Abstract
Expression of the free β-subunit of human chorionic gonadotropin (hCGβ) in malignant tumors is frequently associated with aggressive disease. The pretreatment serum concentration of hCGβ is an independent prognostic variable in renal cell carcinoma (RCC). The three so-called type II genes (hCGβ 3/9, 5, and 8) have been shown to be up-regulated in relation to type I genes (hCGβ 6/7) in some malignant tumors. We developed a reverse transcription-polymerase chain reaction method for quantification of relative levels of the mRNAs for the two types of hCGβ genes and studied the association between the expression in RCC tissue (n = 104) and clinical outcome. hCGβ mRNA expression was detected in 40% (42 of 104) of the tumors, and in 40 of these (93%), this consisted of hCGβ type I mRNA only, whereas type II hCGβ mRNA was detected in two samples. hCGβ mRNA expression was significantly associated with a shorter disease-specific (log-rank P = 0.023; median survival 1.4 versus 7.9 years) and overall survival (log-rank P = 0.011). In a Cox regression model, stage (P < 0.0001) and hCGβ mRNA expression (P < 0.0001) were independent prognostic variables. We conclude that expression of type I hCGβ genes indicates adverse prognosis in RCC.
Renal cell carcinoma (RCC) is the most common malignant tumor of the kidney, accounting for approximately 2% of all cancers. The great majority are conventional RCCs (CRCC), which together with papillary tumors account for 85 to 90% of all kidney tumors. Chromophobe and unclassified tumors account for approximately 5% each and collecting duct carcinoma for less than 1%.1 Clinical stage and nuclear grade are the most important prognostic factors in RCC,2 but the outcome is highly variable within a given stage, and the response to therapy cannot be predicted on the basis of stage and grade.3 Surgery is the treatment of choice, but approximately one third of the tumors recur even after radical surgery. Multiple genes are up- or down-regulated in RCC, and expression profiling is an option for the distinction between histological subtypes and clinical outcome1,4,5,6,7,8,9 as well as a potential tool in the search for treatment of RCCs.
The free β-subunit of human chorionic gonadotropin (hCGβ) is frequently expressed in several nontrophoblastic tumors,10,11 and elevated serum concentrations hCGβ are of prognostic value in cancers of the ovary,12 oral cavity,13 and colon.14 Tissue expression detected by immunohistochemistry is associated with therapy-resistant disease in bladder cancer.15,16 hCGβ is encoded by a cluster of six genes, of which the three so-called type II genes (hCGβ 3/9, 5, and 8) have been shown to be up-regulated in relation to type I genes (hCGβ 6/7) in some malignant tumors,17 whereas type I genes can be expressed in normal tissues.18 The protein products of these genes differ only by one amino acid, residue 117, which in type I genes is encoded as alanine and in type II genes as aspartate.
We have previously shown that the preoperative serum concentration of hCGβ is a prognostic indicator in RCC independent of stage and grade,19 but the prognostic significance of hCGβ tissue expression is not known. We have now developed a quantitative reverse transcription-polymerase chain reaction (RT-PCR) method for quantification of the mRNAs for the two groups of hCGβ genes and compared the expression levels with clinicopathological data.
Materials and Methods
Patients and Samples
Data for this retrospective analysis were collected from 104 patients with RCC who underwent radical nephrectomy at the Umeå University Hospital, Sweden, between 1988 and 2000. The study included 54 men and 50 women with a mean age of 65 years (range, 25–85) (Table 1). Informed consent was obtained from all patients. Blood samples were collected before surgery and analyzed for hemoglobin concentration, erythrocyte sedimentation rate, and C-reactive protein, as well as calcium concentration corrected for albumin by routine methods in the Laboratory of Clinical Chemistry, Umeå University Hospital. Staging procedures included physical examination, chest radiography, ultrasonography, and computer tomography. Patients with skeletal symptoms or elevated serum alkaline phosphatase were assessed by bone scintigraphy and skeletal radiography. After nephrectomy, all patients were followed up with clinical and radiological examinations. At the last follow-up, 28 of 104 patients were alive with a median follow-up time of 6.15 years (range, 0.3 to 15.5 years). Among those that had died, 55 had died of RCC and 21 of other causes (Table 1).
Table 1.
