Abstract
Coinfection with Ehrlichia canis, Babesia canis, Hepatozoon canis, Isospora spp., Giardia spp., and Dipylidium caninum were detected in a 6-week-old dog. The effect of multi-pathogen infection was a fatal combination of gastrointestinal and hematologic abnormalities, including diarrhea, vomiting, anorexia, distended painful abdomen, intussusception, severe thrombocytopenia, anemia, and hypoproteinemia.
Résumé
Coïnfestation par de multiples parasites intestinaux transmis par les tiques chez un chiot âgé de 6 semaines. Une coïnfestation par Ehrlichia canis, Babesia canis, Heptatozoon canis, Isospora spp., Giardia spp., et Dipylidium caninum a été identifiée chez un chiot âgé de 6 semaines. Les effets de cette multi-infestation ont résulté en une combinaison mortelle d’anomalies gastrointestinales et hématologiques comprenant diarrhée, vomissement, anorexie, distension douloureuse de l’abdomen, intussusception, thrombocytopénie grave, anémie et hypoprotéinémie.
(Traduit par Docteur André Blouin)
An intact female, 6-week-old, mixed-breed dog was referred to the Hebrew University Veterinary Teaching Hospital (HUVTH) with chief complaints of acute vomiting, anorexia, and intermittent diarrhea of 1-week duration, as well as a distended painful abdomen. The dog had been adopted from an animal shelter where it had been vaccinated against Canine parvovirus and dewormed 1 wk prior to presentation.
Case description
Upon presentation, the puppy had increased body temperature (39.4°C), tachycardia [heart rate 200 beats/min (bpm)], tachypnea [respiratory rate 160 breaths/min (brpm)], and slightly pale mucus membranes. The abdomen was soft, distended, and apparently painful, and bloody diarrhea was observed. Ectoparasites were not noted. A complete blood (cell) count (CBC) revealed normal white blood cell count (WBC) 13.4 × 109/L (reference range, 6 to 17 × 109/L), anemia [packed cell volume (PCV) 0.15 L/L (reference range, 0.258 to 0.552 L/L)], total solids (TS) 58 g/L (reference range, 60 to 80 g/L); red blood cell (RBC) 2.61 × 1012/L (reference range, 2.76 to 8.42 × 1012/L), hemoglobin 52 g/L (reference range, 64 to 189 g/L), and thrombocytopenia [platelets 2 × 109/L (reference range, 200 to 500 × 109/L)]; however, petechia or ecchymoses, were not detected either on the skin or on mucus membranes. Microscopic high power field examination of a giemsa-stained blood smear confirmed the thrombocytopenia and showed mild erythrocyte anisocytosis and polychromasia. The platelet number was estimated to be higher than counted by the automatic cell counter, but lower than normal, due to the presence of platelet clumps in the smear. A Hepatozoon canis gamont, as well as Babesia canis merozoite and trophozoite parasitemia was detected under high power field magnification, with 3% (15/500) of the neutrophils containing H. canis gamonts and 2.6% (13/500) of the erythrocytes parasitized by B. canis (Figure 1). In addition, Ehrlichia canis morulae were detected in several monocytes. Ehrlichia canis DNA was amplified from an EDTA-anticoagulated blood sample, using primers for the E. canis 16SrRNA gene, as previously described (1). A 527 base-pair DNA product was sequenced