Abstract
Ventricular dysrhythmias are more commonly associated with myocardial disease than are supraventricular dysrhythmias. Management of arrhythmias under general anesthesia is difficult because of the dysrhythmogenic effects of the anesthetic drugs. This report describes a severe ventricular dysrhythmia observed in a pony under general anesthesia, with a severe and old myocardial fibrosis found on postmortem examination.
Résumé
Fibrose du myocarde chez un cheval présentant de la tachycardie ventriculaire polymorphe observée au cours d’une anesthésie générale. Les dysrythmies ventriculaires sont plus souvent associées aux maladies du myocarde que ne le sont les dysrythmies supraventriculaires. Les mesures à prendre lors d’arythmie survenant lors d’anesthésie générales sont compliquées par les effets dysrythmogènes des agents anesthésiques. Ce rapport décrit une dysrythmie ventriculaire grave observée chez un poney au cours d’une anesthésie générale et dont on a constaté à la nécropsie une grave et vielle fibrose du myocarde.
(Traduit par Docteur André Blouin)
A 7-year-old gelding pony was admitted to the Equine Clinic, National Veterinary School, Alfort, for a unilateral left purulent nasal discharge associated with left maxillary sinus deformation and halitosis of 5 months’ duration. There was no history of hyperthermia or chronic respiratory disease. The pony was in good condition and was performing in national jumping competitions. One month prior to admission, antibiotic treatment (penicillin G and streptomycin for 1 wk) was instituted, but it did not improve the condition significantly. The horse was referred for surgical removal of the left 1st maxillary molar tooth.
Case description
Initial clinical examination revealed an alert pony in good body condition (370 kg), rectal temperature 37.5°C, heart rate 40 beats/min (bpm), and respiratory rate 16 breaths/min. Pulse quality was strong and regular. Mucous membranes were pink and moist, and the capillary refill time was normal. Cardiac and respiratory auscultations were considered normal. A foul smelling discharge from the left nostril and deformation of the left maxillary sinus were observed. Percussion of the sinus was also modified. The left retropharyngeal lymph node was enlarged and painful.
A complete blood (cell) count (CBC) and serum biochemical analysis were performed before surgery; values were within the normal range. Dorsoventral and lateral radiographs of the skull revealed a left maxillary sinusitis associated with an alveolar abscess as a result of a fracture of the 1st maxillary molar tooth.
The pony was sedated with romifidine (Sedivet; Boheringer Ingelheim France, Reims, France), 0.06 mg/kg bodyweight (BW), IV. Anesthesia was induced with ketamine (Imalgène 1000; Merial, Lyon, France), 2.2 mg/kg BW, IV, mixed with diazepam (Valium Roche; Produits Roche, Neuilly-sur-Seine, France), 0.03 mg/kg BW, IV, and the pony was placed in right lateral recumbency. Anesthesia was maintained with halothane in oxygen (Halothane Veterinaire Belamont; Laboratoire Belamont, Boulogne-Billancourt, France) after orotracheal intubation, using a 24-mm endotracheal tube. Ventilation was controlled and the animal was perfused with a lactate Ringer’s solution (approximatively 10 mL/kg BW/h). Electrocardiographic leads were placed to obtain base-apex lead tracings during anesthesia. The initial indirect mean arterial pressure (oscillatory method) was below 60 mmHg, and on the basis of this low blood pressure, dobutamine (Dobutamine Aguettant; Laboratoire Aguettant, Lyon, France) infusion was started at an initial rate of 1 μg/kg BW/min to improve cardiac output. As soon as the invasive arterial blood pressure was obtained, dobutamine infusion was adjusted to maintain mean arterial blood pressure over 70 mmHg.