Clinical Characteristics of RCC Patients
| Patients | 104 |
| Males | 54 |
| Females | 50 |
| Dead of RCC | 55 |
| Alive/dead of other cause | 28/21 |
| Tumor stage | |
| I | 49 |
| II | 7 |
| III | 20 |
| IV | 28 |
| Tumor grade | |
| 1 | 7 |
| 2 | 15 |
| 3 | 58 |
| 4 | 21 |
| NA | 2 |
| Blood parameters; median (range) | |
| Serum calcium (mmol/L) | 2.4 (2.2–3.3) |
| Hemoglobin (g/L) | 127 (77–162) |
| C-reactive protein (mg/L) | 24 (1–212) |
| Erythrocyte sedimentation rate (mm/h) | 44 (4–140) |
Tumor Staging
Tumor staging was performed according to 2002 TNM stage classification.20 Among the 104 patients, 56 had TNM stage I and II (pT1-pT2, N0, M0), 20 were of stage III (pT1-T3a-c, N0-N1, M0), and 28 were of stage IV (pT1-T4, N0-N3, M1). Nuclear grading was performed according to Skinner et al,21 and RCC type classification was performed according to the Heidelberg consensus conference.1
RNA Isolation
Tumor tissue samples from 104 patients were snap frozen in liquid nitrogen directly after nephrectomy and stored at −80°C until analysis. Control tissue was dissected from adjacent normal kidney cortex (n = 7). Total RNA was isolated from the frozen specimens by the TRIzol method (Life Technologies, Stockholm, Sweden). Total RNA concentrations were measured spectrophotometrically at 260 nm (Lambda 2 UV/VIS; PerkinElmer Life and Analytical Sciences, Boston, MA).
cDNA Constructs
For preparation of the internal standard (IS), a deletion of nucleotide 775 (dATP) of hCGβ5 was made by site-directed mutagenesis22 of cDNA (GenBank accession number NM_033043) isolated from placental tissue. Wild-type hCGβ and mutated hCGβ (IS) were cloned from nucleotide 144 to 827 into a pCR II plasmid vector (Invitrogen Corp., San Diego, CA) as described by the manufacturer.
Oligonucleotide Primers for RT-PCR
The oligonucleotide primer sequences for RT-PCR are shown in Table 2. The primers match completely with hCGβ 3/9, 5, 8, and 6/7 mRNAs but contain one or more mismatches with the sequences of LHβ and hCGβ 1 and 2.
Table 2.
Nucleotide Sequences of the Primers Used to Study hCGβ mRNA Expression
| Primer | Sequence | Exon (#) | nt* |
|---|---|---|---|
| Outer sense | 5′-TCACTTCACCGTGGTCTCCG-3′ | 1 | 139–158 |
| Outer antisense | 5′-GAGGGGCCTTTGAGGAAGAG-3′ | 3 | 779–798 |
| Nested sense | 5′-Bio-ACCCTGGCTGTGGAGAAGG-3′ | 2 | 468–486 |
| Nested antisense; minisequencing | 5′-GCCTTTGAGGAAGAGGAG-3′ | 3 | 776–793 |
nt, nucleotides.
Accession numbers for hCGβ 3/9, 5, 6/7 and 8 are NM_000737.2, NM_033043.1, NM_033142.1, and NM_033183.1.
RT-PCR
One μg of total RNA was reverse-transcribed with SuperScriptII RNase H− Reverse Transcriptase (GibcoBRL Life Technologies, Gaithersburg, MD), according to the manufacturer’s instructions, using a gene-specific outer antisense primer. For the PCR reaction, 20% of the sample cDNA and 100 molecules of IS cDNA were coamplified in a 50-μl reaction volume using 20 pmol of both sense and antisense primers and 2 U of DynaZyme II DNA polymerase (Finnzymes, Espoo, Finland), initially at 95°C for 5 minutes and then with 45 cycles at 95°C for 1 minute and at 65°C for 1 minute. Three μl of the first PCR product was further amplified in a reaction volume of 75 μl with nested primers (20 pmol of biotinylated sense and 100 pmol of antisense) and 3 U of Dynazyme II DNA polymerase initially at 95°C for 5 minutes and then with 35 cycles at 95°C for 1 minute and at 53°C for 1 minute. All samples were tested at least three times, and water was used as a negative control. Fifteen μl of the product was electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining.