and found to be 99% identical with the Japanese Kagoshima E. canis strain (GenBank accession no. AF536827) by basic local alignment search tool (BLAST) analysis. Blood chemical abnormalities indicated hyponatremia [sodium (Na) 132 mmol/L (reference range, 146 to 156 mmol/L)], hypochloremia [chloride (Cl) 102.7 mmol/L (reference range, 109 to 122 mmol/L)], and hypokalemia [potassium (K+) 3.07 mmol/L (reference range, 3.8 to 5.1 mmol/L)]. Arterial blood gas abnormalities included alkalemia (arterial pH 7.658; reference range, 7.35 to 7.45), decreased bicarbonate concentration [calculated bicarbonate (HCO3 calc) 15.4 mmol/L (reference range, 25 to 35 mmol/L)] and hypocapnia [arterial CO2 partial pressure (PaCO2) 14 mm Hg (reference range, 29 to 36 mm Hg)]. Alkalemia was judged to be due to mixed acid base disturbance that consisted of primary respiratory alkalosis and primary metabolic acidosis. A direct fecal smear revealed numerous Isospora spp. oocysts and Dipylidium caninum eggs. Abdominal radiography and ultrasonography revealed gas-filled intestines with no evidence of obstruction, peritoneal effusion, or organo-megaly. The dog was hospitalized and treatment was initiated. A combination of antibiotics [trimethoprim sulfamethoxazole (Resprim; Teva Pharmaceutical Industries, Jerusalem, Israel), 15 mg/kg body weight (BW), PO, q12h; metronidazole (Metronidazole; B. Brown, Melsungen AG, Germany), 10 mg/kg BW, IV, q12h; doxycycline (Doxylin; Dexxon, Or-Akiva, Israel), 10 mg/kg BW, PO, q24h] was given to clear gastrointestinal infection of coccidian spp., for potential intestinal protozoa (giardiosis), as well as against anaerobic intestinal bacteria, and to treat tick-borne ricketsial infection. Other treatments given were the antiprotozoal drug imidiocarb dipropionate (Imizol; Schering-Plough, Kenilworth, New Jersey, USA), 5 mg/kg BW, IM, q14d, for a total of 2 treatments; the anthelmintics ivermectin (Ivomec; Merial, Lyon, France), 0.2 mg/kg BW, SC, single dose; and praziquantel-pyrantel-febantel (Drontal Plus; Bayer AG, Wuppertal, Germany), 1 tab/10 kg BW, PO, q10d; the IV fluid lactated Ringer’s solution (LRS) (Teva medical, Jerusalem, Israel), supplemented with 5% dextrose and 20 mmol/L K+ at 5 mL/kg BW/h; 10 mL/kg BW of whole blood transfused over a period of 4 h, the antiemetic metoclopramide (Pramin; Rafa Laboratories, Jerusalem, Israel), 0.4 mg/kg BW, SC, q8h; and the gastric protectant cimetidine (Tagamet; Wulfing Pharma, Gronau, Germany), 5 mg/kg BW, IV, q8h. It was discharged 7 d later with instructions to the owners to administer trimethoprim/sulfamethoxazole at 15 mg/kg BW, PO, q12h for 7 d; metronidazole at 10 mg/kg BW, PO, q12h for 5 d; doxycycline at 10 mg/kg BW, PO, q24h for 10 d; and metoclopramide 0.4 mg/kg BW, PO, q8h for 3 d; and to arrange for a 2nd IM injection of imidiocarb dipropionate in 7 d. Hematologic parameters on discharge were PCV 0.27 L/L, TS 60 g/L, and a platelet count of 376 × 109/L.
Figure 1.
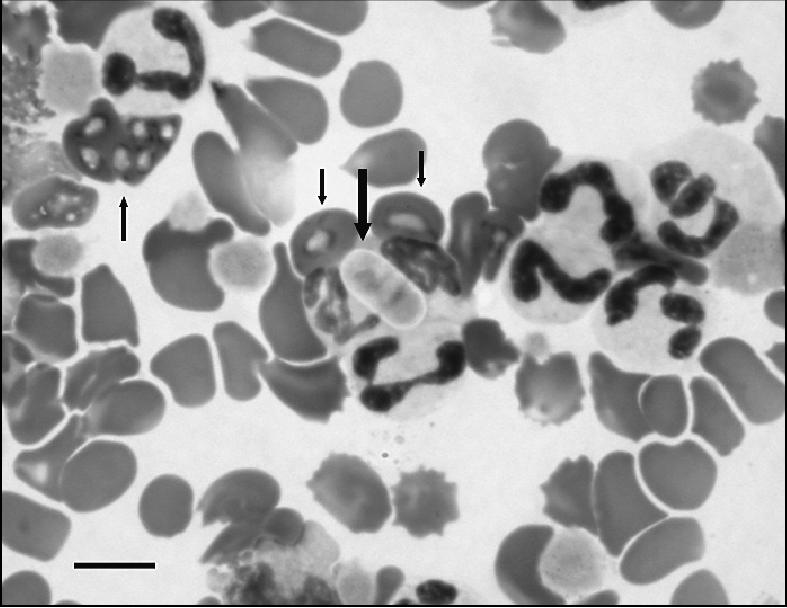
Giemsa-stained blood smear showing concurrent Hepatozoon canis (wide arrow) and Babasia canis (thin arrows) parasitemia in a 6-week-old dog on the initial day of admission. Bar = 10 microns.
