | 66/F | U | idiopathic | + ve | − ve | + ve | NA | 0.8 | 4 | no |
| 11 | 55/F | U | SSM | + ve | + ve | NA | NA | 0.9 | 4 | no |
| 12 | 49/F | U | idiopathic | + ve | + ve | NA | NA | 2 | 4 | no |
| 13 | 37/F | U | post meningitis | + ve | + ve | + ve | NA | 1.7 | 3 | no |
| 14 | 34/F | U | idiopathic | NA | + ve | + ve | NA | 1.5 | 4 | no |
| 15 | 41/F | B | idiopathic | + ve | + ve | NA | NA | 2 | 5 | no |
| 16 | 47/M | U | post surgical | + ve | + ve | NA | NA | 1.6 | 4 | no |
| 17 | 68/M | U | SSM | NA | + ve | + ve | NA | 1.5 | 4 | no |
| 18 | 52/M | U | idiopathic | + ve | + ve | NA | + ve | 2 | 4 | no |
| 19 | 46/M | B | idiopathic | + ve | + ve | NA | NA | 1.6 | 4 | no |
| 20 | 41/M | U | post meningitis | + ve | + ve | + ve | NA | 1 | 4 | no |
| 21 | 50/M | U | idiopathic | − ve | + ve | + ve | + ve | 1.2 | 3 | no |
Preoperative Evaluation
When possible, rhinorrhea was collected for β2-transferrin analysis to confirm the presence of a leak. Endoscopic evaluation in the office did not localize the site of the leak in any case. Preoperative radiographic studies were used in various combinations to localize the precise site of CSF leaks. Computed tomography (CT) cisternography was performed in all 21 cases. High-resolution CT of the paranasal sinuses or temporal bone was performed in 11 cases and magnetic resonance cisternography in 5 cases. Perioperative management often included placement of a lumbar drain, placement of a ventriculoperitoneal (VP) shunt, antibiotics, and bed rest.
Surgical Repair
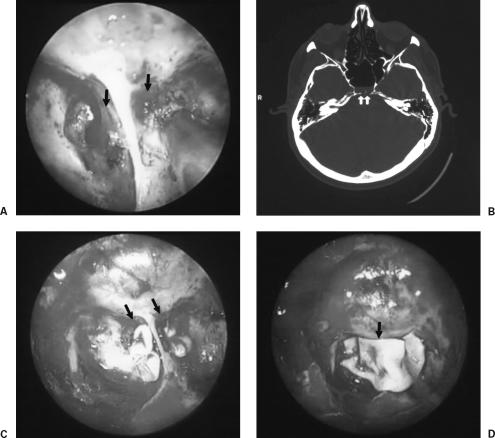
All leaks were repaired with an underlay multilayer technique using acellular dermis (AlloDerm, LifeCell Corporation, Branchburg, NJ, USA) to close the fistula site, followed by fibrin glue to fix the AlloDerm in place. An additional layer of AlloDerm, followed by a fat graft, was used to obliterate the sphenoid sinus with injection of more fibrin glue (Fig. 1). Absorbable Gelfoam® (Pharmacia and Upjohn, Kalamazoo, MI, USA) was then placed on the surface of the fat graft and nasal packing with Merocel® (Medtronic Merocel, Mystic, CT, USA) for 24 to 48 hours.
Figure 1.
(A) Intraoperative endoscopic view of the fistulous sites (arrows). (B) High-resolution CT scan of the skull shows bilateral fistula at the posterior surface of the sphenoid sinus (arrows). (C) AlloDerm packing of the fistulous site. (D) A second layer of Alloderm (arrow) covers the fistulous sites.
Perioperative management often included the use of a lumbar drain. A VP shunt was used when hydrocephalus was diagnosed. Lumbar drains were used in all cases. The average length of lumbar drainage was 4 days. Intravenous antibiotics were used an average of 5 days.
RESULTS
Surgical Outcome
Of the 21 patients, CSF leak sites were successfully repaired in 19 patients (90.5%) with a single-stage endoscopic surgery. Two patients (9.5%) underwent successful repair after a second operation. Therefore, the overall rate of successful repairs was 100%. In these two patients who needed a second operation to place a VP shunt, hydrocephalus was the cause of their persistent CSF leak. Follow-up ranged from 8 to 24 months (mean, 16 mos). No surgical complications were encountered in any of the patients.
DISCUSSION
Recent studies have reported the success of extracranial endoscopic repair of CSF leaks. Over the past decade, rates of success after initial attempt at repair have ranged from 76 to 97%. Success rates after the second attempt at repair have ranged from 86 to 100%.12,15,17,18,19,20 These results are consistent with those of our study.
