Abstract
Our aim was to assess the feasibility and accuracy of contrast-enhanced ultrasound (CEUS) of the breast with SonoVue microbubbles for pre-operative size measurement of invasive breast carcinomas. Seven patients diagnosed with nine invasive breast carcinomas prospectively underwent gray-scale ultrasound and CEUS of the breast according to a standardized protocol. CEUS of the breast was performed by a Philips iU22 scanner equipped with a 4–8 MHz linear array transducer. We used a single dose of 2.4 ml SonoVue as contrast agent. Breast lesion morphology was scored according to the sonographic BI-RADS lexicon criteria and classified accordingly. The greatest tumor dimensions on gray-scale ultrasound and CEUS of the breast were finally compared with the greatest histopathologic tumor sizes. Gray-scale ultrasound underestimated the histopathologic tumor size in 6/9 cases (67%), whereas CEUS of the breast underestimated tumor size in only 3/9 (33%) cases. CEUS of the breast was significantly more accurate for tumor size assessment. Greatest tumor dimension as measured with gray-scale ultrasound of the breast was within 2 mm of the pathologic tumor size in only 2/9 cases (22%), whereas CEUS of the breast accurately assessed tumor size within 2 mm of pathologic tumor size in 6/9 (67%) of the cases (P<0.05). CEUS of the breast proved to be a feasible and safe procedure. It is more accurate than gray-scale ultrasound of the breast for pre-operative size assessment of invasive ductal breast carcinomas.
Keywords: Accuracy, breast cancer, contrast-enhanced ultrasound, SonoVue, tumor size
Introduction
Breast ultrasound has become a standard breast-imaging procedure in addition to mammography, for work-up of patients referred with a palpable mass or with a suspicious lesion detected on the mammogram[1]. Gray-scale breast ultrasound offers the ability to visualize the breast tumor in three dimensions and permits direct measurement of tumor size without magnification. To standardize lesion characterization on ultrasound, the American College of Radiology (ACR) developed a lexicon of sonographic descriptors of breast masses with attendant assessment categories, i.e. the sonographic Breast Imaging Reporting and Data System (BI-RADS) lexicon[2].
Technologic advances over the last decade have fueled research in the field of minimal-invasive image-guided ablation techniques for treatment of patients with limited-stage breast cancer. Techniques that have been studied include radiofrequency ablation, cryoablation, focused ultrasound and laser ablation of breast tumors[3,4]. Different imaging modalities are used to guide the instruments, to monitor the therapeutic procedure and to assess treatment response. Of all imaging modalities, gray-scale breast ultrasound is most often used for breast tumor visualization and real time monitoring of the ablation process[4].
Accurate assessment of breast tumor size is important for planning surgical and minimal-invasive image-guided ablation procedures. Extensive surgical treatment may result in poor cosmetic results, whereas small tumor-free margins may influence the local recurrence rate4–6.
Several previous studies have assessed the accuracy of gray-scale ultrasound for breast tumor size measurement[7–14]. Overall, these studies concluded that gray-scale ultrasound of the breast is a reliable method for determining tumor size and favors mammography, but in general true tumor size is underestimated with this technique[7–14].
In recent years, ultrasound contrast agents have been developed that increase blood echogenicity and improve ultrasound image quality by detection of slow and low-volume blood flow in small tumor vessels (<5 mm). Contrast-enhanced ultrasound (CEUS) of the breast has recently been studied for characterization of indeterminate breast lesions[15–20]. Since breast tumors are strongly vascularized and display neo-angiogenesis in the vital border, it is hypothesized that CEUS of the breast may also be a more accurate modality than gray-scale ultrasound for delineation of breast tumor boundaries and tumor size assessment [18].
This prospective feasibility study was designed to assess the accuracy of CEUS of the breast for pre-operative tumor size measurement in patients diagnosed with invasive ductal carcinoma of the breast.
Materials and methods
Seven consecutive female patients, 49 years of age (range 42–57 years), with 9 breast lesions were prospectively included in this study. All patients were referred to our department for ultrasound examination and ultrasound (US)-guided large-core needle biopsy of a suspicious breast lesion (BI-RADS IV and V) detected on mammography between June 2005 and June 2006.