Solid-Phase Minisequencing
For quantification of PCR products, the biotinylated nested PCR product was captured on a streptavidin-coated scintillating microtitration plate (ScintiPlate; Wallac, Turku, Finland) for 1.5 hours at room temperature and washed with buffer containing 40 mmol/L Tris-HCl, pH 8.0, 1 mmol/L EDTA, 50 mmol/L NaCl, and 0.1% Tween 20. The bound DNA fragment was denaturated with 50 mmol/L NaOH to remove the unbound DNA strand. Detection of hCGβ type I, type II, or IS cDNA was performed in separate reactions using a common detection primer and a specific 3H-labeled dNTP for each gene type. The minisequencing reaction mixture containing Dynazyme DNA polymerase in PCR buffer, detection primer (Table 2), and the specific 3H-labeled dNTP (Amersham, Aylesbury, UK) was added, and primer annealing and extension were performed simultaneously by incubating in 50°C for 20 minutes. Radioactivity was measured in a scintillation counter. After washing, the proportion of the incorporated radioactive nucleotides of the corresponding hCGβ gene types (types I or II or IS) was calculated. The expression level of the corresponding gene type (I or II) was calculated by dividing its signal with the total IS signal of the sample.
Statistical Analysis
Differences in hCGβ gene expression levels between groups were analyzed by the Mann-Whitney U or Kruskal Wallis tests. The association between the expression level and serum parameters as well as tumor diameter was assessed using Kendall’s tau b test for correlations. Survival probabilities were calculated using the Kaplan-Meier method and compared between quartiles of hCGβ expression using the log-rank test. Cox proportional hazards regression was used for multivariate analysis of variables affecting survival. Follow-up started at the time of nephrectomy. In analysis of disease-specific survival, death from RCC was considered the event. Patients were censored at last follow-up or when dying from unrelated causes. In analysis of overall survival, death from any cause was considered the event. SPSS statistical software (version 12.0.1; Chicago, IL) was used in all calculations. All tests were two-sided, and P values below 0.05 were considered significant.
Results
Reproducibility and Detection Limit of the Assay
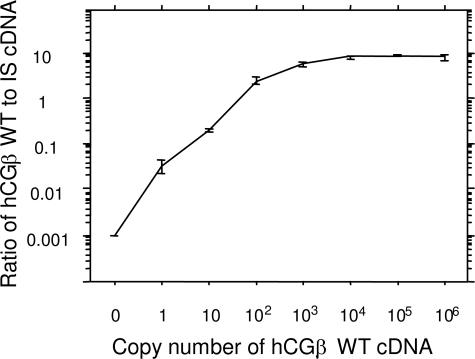
The standard curve for the PCR assay was determined by using triplicate dilutions of hCGβ wild-type cDNA together with 100 molecules of IS. The assay was linear from 1 to 1000 hCGβ wild-type cDNA molecules (Figure 1). The intra-assay coefficient of variation (CV%) was 7 to 32% for 10 to 106 hCGβ cDNA molecules. The detection limit of the assay was defined on the basis of the copy number giving a CV of 15%, which was 12.
Figure 1.
Standard curve of the quantitative RT-PCR assay for hCGβ mRNA. WT, wild-type hCGβ. Whiskers represent standard errors.