Diagnostic Algorithm
The site of leakage can usually be identified with the use of high-resolution CT cisternography after the intrathecal administration of low-osmolar, nonionic iodine contrast media (e.g., metrizamide). Egress of contrast material mixed with CSF through the fistula site can then be potentially visualized on CT. Recent studies have defined the sensitivity, specificity, and accuracy of various radiographic methods for localization and evaluation of sites of CSF leakage.3,4,5,6 The accuracy of cisternography using metrizamide has been in the range of 90% or higher.7
The primary problem with CT cisternography is that identification of the exact location of the leak requires the presence of an active fistula. Intermittent leaks that are temporarily sealed by swelling, inflammation, or prolapsed arachnoid may yield a false-negative result.8 A saline challenge, in which the normal saline solution is injected intrathecally to increase CSF pressure, may prevent a false-negative result. Nothing else can be done to increase the probability of CT cisternography identifying the site of a CSF leak. In contrast, magnetic resonance cisternography does not require the intrathecal administration of a contrast agent. It is noninvasive and its sensitivity, specificity, and accuracy are 87%, 57%, and 96%, respectively, when combined with coronal CT scans.4,8
If CT cisternography fails to localize a leak and the probability of leakage is high, a radionuclide pledget study can be performed. This study does not provide the same degree of anatomic localization as CT cisternography, but it can still help localize the general area of the leak (i.e., ethmoid, sphenoid, right, left).
Analysis of nasal secretions for β2 transferrin may be used to confirm the diagnosis. β2 transferrin is cost-effective for confirming fluid as CSF9; however, there is an increased false-positive rate in cases of cirrhosis or hereditary protein anomalies.10 Recently, β-trace protein has proved to be more sensitive and more specific than β2 transferrin (100 to 93% sensitivity and 100 to 97% specificity, respectively).11
Techniques and Materials for Repair of CSF Leaks
Various techniques have been described for the repair of CSF leaks of the anterior skull base and sphenoid sinus. Surgical repair may be achieved intracranially through a bifrontal craniotomy or extracranially through the paranasal sinuses. Historically, intracranial approaches have been used to repair refractory sinonasal CSF leaks. Obvious disadvantages of this strategy include the need for a large external incision, the risk of anosmia from trauma to the olfactory tracts and filaments in the cribriform region, possible cerebral edema, encephalomalacia from frontal lobe retraction, and intracranial hemorrhage.12,13 Despite the merits of direct visualization and the use of vascularized pericranial flaps, success rates associated with treatment of sinonasal CSF leaks through a frontal craniotomy are about 80%.14
In 1948 Dohlman described the first extracranial approach for the repair of CSF rhinorrhea using a naso-orbital incision. Subsequently, others developed a variety of endonasal approaches using septal or turbinate flaps to repair the fistulous site.15 Extracranial approaches typically employ mastoid surgery for temporal bone leaks, transseptal sphenoid obliteration for sphenoid leaks, or endoscopic endonasal repair through the sphenoid or ethmoid sinuses. Because these approaches have equal or superior success rates and are typically less morbid than intracranial procedures, they have become increasingly popular for the repair of skull base CSF leaks. Recent studies have reported success rates from 86 to 100% with endoscopic repair of CSF leaks of the sinonasal tract,12,15,16,17,18,19,20,21 regardless of the graft material or technique used (Table 2).22 There is little controversy about the usefulness of such approaches as long as preoperative localization and intraoperative visualization are precise.8
Table 2.
Materials Used for CSF Leak Repair
| Materials Used for Closure of CSF Fistula Site |
|---|
| Flap (vascularized and nonvascularized) |
| Mucosa |
| Allograft |
| Lyophilized dura |
| Fascia lata (autogenous or lyophilized) |
| Temporal fascia |
| Autogenous fat |
| Hydroxyapatite |
| Others (fibrin glue, dissolving pack, cautery) |
Since Wigand described the endoscopic skull base repair in 1981,23 the popularity of this technique has grown. Many series have been reported, with success rates ranging from 83 to 100%.12,21,24,25 It has been suggested that endoscopic repair be limited to defects less than 1.5 cm in size.22 Other authors2,15 have found no correlation between success of endoscopic repair and the size of the defect. Others have used cartilage or bone to repair larger defects and have reported successful repair of defects larger than 2 cm.17,21 In each case, a multilayer repair was performed, using temporalis fascia and/or muscle, septal cartilage, and/or bone and tissue glue. Each repair was supported with packing for 14 days to achieve stability. During this initial period, the head of the bed was elevated. Lumbar drains were used in all cases.17,22,26
We describe a multilayer technique for the closure of CSF fistula. An AlloDerm graft for closure of the fistula site and another layer of AlloDerm with fibrin glue were used to seal the site. A free abdominal fat plug was then placed to fill the entire sphenoid sinus cavity. We found that this technique ensured that the fistulous site would be sealed and improved the chance of closing the leak in one session. The choice was mainly based on our experience with AlloDerm and the use of fat grafts. Kirtane et al27 reported their technique of sealing the sphenoid sinus with fascia and a fat plug without the use of fibrin glue. We believe that using a biological adhesive material such as fibrin glue enhances the healing process and ensures sealing of the fistulous site. The senior author (PDC) abandoned the use of hydroxyapatite cement in the management of CSF leak28,29 after many patients complained of severe postoperative headaches.