The diagnosis of invasive ductal breast cancer was confirmed in all patients by US-guided large-core needle biopsy (14 gage). Eligible patients for pre-operative tumor size measurement with contrast-enhanced ultrasound (CEUS) of the breast had no history of previous breast surgery. Patients were excluded if use of the ultrasound contrast agent (SonoVue, Bracco Spa, Milan, Italy) was contra-indicated, due to a history of cardiac failure, right to left shunt, severe pulmonary hypertension, uncontrolled systemic hypertension, adult respiratory disorders and hypersensitivity[19]. Written informed consent was obtained from all patients and the study was performed in accordance with a protocol approved by our institutional panel.
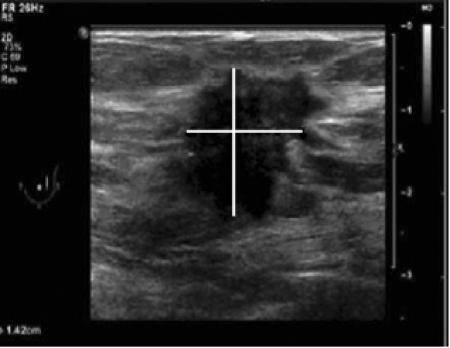
All eligible patients prospectively underwent both gray-scale ultrasound and CEUS of the breast according to a standardized protocol. High-frequency gray-scale ultrasound examination of the breast was performed first with a Philips iU22 scanner (Philips Medical Systems, Best, the Netherlands) equipped with a 11 MHz linear array transducer. The probe was held orthogonal to the skin. Breast lesion morphology was scored according to the sonographic BI-RADS lexicon criteria[2] and classified accordingly. Tumors size (expressed in millimeters) was documented in three dimensions (length, width, and height). For measurements, the tumor edge was defined as the end of the hypoechoic mass before the hyperechoic transition border (so called ‘echogenic interface’) between tumor and healthy surrounding tissue (Fig. 1)[11]. The maximum dimension on gray-scale ultrasound was finally compared with the maximum histopathologic tumor size.
Figure 1.
Gray-scale ultrasound image of an irregular, not parallel oriented, spiculated breast lesion, classified as BI-RADS V in the upper outer quadrant of the left breast. For measurements (see lines), the tumor edge was defined as the end of the hypoechoic mass before the hyperechoic transition border (so-called ‘echogenic interface’) between tumor and healthy surrounding tissue.
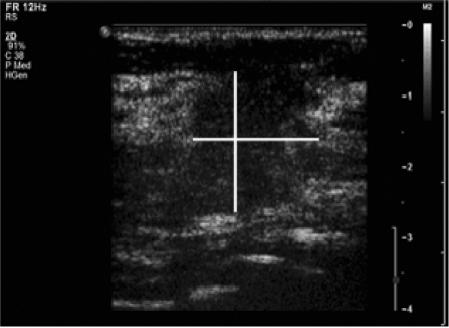
Additionally, patients underwent CEUS of the breast according to the following protocol. Nonlinear harmonic imaging using a Philips iU22 scanner (Philips Medical Systems, Best, the Netherlands) equipped with a 4–8 MHz linear array transducer was performed at baseline with a low mechanical index of 0.1, chosen to avoid gas bubble destruction. SonoVue was provided as lyophilized powder contained in a septum-scaled vial. A suspension of sulfur hexafluoride (SF6) microbubbles was obtained by adding 5 ml saline (0.9% sodium chloride) to 25 mg of the powder, followed by hand agitation[15]. We used a single dose of 2.4 ml SonoVue as contrast agent (SF6 volume in a 2.4 ml dose in 0.02 ml), which was intravenously administrated via a 20 gauge canula placed in an arm vein followed by a flushing of 10 ml standard saline. Directly after SonoVue administration the microcirculation was studied by recording with clip function for 60 s, without chancing the transducer. QLAB software (Philips Medical System, Best, The Netherlands) was used to quantify enhancement on the CEUS images in time. By using the region of interest quantification method we assessed time intensity curves of vascular enhancement at the margin of the tumor. When enhancement was at peak level (around 10 s) breast tumor size was recorded and measured again in three dimensions. For the measurements, the tumor edge was defined as the end of the hyperechoic mass at the time of maximal contrast enhancement of the lesion (Fig. 2). The maximum dimension of the tumor on CEUS of the breast was finally compared with the maximum histopathologic tumor size. Both gray-scale and CEUS breast ultrasound examination were performed by the same experienced breast radiologist in all cases.