hCGβ mRNA Expression in Tumor Tissue
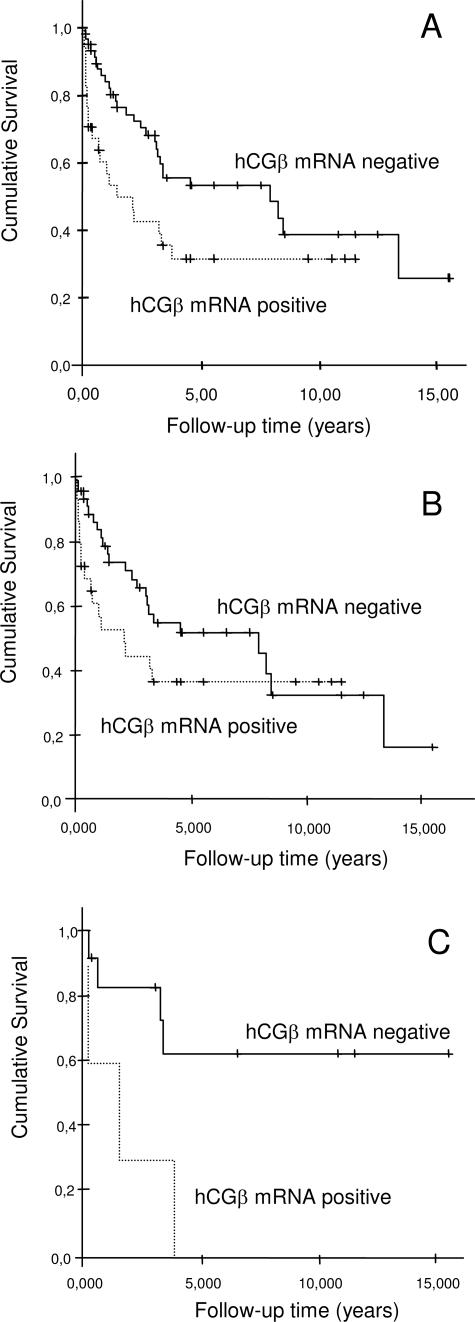
hCGβ mRNA was detected in 40% (42/104) of the tumor samples (Table 3) but not in benign renal tissue (n = 7). All of the positive tumors expressed type I hCGβ mRNA (range, 12 to 475 copies), whereas type II hCGβ mRNA (range, 39 to 56 copies) was detected in only two of these (2%). Thus, expression of hCGβ mRNA was mainly caused by expression of type I genes (Kendall’s tau r = 0.868, P < 0.0001), and isolated expression of type II hCGβ mRNA was not detected. Expression of hCGβ mRNA was associated with a shorter disease-specific survival (median survival 1.4 versus 7.9 years; log-rank P = 0.023 and Breslow P = 0.0029) and overall survival (median 0.6 versus 3.2 years; log-rank P = 0.011 and Breslow P = 0.0028). The relative risk of dying from RCC was 1.9 (95% confidence interval 1.1 to 3.4) in patients with hCGβ mRNA-positive tumors compared with those with undetectable expression (log-rank P = 0.023, Figure 2).
Table 3.
hCGβ mRNA Expression, Cancer Specific Mortality, and Number of Type I hCGβ Transcripts in Various Tumor Types
| Type of RCC | hCGβ mRNA in tissue (%) | Dead of RCC
|
Type I hCGβ mRNA number of copies; median (range) | |
|---|---|---|---|---|
| hCGβ mRNA postitive cases | hCGβ mRNA negative cases | |||
| CRCC | 33/79 (42) | 21/33 (64) | 23/46 (50) | 9 (0–475) |
| Chromophobe | 2/5 (40) | 1/2 (50) | 0/3 (0) | 10 (0–80) |
| Papillary | 5/16 (31) | 4/5 (80) | 5/11 (45) | 2 (0–143) |
| Unclassified | 2/4 (50) | 1/2 (50) | 0/2 (0) | 11 (8–14) |
| All | 42/104 (40) | 27/42 (64) | 28/62 (45) | 8 (0–475) |
Figure 2.
Disease-specific survival of RCC patients with type I hCGβ mRNA-positive tumors (n = 42) compared with those with hCGβ mRNA-negative tumors (n = 62; log-rank P = 0.0234; Breslow P = 0.0029 (A), disease-specific survival in CRCCs (n = 79; log-rank P = 0.15 and Breslow P = 0.026) (B), and papillary chromophobe as well as unclassified tumors (n = 25; log-rank P = 0.017; Breslow P = 0.019) (C).