No radiological or anatomical findings on postoperative studies were related to these patients' complaints. The one variable that correlated with persistent CSF leakage was the presence of hydrocephalus. High-pressure hydrocephalus should be expected when a CSF leak is persistent and should be ruled out by measuring the opening pressure of a lumbar puncture.15 Of our 21 patients, 2 have experienced a recurrent CSF leak after operative repair. In both cases hydrocephalus was the cause, and the condition resolved with the placement of a VP shunt. Although CSF leaks traditionally have been managed through an intracranial approach, this technique carries a risk of substantial morbidity.13,14,15 The use of an allogenic graft may help decrease this morbidity and provides an alternative to the autogenous fascial layer.15
CONCLUSION
The multilayer repair of the CSF leak is an effective and successful procedure for the surgical repair of a CSF fistula. Neither the number, size, nor cause of a CSF fistula appears to affect surgical outcomes. However, the presence of hydrocephalus did affect surgical outcomes and two patients with increased intracranial pressure required placement of a VP shunt and reconstruction of the CSF fistulous site. The endoscopic transnasal repair of a CSF leak involving the sphenoid sinus is a direct and fast approach associated with good outcomes and minimal complications.
REFERENCES
- Stankiewicz J A. Cerebrospinal fluid fistula and endoscopic sinus surgery. Laryngoscope. 1991;101:250–256. doi: 10.1288/00005537-199103000-00006. [DOI] [PubMed] [Google Scholar]
- Church C A, Chiu A G, Vaughan W C. Endoscopic repair of large skull base defects after powered sinus surgery. Otolaryngol Head Neck Surg. 2003;129:204–209. doi: 10.1016/S0194-5998(03)00521-7. [DOI] [PubMed] [Google Scholar]
- Johnson D B, Brennan P, Toland J, O'Dwyer A J. Magnetic resonance imaging in the evaluation of cerebrospinal fluid fistulae. Clin Radiol. 1996;51:837–841. doi: 10.1016/s0009-9260(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Shetty P G, Shroff M M, Fatterpekar G M, Sahani D V, Kirtane M V. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. AJNR Am J Neuroradiol. 2000;21:337–342. [PMC free article] [PubMed] [Google Scholar]
- El Gammal T, Sobol W, Wadlington V R, et al. Cerebrospinal fluid fistula: detection with MR cisternography. AJNR Am J Neuroradiol. 1998;19:627–631. [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Goyal M, Mishra N K, Sharma A, Gaikwad S B. Positional MRI: a technique for confirming the site of leakage in cerebrospinal fluid rhinorrhoea. Neuroradiology. 1997;39:818–820. doi: 10.1007/s002340050512. [DOI] [PubMed] [Google Scholar]
- Eljamel M S, Pidgeon C N, Toland J, Phillips J B, O'Dwyer A A. MRI cisternography, and the localization of CSF fistulae. Br J Neurosurg. 1994;8:433–437. doi: 10.3109/02688699408995111. [DOI] [PubMed] [Google Scholar]
- Zapalac J S, Marple B F, Schwade N D. Skull base cerebrospinal fluid fistulas: a comprehensive diagnostic algorithm. Otolaryngol Head Neck Surg. 2002;126:669–676. doi: 10.1067/mhn.2002.125755. [DOI] [PubMed] [Google Scholar]
- Meurman O H, Irjala K, Suonpaa J, Laurent B. A new method for the identification of cerebrospinal fluid leakage. Acta Otolaryngol. 1979;87:366–369. doi: 10.3109/00016487909126434. [DOI] [PubMed] [Google Scholar]
- McMains K C, Gross C W, Kountakis S E. Endoscopic management of cerebrospinal fluid rhinorrhea. Laryngoscope. 2004;114:1833–1837. doi: 10.1097/00005537-200410000-00029. [DOI] [PubMed] [Google Scholar]
- Arrer E, Meco C, Oberascher G, Piotrowski W, Albegger K, Patsch W. beta-Trace protein as a marker for cerebrospinal fluid rhinorrhea. Clin Chem. 2002;48:939–941. [PubMed] [Google Scholar]