Figure 2.
Image of the same lesions with CEUS of the breast, lesion has become more hyperechoic due to centripetal contrast enhancement. For measurements (see lines) the tumor edge was defined as the end of the hyperechoic mass at time of maximal contrast enhancement of the lesion.
Surgical resection of the tumor was performed in all patients within 2 weeks. Histologic sections of the resected tumors after hematoxylin and eosin (H&E) staining were examined under a light microscope and used for measurement of histopathologic tumor size in millimeters in three dimensions. Margin status was recorded as involved or not involved. For those patients who underwent re-excision for tumor-involved margins, the extent of invasive ductal carcinoma in the re-excision specimen was recorded.
To assess the difference between the three methods of tumor size measurement, data were analyzed in a SPSS database (version 9.0). The greatest lesion diameters (mm) obtained by gray-scale breast ultrasound and CEUS of the breast were compared with the greatest lesion diameter (mm) on pathology. Tumor size measured in greatest dimension was compared between the groups by using Student t-test analysis (P<0.05). Furthermore, the percentage greatest tumor diameter as assessed by gray-scale ultrasound and CEUS was within 2 mm of pathologic tumor size, which is considered accurate[8]. The percentage of over- and underestimated tumor size for each modality was calculated using Fisher's exact test, with a P-value <0.05 considered to be significant.
Results
The mean patient age in this study was 49 years (range 42–57 years). Two patients presented with two breast lesions in the same quadrant (multifocal disease), and the remaining five patients presented with a solitary breast lesion. The sonographic characteristics of the lesions according to the sonographic BI-RADS criteria are presented in Table 1. Definitive diagnosis on pathology was invasive ductal carcinoma in all cases, with an additional ductal carcinoma in situ (DCIS) component in two cases.
Table 1.
Baseline characteristics of 10 breast tumors according to sonographic BI-RADS lexicon criteria
| Tumor | Shape | Orientation | Margin | Lesion boundary | Echo pattern | Posterior acoustic features | Surrounding tissue | BI-RADS class |
|---|---|---|---|---|---|---|---|---|
| 1 | Irregular | Parallel | Microlobulated | Echogenic interface | Hypoechoic | Combined patterna | Architectural distortion | IV |
| 2 | Irregular | Not parallel | Indistinct | Echogenic interface | Hypoechoic | Shadowing | Architectural distortion | V |
| 3 | Irregular | Not parallel | Indistinct | Echogenic interface | Hypoechoic | Shadowing | Architectural distortion | V |
| 4 | Irregular | Not parallel | Indistinct | Echogenic interface | Hypoechoic | Shadowing | Architectural distortion | IV |
| 5 | Irregular | Parallel | Microlobulated | Echogenic interface | Hypoechoic | No posterior alteration | Architectural distortion | IV |
| 6 | Oval | Not parallel | Circumscribed | Echogenic interface | Hypoechoic | Combined pattern | Architectural distortion | IV |
| 7 | Irregular | Not parallel | Spiculated | Echogenic interface | Hypoechoic | Combined pattern | Architectural distortion | V |
| 8 | Oval | Parallel | Indistinct | Echogenic interface | Hypoechoic | Combined pattern | Architectural distortion | IV |
| 9 | Irregular | Not parallel | Indistinct | Echogenic interface | Hypoechoic | Combined pattern | Architectural distortion | V |
a Combined pattern implied both posterior acoustic shadowing and enhancement.