There was no significant difference in hCGβ expression levels or survival between the distinct tumor types (Table 3). Because CRCCs are generally of higher stage at nephrectomy, and thus have a worse prognosis than papillary and chromophobe tumors, we also analyzed these separately (n = 79) and with the latter two as one group (n = 21). hCGβ mRNA positivity was more strongly associated with shorter survival in the group of papillary and chromophobe tumors, with a median survival of 0.04 years in the hCGβ mRNA-positive tumors versus >15 years in the negative tumors (log-rank P = 0.035 and Breslow P = 0.025). Median survival in hCGβ mRNA-positive CRCCs was 2.1 years compared with 7.9 years in the negative ones (log-rank P = 0.15 and Breslow P = 0.026; Figure 2). The highest hCGβ mRNA expression levels occurred in stage 2 (mean 90 copies, median 32) followed by stage 1 (mean 17 copies, median 0 copies). When analyzed by stage, hCGβ mRNA positivity was associated with shorter survival in stage 1 (log-rank P = 0.086 and Breslow P = 0.025) but not in the other stages. The expression levels were not associated with grade, age, and gender of the patients; hemoglobin concentration; erythrocyte sedimentation rate; or C-reactive protein. However, serum calcium concentration was significantly associated with total (Kendall’s tau r = 0.198, P = 0.012) and type I (Kendall’s tau r = 0.197, P = 0.012) hCGβ mRNA expression level. In univariate analysis using Cox regression, clinical stage, tumor diameter, hemoglobin, serum calcium concentration, and hCGβ, mRNA expression levels were significantly associated with survival (Table 4). In multivariate analysis, only hCGβ mRNA expression and tumor stage remained significant, independent predictors of disease-specific survival (Table 4).
Table 4.
Relative Risk of Dying from RCC as a Function of Stage, Tumor Diameter, Serum Calcium and Hemoglobin Concentration, and hCGβ mRNA Expression Level
| Variable | Unadjusted
|
Mutually adjusted
|
||
|---|---|---|---|---|
| P | Relative risk (95% CI) | P | Relative risk (95% CI) | |
| TNM stage >2 | <0.0001 | 10.27 (5.32–19.87) | <0.0001 | 11.61 (5.10–26.41) |
| Tumor diameter >7 cm | 0.024 | 2.31 (1.12–4.76) | 0.460 | 1.35 (0.603–3.04) |
| Calcium >2.4 mmol/L | 0.017 | 2.18 (1.15–4.13) | 0.760 | 0.90 (0.44–1.84) |
| Hemoglobin <100 g/l | 0.022 | 2.28 (1.12–4.64) | 0.250 | 1.74 (0.68–4.49 |
| hCGβ mRNA (per 100 copies) | <0.0001 | 2.03 (1.37–3.01) | <0.0001 | 2.61 (1.62–4.21) |
ESR and CRP were not significantly associated with survival (not shown).
CI, confidence interval.
Discussion
We have previously shown that the serum level of hCGβ is an independent prognostic marker in patients with RCC. In an earlier study using a nonquantitative RT-PCR assay that detected both types of hCGβ mRNA, we found mRNA expression in 8 of 20 tumors (40%) and hCGβ protein in 35 of 229 (15%) tumors by immunohistochemistry, but in this study, tissue expression was not associated with prognosis.23 We have now developed a quantitative RT-PCR technique that allows us to accurately measure the expression of the two types of hCGβ mRNA. By this method we also detected hCGβ mRNA in 40% of the tumors, and this is an independent indicator of adverse prognosis. Interestingly, the prognostic significance was accentuated in papillary and chromophobe tumors, which generally bear a more favorable prognosis than CRCCs. Furthermore, mainly type I hCGβ genes are expressed, and, therefore, the proposed hypothesis of hCGβ type I genes representing “benign” genes expressed in nonmalignant tissues18 is not true for RCC.
Our quantitative RT-PCR method is designed for quantification of the expression and comparison of relative expression levels of two homologous genes. The mRNA transcripts of the homologous genes are reverse-transcribed, coamplified with the very same primer pair, and quantified by solid-phase minisequencing. To achieve quantification we added 100 molecules of an internal cDNA standard to each sample and coamplified standard and target genes in the same reaction. With this type of experimental setup the relative amounts of the amplification products remain unchanged throughout amplification and reflect the proportions of mRNA transcripts originally present in the sample.24,25 Our method is linear from 1 to 10,000 copies of target cDNA, allowing analysis of low-level variation in the expression of the hCGβ genes.
Expression profiling with microarrays is a powerful tool for the screening of cancer-related alterations in gene expression, but to our knowledge, changed expression levels of hCGβ genes have not been identified in RCC by this method.4,5,6,7,8,9,26 This may be explained by the low expression level of hCGβ mRNA, being twofold the detection limit of our very sensitive assay in only a third of the tumor samples. Interestingly, this low level of expression was strongly associated with adverse outcome.