Three days after discharge, the dog was presented again with the complaint of vomiting, diarrhea, anorexia, and painful, distended abdomen that had become apparent the night before. A CBC revealed a normal white blood cell count 13.7 × 109/L, a normocytic normochromic anemia (PCV 0.21 L/L, TS 38 g/L, red blood cell 3.32 × 1012/L, and Hgb 68 g/L), and H. canis parasitemia (2% of the neutrophils) were still evident. Ehrlichia canis morulae or B. canis merozoites were not detected. A partial biochemical panel revealed hypoalbuminemia [albumin (Alb) 19.8 g/L; reference range, 26 to 43 g/L] and hyponatremia (Na — 139 mmol/L). Large numbers of Isospora spp. oocysts and Giardia spp. trophozoites were detected on a direct fecal smear. Abdominal radiographs revealed loss of serosal details, which raised the suspicion of abdominal effusion. Therefore, a blinded abdominocentesis was performed and yielded a peritoneal effusion, characterized as transudate (TS 0 g/L, acellular, creatinine < 42 μmol/L). Therapy was continued with antibiotics [trimethoprim sulfamethoxazole at 75 mg/kg BW, PO, q12h; metronidazole at 25 mg/kg BW, IV, q12h], an antiprotozoal [imidiocarb dipropionate at 5 mg/kg BW, IM, — second injection], an anthelmintic [pyrantel pamoate (Combantrin; Pfizer, Markham, Ontario), 5 mg/kg BW, PO, — single treatment], and supportive care (LRS supplemented with 5% dextrose and 20 mmol/L K+ at 5 mL/kg BW/h). On day 11 from the initial presentation, signs of bloody diarrhea appeared, and abdominal palpation indicated an intussusception. The dog underwent an exploratory laparotomy and a 15-cm long reducible ileal intussusception was observed and reduced by gentle traction. Three days later, a cloudy fluid that contained bacteria within degenerated neutrophils was aspirated from the abdominal cavity. The dog was diagnosed with septic peritonitis and the owners opted for euthanasia, but denied a postmortem examination.
Discussion
This case demonstrates the complexity and clinically challenging multiple intestinal and hemoparasitic coinfections, as well as the potential complications encountered during the management of such cases. The wide range of clinical signs found in such coinfections often leads to difficulties in the diagnosis and clinical management (2). The initial observation of some pathogens may hinder the diagnosis of other potentially more virulent infections or clinical diagnoses requiring specific therapy. Furthermore, coinfections often lead to a more serious disease course in pediatric patients, since at 3–6 wk of age, puppies may not have sufficient nutritional reserves to accommodate large parasitic burdens (3).
Puppies have an immature immune system and, thus, are predisposed to infection through environmental exposure and contact with adult animals that harbor infections. Environmental factors such as crowding in animal shelters, kennels, and pet shops may increase the exposure of young animals to pathogens (4). Fifty-four percent of the animals adopted from shelters in Perth, Australia, were reported to become ill and suffer from respiratory or gastrointestinal signs within 14 d of their acquisition, and 92% of the pet owners that returned their newly adopted animal to the shelter did so due to such illness (5). Hemoparasitic and gastrointestinal pathogens, such as ascarids, hook worms, cestodes, coccidiae, and Giardia spp., have been reported to be prevalent in dogs housed in crowded conditions (3,4). Puppies 3 to 6 wk of age are especially susceptible to internal and external parasitic infection (6).
Puppies infected with tick-transmitted pathogens, such as B. canis and H. canis, can exhibit more severe clinical signs than older dogs (3,7). Moreover, E. canis infection has been reported to predispose dogs to opportunistic pathogens, such as B. canis and H. canis (8,9). Multiple tick-transmitted pathogen coinfections in dogs have been documented and associated with severe and fatal disease (9).