Gray-scale ultrasound of the breast showed a mean greatest tumor diameter of 15.5 mm (range 10.1–20.6 mm), compared to 16.5 mm (range 11.5–18.5 mm) in the CEUS group. Mean greatest histopathologic tumor diameter was 15.6 mm (range 9.0–25.0 mm). Table 2 shows the greatest tumor diameter as measured with each modality compared to pathologic tumor size. Mean greatest tumor diameter as assessed with both ultrasound techniques did not significantly differ (P = 0.23). However, gray-scale ultrasound underestimated tumor size in 6/9 (67%) cases, whereas CEUS of the breast underestimated tumor size only in 3/9 (33%) cases. Consequently, the accuracy of both imaging techniques for tumor size assessment differed significantly. Tumor as measured with gray-scale ultrasound of the breast was within 2 mm of the pathologic tumor size in only 2/9 cases (22%), whereas CEUS of the breast accurately assessed tumor size within 2 mm of pathologic tumor size in 6/9 (67%) of the cases (P < 0.05). No complications due to SonoVue administration were noted. Margin status was recorded as not involved in all nine cases; none of the patients underwent re-excision.
Table 2.
Greatest tumor diameter according to different modalities
| Tumor | Maximum diameter (mm) gray-scale US | Maximum diameter (mm) CEUS | Maximum diameter (mm) pathology | Tumor histology |
|---|---|---|---|---|
| 1 | 20.5 | 18.5 | 12.0 | Invasive ductal carcinoma and DCIS |
| 2 | 20.6 | 19.0 | 18.0 | Invasive ductal carcinoma |
| 3 | 10.1 | 11.5 | 13.0 | Invasive ductal carcinoma |
| 4 | 15.7 | 17.2 | 18.0 | Invasive ductal carcinoma and DCIS |
| 5 | 16.7 | 17.0 | 25.0 | Invasive ductal carcinoma |
| 6 | 15.9 | 18.0 | 17.0 | Invasive ductal carcinoma |
| 7 | 12.0 | 12.6 | 9.0 | Invasive ductal carcinoma |
| 8 | 10.3 | 12.0 | 11.0 | Invasive ductal carcinoma |
| 9 | 18.0 | 23.0 | 22.0 | Invasive ductal carcinoma |
Discussion
To our knowledge this is the first study to assess the accuracy of CEUS for pre-operative size assessment of invasive ductal carcinomas of the breast. In this prospective feasibility study CEUS of the breast proved to be a safe and technically feasible procedure. CEUS of the breast was significantly more accurate than gray-scale ultrasound of the breast for tumor size measurement. The maximal tumor diameter as assessed by CEUS of the breast was within 2 mm of pathologic tumor size in 67% of cases, compared with 22% in the gray-scale ultrasound group (P < 0.05).
The ability to accurately and reliably measure breast tumor size prior to any surgical treatment or primary medical treatment is essential[4–6]. As a consequence previous research focused on different methods enabling non-invasive tumor size measurements, including clinical examination (palpation), mammography, gray-scale ultrasound, and magnetic resonance imaging (MRI) of the breast[7–14]. Of these methods palpation proved to have the lowest accuracy, because it is influenced by skin thickening, breast edema, and obesity, and is prone to overestimation of tumor size[7,9]. Mammography proved to be more accurate than palpation, however it is taken in two standard projections (cranio-caudal and medio-lateral-oblique) not necessarily expressing the largest dimension of the tumor[7,9,12,13]. As a consequence, mammography in general underestimates tumor size. Although all studies showed significant correlation between mammographic and gray-scale ultrasound measurements, the latter technique is considered the most accurate for breast tumor size measurement. Previous studies that compared maximum breast tumor size as measured with gray-scale ultrasound of the breast to tumor size on pathology, reported correlation coefficients in the range of 40–84%[7–14]. However, all concluded that gray-scale ultrasound of the breast still underestimates tumor size in more than half of the patients. MRI of the breast has been reported to be the most accurate imaging modality for non-invasive tumor size assessment. In a prospective study including 111 women with 177 breast lesions, MRI was reported to have an accuracy of 85% for evaluation of disease extent.