- Lanza D C, O'Brien D A, Kennedy D W. Endoscopic repair of cerebrospinal fluid fistulae and encephaloceles. Laryngoscope. 1996;106:1119–1125. doi: 10.1097/00005537-199609000-00015. [DOI] [PubMed] [Google Scholar]
- Hughes R G, Jones N S, Robertson I J. The endoscopic treatment of cerebrospinal fluid rhinorrhoea: the Nottingham experience. J Laryngol Otol. 1997;111:125–128. doi: 10.1017/s0022215100136643. [DOI] [PubMed] [Google Scholar]
- Park J I, Strelzow V V, Friedman W H. Current management of cerebrospinal fluid rhinorrhea. Laryngoscope. 1983;93:1294–1300. doi: 10.1002/lary.1983.93.10.1294. [DOI] [PubMed] [Google Scholar]
- Zweig J L, Carrau R L, Celin S E, et al. Endoscopic repair of cerebrospinal fluid leaks to the sinonasal tract: predictors of success. Otolaryngol Head Neck Surg. 2000;123:195–201. doi: 10.1067/mhn.2000.107452. [DOI] [PubMed] [Google Scholar]
- Dodson E E, Gross C W, Swerdloff J L, Gustafson L M. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea and skull base defects: a review of twenty-nine cases. Otolaryngol Head Neck Surg. 1994;111:600–605. doi: 10.1177/019459989411100510. [DOI] [PubMed] [Google Scholar]
- Mattox D E, Kennedy D W. Endoscopic management of cerebrospinal fluid leaks and cephaloceles. Laryngoscope. 1990;100:857–862. doi: 10.1288/00005537-199008000-00012. [DOI] [PubMed] [Google Scholar]
- Casiano R R, Jassir D. Endoscopic cerebrospinal fluid rhinorrhea repair: is a lumbar drain necessary? Otolaryngol Head Neck Surg. 1999;121:745–750. doi: 10.1053/hn.1999.v121.a98754. [DOI] [PubMed] [Google Scholar]
- Mao V H, Keane W M, Atkins J P, et al. Endoscopic repair of cerebrospinal fluid rhinorrhea. Otolaryngol Head Neck Surg. 2000;122:56–60. doi: 10.1016/S0194-5998(00)70144-6. [DOI] [PubMed] [Google Scholar]
- Wax M K, Ramadan H H, Ortiz O, Wetmore S J. Contemporary management of cerebrospinal fluid rhinorrhea. Otolaryngol Head Neck Surg. 1997;116:442–449. doi: 10.1016/S0194-59989770292-4. [DOI] [PubMed] [Google Scholar]
- Burns J A, Dodson E E, Gross C W. Transnasal endoscopic repair of cranionasal fistulae: a refined technique with long-term follow-up. Laryngoscope. 1996;106:1080–1083. doi: 10.1097/00005537-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Wigand M E. Transnasal ethmoidectomy under endoscopical control. Rhinology. 1981;19:7–15. [PubMed] [Google Scholar]
- Schick B, Ibing R, Brors D, Draf W. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001;110:142–147. doi: 10.1177/000348940111000209. [DOI] [PubMed] [Google Scholar]
- Wormald P J, McDonogh M. “Bath-plug” technique for the endoscopic management of cerebrospinal fluid leaks. J Laryngol Otol. 1997;111:1042–1046. doi: 10.1017/s0022215100139295. [DOI] [PubMed] [Google Scholar]
- Gjuric M, Goede U, Keimer H, Wigand M E. Endonasal endoscopic closure of cerebrospinal fluid fistulas at the anterior cranial base. Ann Otol Rhinol Laryngol. 1996;105:620–623. doi: 10.1177/000348949610500806. [DOI] [PubMed] [Google Scholar]
- Kirtane M V, Gautham K, Upadhyaya S R. Endoscopic CSF rhinorrhea closure: our experience in 267 cases. Otolaryngol Head Neck Surg. 2005;132:208–212. doi: 10.1016/j.otohns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Costantino P D, Hiltzik D H, Sen C, et al. Sphenoethmoid cerebrospinal fluid leak repair with hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 2001;127:588–593. doi: 10.1001/archotol.127.5.588. [DOI] [PubMed] [Google Scholar]
- Catalano P J, Post K, Sen C, Costantino P, Friedman C. Prevention of cerebrospinal fluid rhinorrhea in neurotologic surgery. Am J Otol. 2000;21:265–269. doi: 10.1016/s0196-0709(00)80020-4. [DOI] [PubMed] [Google Scholar]