The current possibilities for the rapid transfer of animals between different countries and habitats create opportunities for invasion of new pathogens and disease vectors to nonendemic regions via automobiles, airplanes, and ships. In addition, exotic diseases are often detected in animals that return from travel outside their native environment. For these reasons, veterinary practitioners need to be alert and to consider nonendemic infections in their list of differential diagnoses for some disease conditions.
Anemia and thrombocytopenia are common findings in babesiosis and ehrlichiosis (9,10). Electrolyte abnormalities are frequent in animals with vomiting and diarrhea. Pain and anxiety combined with dehydration were probably responsible for the mixed acid base abnormalities that included respiratory alkalosis and metabolic acidosis. Protein losing enteropathy, maldigestion, and malabsorption, secondary to giardiasis, coccidiosis, and helminth infestation, were the likely causes of the panhypoproteinemia, and the latter probably led to the ascitic transudate. Intussusception is a well-documented sequel to severe, continuous diarrhea or intestinal parasitic infestation altering the gut motility (11). The final complication of septic peritonitis might have been caused by a devitalized intestinal segment involved in the intussusception or perforation of the intestine after surgery.
The brown dog tick, Rhipicephalus sanguineus, is the main vector of B. canis vogeli, H. canis, and E. canis. The acquired E. canis and B. canis infections probably resulted from a R. sanguineus bite. Hepatozoon canis is transmitted by the same tick vector but infects dogs via a different route. Hepatozoon canis sporozoites present in the host tick’s haemocoel need to be ingested by the dog for infection to develop (12). The time interval from H. canis sporozoite ingestion to gamont parasitemia has been shown to be 28 d (12). Thus, the presence of gamont parasitemia upon presentation suggests that tick ingestion had occurred at least 4 wk before. Such an event, although possible, is unlikely. However, both H. canis and B. canis can be transmitted in-utero (13,14), and this was likely to have been the route of infection for H. canis and, possibly, B. canis in this case. In contrast, to date, there is no evidence that E. canis can be transmitted in-utero. Therefore, for the puppy to have ehrlichiosis during hospitalization, it had to have been exposed to the tick prior to admission to hospital. The incubation periods from bite to clinical signs with E. canis and B. canis has been shown to be 8 to 20 and 10 to 21 d, respectively (15,16). Thus the puppy could potentially have been infected after birth with E. canis and B. canis. Given that R. sanguineus is a 3-host tick and leaves the host for molting, the absence of ticks on physical examination does not rule out the possibility for tick-borne infections.
Giardia spp. and Isospora spp. are transmitted fecoorally. Giardiasis can be manifested in a wide spectrum of clinical signs, from none to those through mild to severe infection. It can induce acute to chronic vomiting, diarrhea, or both, as well as protein losing enteropathy (17). Giardial infection is often associated with young age and poor hygienic conditions, both in dogs and humans (18). Young dogs, as well as young children, are more prone to giardiasis and tend to develop a more severe disease (4,18). Isospora infection is usually self-limiting and without clinical signs, although puppies and immunosuppressed animals may develop mucoid to bloody diarrhea (17). The dog in the present report may have acquired giardiasis and coccidiosis at the animal shelter. Dogs in animal shelters are often housed in crowded and inadequate hygienic conditions, with no separation between young and adult animals. Under these conditions, the potential for the spread of infectious agents is increased. These factors were probably involved in the development of the presently described coinfection.
To the best of our knowledge, this is the first description of coinfection with all 6 of these pathogens. Puppies, especially when acquired from an animal shelter, should be carefully examined when transferred to a new environment, because they may harbor multiple infections that potentially can lead to a complex illness with mixed clinical signs. In addition, routine prophylactic measures should be taken, including ectoparasite prevention treatments, deworming, and vaccination. Lastly, when a vector-borne disease is diagnosed, other potential coexisting infections transmitted by the same arthropod vector should be ruled out. CVJ
Footnotes
Dr. Gal’s current address is College of Veterinary Medicine, University of Illinois at Urbana-Champaign, 1008 West Hazelwood Drive, Urbana, Illinois 61802, USA.