Since, breast MRI is expensive, time-consuming and still not available in every institute, we wanted to tackle the problem of tumor size underestimation rate on gray-scale ultrasound, by using contrast-enhanced ultrasound of the breast for tumor size measurement. CEUS of the breast is performed with the second generation contrast agent SonoVue, which is made of microbubbles stabilized by phospholipids and containing the inert gas, SF6[17]. The microbubbles have a high reflectivity and are not extravasated from the vessel lumen, and as a consequence act like true blood pool agents. The effective vessel diameter from which an echo can be detected is in the range of a capillary. It has been postulated that this technique reliably visualizes the neovascularization within and around the tumor, and can potentially be used for tumor boundary identification and lesion characterization[18,20]. Several studies have proven the potential of CEUS of the breast in differentiating malignant from benign breast lesions, with varying sensitivities (67–95%) and specificities (58–82%) depending on the patient population being studied, the type of equipment, and the criteria used for interpretation of the CEUS images[16,18,20].
To date, no studies have been performed evaluating the accuracy of CEUS of the breast for size measurement of malignant breast tumors. Our study results showed that both gray-scale ultrasound and CEUS of the breast underestimated tumor size. Gray-scale ultrasound of the breast underestimated tumor size in 6/9 (67%) cases, which is in agreement with previous literature. However, CEUS of the breast underestimated tumor size only in 3/9 (33%) of cases. Although the numbers in this study are small, a trend towards lower tumor size underestimation rate with CEUS of the breast was found. More important, the accuracy of CEUS of the breast for determining tumor size within 2 mm of pathologic tumor size was 6/9 (67%), compared with 2/9 (22%) for gray-scale ultrasound. This implies that CEUS of the breast is a more accurate technique than gray-scale ultrasound of the breast for breast tumor lesion size measurement.
A possible limitation of the study is the small number of patients included; as a consequence a well-founded statistical analysis and firm conclusions cannot be made. Furthermore, subgroup analysis reporting accuracy of tumor size assessment for different tumor subtypes could not be made. Second, it is known that ultrasound examination is operator dependent; both the quality of ultrasound and the accuracy of tumor size estimation may depend on the clinician's experience. Since, in our study all the ultrasound measurements were performed by the same breast imaging radiologist, no inter- and intra-observer bias of CEUS for lesion size measurement could be calculated. Third, whether to include the echogenic interface (halo) around the hypoechoic lesion for measurement purposes on gray-scale breast ultrasound examination is still a matter of debate in the current literature[11]. In our study the halo was not included for tumor size measurement, which is in agreement with most other previous studies. Finally, currently no standard with regard to lesion size measurement on CEUS of the breast exists. Because it is a novel breast imaging modality, it is only rationally assumed that lesion size should be measured at the time of maximal contrast enhancement of the lesion, and that edges are defined as the end of the hyperechoic mass at that time. As a consequence, larger studies focusing on CEUS tumor size measurement will be needed in the future.
This feasibility study proves that CEUS of the breast is a feasible and safe technique for breast tumor size measurement. In this small group of patients with invasive ductal carcinoma of the breast we showed that CEUS of the breast was more accurate than gray-scale ultrasound of the breast for tumor size assessment. The maximal tumor diameter as assessed by CEUS of the breast was within 2 mm of pathologic tumor size in 67% of cases, compared with 22% in the gray-scale ultrasound group.