One hour after induction, cardiac dysrhythmia was observed. A diagnosis of premature ventricular complexes (PVC) was made on the basis of an early appearing, abnormally shaped QRS, not preceded by a P-wave. Abnormal QRS complexes were coupled to the preceding normal sinus beat, followed by a characteristic compensatory pause, or inserted between 2 normal beats. The coupling interval between a PVC and the preceding sinus QRS complex was regular (T-R interval: 0.4 s). The direction of the T-wave was either normal or in the opposite direction. Episodes of PVC were intermittent and polymorphic (2 forms of PVC in opposite direction), with the PVC observed either singly, coupled with normal complexes, in pairs, or in runs. These episodes were followed by a period of regular sinusal rhythm (heart rate: 30–35 bpm). The heart rate during episodes of PVCs was faster (70–80 bpm). Despite some brief episodes of hypotension during sinusal rhythm, the arterial blood pressure was normal. Based on the absence of effect on arterial blood pressure during PVC episodes and the moniform shape of the PVC, no antidysrythmic treatment was attempted. Halothane was adjusted according to the level of anesthesia, ventilation was adapted according to the arterial blood gas values (normal blood gas values were obtained). Two hours after induction, runs of more than 4 PVCs successively were observed (Figure 1), being first paroxysmal with the rhythm strip alternatively sinusal and ventricular. An underlying sinusal rhythm was present (P-wave during episodes of PVCs). Treatment with an IV bolus of lidocaine (Lurocaine; Vetoquinol, Paris, France), 0.5 mg/kg BW (200 mg), IV, was initiated, allowing a stabilization of a sinusal rhythm but inducing a 20 mmHg decrease in mean arterial blood pressure. Ten minutes later ventricular tachycardia reappeared and another bolus of lidocaine, 0.3 mg/kg BW, IV, was administered, followed by 25 μg/kg BW/min continuous administration. The cardiac rhythm persisted intermittently dysrhythmic, being alternatively sinusal, associated with moderate hypotension (mean arterial pressure: 60 mmHg), and ventricular, with marked increase of heart rate (60–70 bpm) and hypertension (mean arterial pressures reaching 100 mmHg).
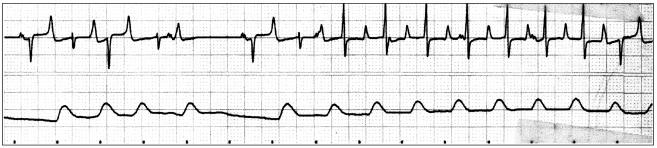
Figure 1.
Base-apex lead ECG (upper trace) (12.5 mm/s) and arterial pressure (lower trace) recordings 2 hours after induction of anesthesia. Normal sinus complexes (1st, 3rd, and 5th beats) are observed at the beginning of the strip, coupled with PVCs of the same morphology (2nd, 4th, and 6th beats). The 7th beat and thereafter correspond to a 2nd population of PVCs (opposite direction) and form together paroxysmal ventricular tachycardia (7 PVCs successively). A P-wave is intercalated between the 5th QRS complex and its T wave.
The pony was still unstable when transferred to the recovery box. The electrocardiogram (ECG) was not maintained for 15 min because of the limited access in the recovery stall. Respiration was efficient and the oxygen demand valve was connected to the endotracheal tube to assist spontaneous ventilation. Fifteen minutes after the transfer to the recovery box, ECG tracings were obtained and ventricular fibrillation was observed. A lidocaine bolus, 0.3 mg/kg BW, IV, was administered, but cardiac arrest occurred 30 min after withdrawal of the halothane administration. Medical reanimation with epinephrine (Adrenaline Aguettant; Laboratoire Aguettant), 10 mg, IV, and atropine (Atropine Lavoisier; Laboratoires Chaix et Du Marais, Paris, France), 5 mg, IV, cardiac massage, and ventilation was attempted for 20 min without success.
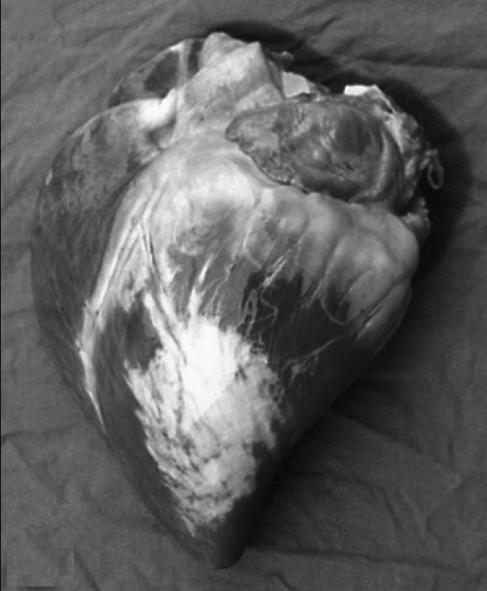
Macroscopic evaluation of the heart (Figure 2) revealed multifocal, wide white-grey areas on the epicardium of the left ventricle. Similar lesions were present throughout the myocardium. Upon microscopic evaluation, severe diffuse mature fibrosis was observed spreading from the epicardial surface into the myocardium of the left ventricle. In this latter location, the fibrosis was less severe and had a multifocal distribution. Entrapped cardiomyocytes were degenerated. A slight lymphocytic inflammation was present in the perivascular areas. Similar lesions were also present in the interventricular septum. In both atria, a slight perivascular inflammation and endocardial edema were observed.