References
- 1.Sparagano OA, de Vos AP, Paoletti B, et al. Molecular detection of Anaplasma platys in dogs using polymerase chain reaction and reverse line blot hybridization. J Vet Diagn Invest. 2003;15:527–534. doi: 10.1177/104063870301500604. [DOI] [PubMed] [Google Scholar]
- 2.Kordick SK, Breitschwerdt EB, Hegarty BC, et al. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–2638. doi: 10.1128/jcm.37.8.2631-2638.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinkenbeard KD, Cowell RL, Meinkoth JH, Decker LS. The hemato-poietic and lymphoid systems. In: Hoskins JD, ed. Veterinary Pediatrics Dogs and Cats from Birth to Six Months. 3rd ed. Philadelphia: WB Saunders, 2001:314–315.
- 4.Bugg RJ, Robertson ID, Elliot AD, Thompson RCA. Gastrointestinal parasites of urban dogs in Perth, Western Australia. Vet J. 1999;157:295–301. doi: 10.1053/tvjl.1998.0327. [DOI] [PubMed] [Google Scholar]
- 5.Wells DL, Hepper PG. Prevalence of disease in dogs purchased from an animal rescue shelter. Vet Rec. 1999;144:35–38. doi: 10.1136/vr.144.2.35. [DOI] [PubMed] [Google Scholar]
- 6.Macintire DK. Intensive care management. In: Hoskins JD, ed. Veterinary Pediatrics Dogs and Cats from Birth to Six Months. 3rd ed. Philadelphia: WB Saunders, 2001:65–66.
- 7.Baneth G, Aroch I, Presentey B. Hepatozoon canis infection in a litter of Dalmatian dogs. Vet Parasitol. 1997;70:201–226. doi: 10.1016/s0304-4017(96)01152-1. [DOI] [PubMed] [Google Scholar]
- 8.Baneth G, Mathew JS, Shkap V, Macintire DK, Barta JR, Ewing SA. Canine hepatozoonosis: Two disease syndromes caused by separate Hepatozoon spp. Trends Parasitol. 2003;19:27–31. doi: 10.1016/s1471-4922(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 9.Breitschwerdt EB. Obligate intracellular bacterial pathogens. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, Missouri: Elsevier Saunders, 2005:631–636.
- 10.Jacobson LS, Clark IA. The pathophysiology of canine babesiosis: New approaches to an old puzzle. J S Afr Vet Assoc. 1994;65:134–145. [PubMed] [Google Scholar]
- 11.Washabau JR, Holt DE. Diseases of the large intestine. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, Missouri: Elsevier Saunders, 2005:1378–1408.
- 12.Baneth G, Samish M, Alekseev E, Aroch I, Shkap V. Transmission of Hepatozoon canis by naturally-fed or percutaneously-injected Rhipicephalus sanguineus ticks. J Parasitol. 2001;87:606–611. doi: 10.1645/0022-3395(2001)087[0606:TOHCTD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Murata T, Inoue M, Tateyama S, Taura Y, Nakama S. Vertical transmission of Hepatozoon canis in dogs. J Vet Med Sci. 1993;55:867–868. doi: 10.1292/jvms.55.867. [DOI] [PubMed] [Google Scholar]
- 14.Taboada J, Lobetti R. Babesiosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 3rd ed. St. Louis, Missouri: Elsevier Saunders, 2006:722–735.
- 15.Waner T, Rosner M, Harrus S, Naveh A, Zass R, Keysary A. Detection of ehrlichial antigen in plasma of beagle dogs with experimental acute Ehrlichia canis infection. Vet Parasitol. 1996;63:331–335. doi: 10.1016/0304-4017(95)00902-7. [DOI] [PubMed] [Google Scholar]
- 16.Boozer AL, Macintire DK. Canine babesiosis. Vet Clin North Am Small Anim Pract. 2003;33:885–904. doi: 10.1016/s0195-5616(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 17.Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, Missouri: Elsevier Saunders, 2005:1332–1377.
- 18.Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. Med J Aust. 1993;158:157–159. doi: 10.5694/j.1326-5377.1993.tb121692.x. [DOI] [PubMed] [Google Scholar]