A strong association between hCGβ expression and adverse outcome has also been observed in many other cancers,12,13,14 but the mechanism behind this is not truly known. hCGβ has been shown to exert a growth-promoting effect in culture,27 and in bladder cancer cells, this has been shown to result from inhibition of apoptosis.28 No receptor for hCGβ has been identified, but based on the structural similarity between hCGβ and the so-called cysteine-knot growth factors,29 eg, transforming growth factor β, platelet-derived growth factor-B, and nerve growth factor, it has been suggested that hCGβ interferes with the growth-inhibiting effect of these through a paracrine or autocrine route.30 The growth-promoting effect of hCGβ can be blocked by antibodies,31 and inhibition of hCGβ mRNA expression with antisense RNA in choriocarcinoma cells suppresses cell proliferation and induces apoptosis.32 Furthermore, induction of an antibody response toward hCGβ has been shown to improve survival in patients with colorectal cancer,33 and hCGβ-targeted immunotherapies have shown promising results in animal studies on breast cancer.34 If these results can be confirmed, methods for sensitive quantification of hCGβ expression will become important.
A high proportion of type II mRNA has been observed in normal tissues with high (placenta) or moderate (testis, pituitary) expression of hCGβ mRNA, whereas only type I mRNA was detected in tissues with low hCGβ mRNA levels, eg, breast, prostate, skeletal muscle, bladder, adrenal, thyroid, colon, and uterus.18,35,36 Type II genes are expressed in breast cancer tissue,17 and increased expression of type II genes has been found to be associated with advanced stage in bladder cancer.35 Based on these findings, malignant transformation has been thought to be associated with type II hCGβ expression. However, earlier studies on RCC cell lines have shown a higher proportion (50 to 85%) of type I hCGβ genes,36 and in the present study, we detected type II gene expression in only two of the 42 positive tumors. Thus, expression of the various hCGβ genes is both tissue and tumor specific, and aggressive tumor growth may be associated with increased expression of either gene type.
We conclude that hCGβ mRNA expression at the tissue level is a marker of adverse prognosis in patients with RCC. This is caused by expression of type I hCGβ genes, whereas increased type II gene expression occurs in most other cancers studied. Measurement of hCGβ expression is likely to become clinically important if therapies targeting its expression32,33,34 prove successful for treatment of various cancers.
Acknowledgments
We thank Mr. Oso Rissanen and Mrs. Maritta Putkiranta for expert technical assistance.
Footnotes
Supported by grants from the Finska Läkaresällskapet.
K.H. and S.L. contributed equally to the manuscript.
References
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, Eble JN, Fleming S, Ljungberg B, Medeiros LJ, Moch H, Reuter VE, Ritz E, Roos G, Schmidt D, Srigley JR, Storkel S, van den Berg E, Zbar B. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–663. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- Golimbu M, Joshi P, Sperber A, Tessler A, Al-Askari S, Morales P. Renal cell carcinoma: survival and prognostic factors. Urology. 1986;27:291–301. doi: 10.1016/0090-4295(86)90300-6. [DOI] [PubMed] [Google Scholar]
- Young AN, Amin MB, Moreno CS, Lim SD, Cohen C, Petros JA, Marshall FF, Neish AS. Expression profiling of renal epithelial neoplasms: a method for tumor classification and discovery of diagnostic molecular markers. Am J Pathol. 2001;158:1639–1651. doi: 10.1016/S0002-9440(10)64120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Rhodes DR, Furge KA, Kanayama H, Kagawa S, Haab BB, Teh BT. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci USA. 2001;98:9754–9759. doi: 10.1073/pnas.171209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasselli JR, Shih JH, Iyengar SR, Maranchie J, Riss J, Worrell R, Torres-Cabala C, Tabios R, Mariotti A, Stearman R, Merino M, Walther MM, Simon R, Klausner RD, Linehan WM. Predicting survival in patients with metastatic kidney cancer by gene-expression profiling in the primary tumor. Proc Natl Acad Sci USA. 2003;100:6958–6963. doi: 10.1073/pnas.1131754100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Yang XJ, Sugimura J, Backdahl J, Tretiakova M, Qian CN, Gray SG, Knapp R, Anema J, Kahnoski R, Nicol D, Vogelzang NJ, Furge KA, Kanayama H, Kagawa S, Teh BT. Molecular subclassification of kidney tumors and the discovery of new diagnostic markers. Oncogene. 2003;22:6810–6818. doi: 10.1038/sj.onc.1206869. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Shinghal R, Gill H, Reese JH, Terris M, Cohen RJ, Fero M, Pollack JR, van de Rijn M, Brooks JD. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am J Pathol. 2003;162:925–932. doi: 10.1016/S0002-9440(10)63887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ljungberg B, Grankvist K, Rasmuson T, Tibshirani R, Brooks JD. Gene expression profiling predicts survival in conventional renal cell carcinoma. PLoS Med. 2005;3:e13. doi: 10.1371/journal.pmed.0030013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfthan H, Haglund C, Roberts P, Stenman UH. Elevation of free beta subunit of human choriogonadotropin and core beta fragment of human choriogonadotropin in the serum and urine of patients with malignant pancreatic and biliary disease. Cancer Res. 1992;52:4628–4633. [PubMed] [Google Scholar]
- Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhomme C, Rougier P. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901–3907. [PubMed] [Google Scholar]
- Vartiainen J, Lehtovirta P, Finne P, Stenman UH, Alfthan H. Preoperative serum concentration of hCGbeta as a prognostic factor in ovarian cancer. Int J Cancer. 2001;95:313–316. doi: 10.1002/1097-0215(20010920)95:5<313::aid-ijc1054>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Hedström J, Grenman R, Ramsay H, Finne P, Lundin J, Haglund C, Alfthan H, Stenman UH. Concentration of free hCGbeta subunit in serum as a prognostic marker for squamous-cell carcinoma of the oral cavity and oropharynx. Int J Cancer. 1999;84:525–528. doi: 10.1002/(sici)1097-0215(19991022)84:5<525::aid-ijc14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lundin M, Nordling S, Carpelan-Holmström M, Louhimo J, Alfthan H, Stenman UH, Haglund C. A comparison of serum and tissue hCG beta as prognostic markers in colorectal cancer. Anticancer Res. 2000;20:4949–4951. [PubMed] [Google Scholar]
- Moutzouris G, Yannopoulos D, Barbatis C, Zaharof A, Theodorou C. Is beta-human chorionic gonadotrophin production by transitional cell carcinoma of the bladder a marker of aggressive disease and resistance to radiotherapy? Br J Urol. 1993;72:907–909. doi: 10.1111/j.1464-410x.1993.tb16294.x. [DOI] [PubMed] [Google Scholar]
- Martin JE, Jenkins BJ, Zuk RJ, Oliver RT, Baithun SI. Human chorionic gonadotrophin expression and histological findings as predictors of response to radiotherapy in carcinoma of the bladder. Virchows Arch A Pathol Anat Histopathol. 1989;414:273–277. doi: 10.1007/BF00822032. [DOI] [PubMed] [Google Scholar]
- Giovangrandi Y, Parfait B, Asheuer M, Olivi M, Lidereau R, Vidaud M, Bieche I. Analysis of the human CGB/LHB gene cluster in breast tumors by real-time quantitative RT-PCR assays. Cancer Lett. 2001;168:93–100. doi: 10.1016/s0304-3835(01)00496-7. [DOI] [PubMed] [Google Scholar]
- Bellet D, Lazar V, Bieche I, Paradis V, Giovangrandi Y, Paterlini P, Lidereau R, Bedossa P, Bidart JM, Vidaud M. Malignant transformation of nontrophoblastic cells is associated with the expression of chorionic gonadotropin beta genes normally transcribed in trophoblastic cells. Cancer Res. 1997;57:516–523. [PubMed] [Google Scholar]