References
- 1.Del Cura JL, Elizagaray E, Zabala R, Legorburu A, Grande D. The use of unenhanced Doppler sonography in the evaluation of solid breast lesions. AJR Am J Roentgenol. 2005;184:1788–94. doi: 10.2214/ajr.184.6.01841788. [DOI] [PubMed] [Google Scholar]
- 2.American College of Radiology. Breast imaging reporting and data system: ultrasound. 4th. Reston, VA: American College of Radiology; 2003. edn. [Google Scholar]
- 3.Hall-Craggs MA, Vaidya JS. Minimally invasive therapy for the treatment of breast tumours. Eur J Radiol. 2002;42:52–7. doi: 10.1016/s0720-048x(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–39. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksson I, Liljegren G, Palm-Sjovall M, et al. Risk factors for local recurrence after breast conserving surgery. Br J Surg. 2003;90:1093–102. doi: 10.1002/bjs.4206. [DOI] [PubMed] [Google Scholar]
- 6.Asgeirsson KS, McCulley SJ, Pinder SE, Macmillan RD. Size of invasive breast cancer and risk of local recurrence after breast-conservation therapy. Eur J Cancer. 2003;39:2462–9. doi: 10.1016/s0959-8049(03)00605-1. [DOI] [PubMed] [Google Scholar]
- 7.Fornage BD, Toubas O, Morel M. Clinical, mammographic, and sonographic determination of preoperative breast cancer size. Cancer. 1987;60:765–71. doi: 10.1002/1097-0142(19870815)60:4<765::aid-cncr2820600410>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Tresserra F, Feu J, Grases PJ, et al. Assessment of breast cancer size: sonographic and pathologic correlation. J Clin Ultrasound. 1999;27:485–91. doi: 10.1002/(sici)1097-0096(199911/12)27:9<485::aid-jcu1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Allen SA, Cunliffe WJ, Gray J, et al. Pre-operative estimation of primary breast cancer size: a comparison of clinical assessment, mammography and ultrasound. Breast. 2001;10:299–305. doi: 10.1054/brst.2000.0255. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson CA, MacDermott TA. Ultrasound can estimate the pathologic size of infiltrating ductal carcinoma. Arch Surg. 2000;135:158–9. doi: 10.1001/archsurg.135.2.158. [DOI] [PubMed] [Google Scholar]
- 11.Shoma A, Moutamed A, Ameen M, Abdelwahab A. Ultrasound for accurate measurement of invasive breast cancer tumor size. Breast J. 2006;12:252–6. doi: 10.1111/j.1075-122X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- 12.Golshan M, Fung BB, Wiley E, Wolfman J, Rademaker A, Morrow M. Prediction of breast cancer size by ultrasound, mammography and core biopsy. Breast. 2004;13:265–71. doi: 10.1016/j.breast.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233:830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 14.Heusinger K, Lohberg C, Lux MP, et al. Assessment of breast cancer tumor size depends on method, histopathology and tumor size itself. Breast Cancer Res Treat. 2005;94:17–23. doi: 10.1007/s10549-005-6653-x. [DOI] [PubMed] [Google Scholar]
- 15.Kwee RM, Van den Bosch MAAJ, ElOuamari M, et al. Contrast-enhanced breast ultrasonography reveals an unusual breast tumor in a male patient with gynecomastia. J Ultrasound Med. 2006;25:1347–51. doi: 10.7863/jum.2006.25.10.1347. [DOI] [PubMed] [Google Scholar]
- 16.Kook SH, Kwag HJ. Value of contrast-enhanced power Doppler sonography using a microbubble echo-enhancing agent in evaluation of small breast lesions. J Clin Ultrasound. 2003;31:227–38. doi: 10.1002/jcu.10172. [DOI] [PubMed] [Google Scholar]
- 17.Greis C. Technology overview: SonoVue. Eur Radiol. 2004;14(Suppl 8):11–15. [PubMed] [Google Scholar]
- 18.Madjar H. Contrast ultrasound in breast tumor characterization: present situation and future tracks. Eur Radiol. 2001;11(suppl 3):E41–6. doi: 10.1007/pl00014129. [DOI] [PubMed] [Google Scholar]
- 19.Bokor D, Chambers JB, Rees PJ, Mant TG, Luzzani F, Spinazzi A. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol. 2001;36:104–9. doi: 10.1097/00004424-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Caruso G, Ienzi R, Cirino A, et al. Breast lesion characterization with contrast-enhanced US. Work in progress. Radiol Med (Torino) 2002;104:443–50. [PubMed] [Google Scholar]