- Hotakainen K, Ljungberg B, Paju A, Rasmuson T, Alfthan H, Stenman UH. The free beta-subunit of human chorionic gonadotropin as a prognostic factor in renal cell carcinoma. Br J Cancer. 2002;86:185–189. doi: 10.1038/sj.bjc.6600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin LH, Wittekind C. New York: Wiley-Liss,; UICC TNM Classification of Malignant Tumours, ed 4. International Union Against Cancer (UICC) 2002 [Google Scholar]
- Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28:1165–1177. doi: 10.1002/1097-0142(1971)28:5<1165::aid-cncr2820280513>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hotakainen K, Ljungberg B, Haglund C, Nordling S, Paju A, Stenman UH. Expression of the free beta-subunit of human chorionic gonadotropin in renal cell carcinoma: prognostic study on tissue and serum. Int J Cancer. 2003;104:631–635. doi: 10.1002/ijc.11000. [DOI] [PubMed] [Google Scholar]
- Stenman J, Finne P, Stahls A, Grenman R, Stenman UH, Palotie A, Orpana A. Accurate determination of relative messenger RNA levels by RT-PCR. Nature Biotechnol. 1999;17:720–722. doi: 10.1038/10942. [DOI] [PubMed] [Google Scholar]
- Lintula S, Stenman J, Bjartell A, Nordling S, Stenman UH. Relative concentrations of hK2/PSA mRNA in benign and malignant prostatic tissue. Prostate. 2005;63:324–329. doi: 10.1002/pros.20194. [DOI] [PubMed] [Google Scholar]
- Yao M, Tabuchi H, Nagashima Y, Baba M, Nakaigawa N, Ishiguro H, Hamada K, Inayama Y, Kishida T, Hattori K, Yamada-Okabe H, Kubota Y. Gene expression analysis of renal carcinoma: adipose differentiation-related protein as a potential diagnostic and prognostic biomarker for clear-cell renal carcinoma. J Pathol. 2005;205:377–387. doi: 10.1002/path.1693. [DOI] [PubMed] [Google Scholar]
- Gillott DJ, Iles RK, Chard T. The effects of beta-human chorionic gonadotrophin on the in vitro growth of bladder cancer cell lines. Br J Cancer. 1996;73:323–326. doi: 10.1038/bjc.1996.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000;82:1553–1556. doi: 10.1054/bjoc.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapthorn AJ, Harris DC, Littlejohn A, Lustbader JW, Canfield RE, Machin KJ, Morgan FJ, Isaacs NW. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Butler SA, Iles RK. The free monomeric beta subunit of human chorionic gonadotrophin (hCG beta) and the recently identified homodimeric beta-beta subunit (hCG beta beta) both have autocrine growth effects. Tumour Biol. 2004;25:18–23. doi: 10.1159/000077719. [DOI] [PubMed] [Google Scholar]
- Butler SA, Staite EM, Iles RK. Reduction of bladder cancer cell growth in response to hCGbeta CTP37 vaccinated mouse serum. Oncol Res. 2003;14:93–100. doi: 10.3727/000000003108748649. [DOI] [PubMed] [Google Scholar]
- Hamada AL, Nakabayashi K, Sato A, Kiyoshi K, Takamatsu Y, Laoag-Fernandez JB, Ohara N, Maruo T. Transfection of antisense chorionic gonadotropin beta gene into choriocarcinoma cells suppresses the cell proliferation and induces apoptosis. J Clin Endocrinol Metab. 2005;90:4873–4879. doi: 10.1210/jc.2004-2458. [DOI] [PubMed] [Google Scholar]
- Moulton HM, Yoshihara PH, Mason DH, Iversen PL, Triozzi PL. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res. 2002;8:2044–2051. [PubMed] [Google Scholar]
- Yi H, Rong Y, Yankai Z, Wentao L, Hongxia Z, Jie W, Rongyue C, Taiming L, Jingjing L. Improved efficacy of DNA vaccination against breast cancer by boosting with the repeat beta-hCG C-terminal peptide carried by mycobacterial heat-shock protein HSP65. Vaccine. 2006;24:2575–2584. doi: 10.1016/j.vaccine.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Lazar V, Diez SG, Laurent A, Giovangrandi Y, Radvanyi F, Chopin D, Bidart JM, Bellet D, Vidaud M. Expression of human chorionic gonadotropin beta subunit genes in superficial and invasive bladder carcinomas. Cancer Res. 1995;55:3735–3738. [PubMed] [Google Scholar]
- Span PN, Thomas CM, Heuvel JJ, Bosch RR, Schalken JA, vd Locht L, Mensink EJ, Sweep CG. Analysis of expression of chorionic gonadotrophin transcripts in prostate cancer by quantitative Taqman and a modified molecular beacon RT-PCR. J Endocrinol. 2002;172:489–495. doi: 10.1677/joe.0.1720489. [DOI] [PubMed] [Google Scholar]