Figure 2.
Macroscopic appearance of the heart. Note the multifocal wide white-grey areas on the epicardium of the left ventricle.
Discussion
In the horse, ventricular dysrhythmias are less common than atrial dysrhythmias and can be indicative of a primary cardiac disease (1–5). Ventricular arrhythmias have also been associated with systemic disorders, such as hyperthermia, sepsis, electrolyte imbalance, acidosis, hypoxia, and toxemia, or with anesthetic drugs (3,4,6–9). Premature ventricular complexes (PVCs) are the most frequent ventricular dysrhythmias in horses, and can lead to ventricular tachycardia (1,3,10). The clinical signification of intermittent ventricular premature complexes is difficult to ascertain, but persistent ventricular dysrhythmia is indicative of heart disease, systemic disease, or a drug-induced cardiac abnormality. Most reports of ventricular tachycardia emphasize the difficulty of early detection, before the appearance of clinical manifestations (3,11,12). In the case described here, the CBC and acid-base values were within normal limits. Therefore, sepsis and acidosis were not likely responsible for the ventricular arrhythmia.
The dysrhythmogenic potential of anesthetic drugs has already been reported (2,3,8,13–15). Premature ventricular complexes (PVCs) may occur without significant underlying disease (1). Activation of normal potential cardiac pacemakers located in the area of the atrioventricular node could be enhanced by the administration of drugs like xylazine, detomidine, or halogenated anesthetics (halothane). Those drugs cause a sinus node depression, while enhancing the effects of catecholamines on fibers in the area of the atrioventricular node. Therefore, the significant dysrhythmias that occurred under anesthesia required the arrest of drug administration as soon as possible (2). Halothane sensitizes the myocardium to catecholamine-induced arrhythmias (8,9,14,15). It may be helpful to switch the inhalant to isoflurane, as the latter agent causes less myocardial sensitization than halothane. Unfortunately, in this case, isoflurane was not available. Dobutamine also has been reported to induce dysrhythmias during halothane-anesthesia (16), but most of the arrhythmias observed have had a supraventricular or junctional origin (17). In this case, all the anesthetic drugs used (romifidine, ketamine, halothane, dobutamine) might have triggered or contributed to the dysrhythmia.
Induced ischemia or myocardial hypoxia can trigger ventricular arrhythmia, as can a more serious cardiac abnormality, such as myocarditis. Though the effect of dobutamine and tachycardia of any type, if severe enough, can cause myocardial ischemia, the severe and mature myocardial fibrosis found in this case is consistent with an old ventricular infarction. The lesions were considered unlikely to have been induced by the administration of dobutamine or by the tachycardia and, most likely, were related to the ventricular arrhythmia; the anesthetic drugs likely contributed to the development of the PVCs.
The management of ventricular dysrhythmia during anesthesia should focus on controlling the potential contributing factors, such as the anesthetic drugs, acid-base and electrolyte imbalances, hypoxaemia, or abnormal carbon dioxide levels. If myocardial hypoxia is suspected secondary to profound hypotension, treatment should focus on improving the patient’s hemodynamic status. If dobutamine appears to be the culprit, stopping the infusion will quickly resolve the problem, due to dobutamine’s short half-life. Administration of antidysrhythmic drugs should be considered, if significative hemodynamic changes or ventricular persistent tachycardia is present (3,10). Decrease in blood pressure was not observed during ectopic depolarizations, despite the increase in the heart rate. Treatment in this case was pursued on the basis of the frequency of the PVCs, the multiform appearance of the depolarizations, and the increased heart rate.
Lidocaine has been reported as the drug of choice to control ventricular dysrhythmias in horses (1). Lidocaine decreases the conduction of cardiac impulses, abolishes abnormal spontaneous electrical activity originating from diseased heart tissue, and increases the refractory period (1). In the standing horse, the most significant side effects are neurologic signs, secondary to central nervous system stimulation (9), but they are not relevant in the anesthetized animal. When used in combination with anesthetics, lidocaine injections are usually associated with decreased contractility (18). Indeed, a 20 mmHg decrease in blood pressure followed the 2 lidocaine bolus injections. Nevertheless, a continuous injection that induces less cardiovascular depression should be considered, as the effect of bolus administrations is not prolonged.
Myocardial injury may result from toxic, viral, bacterial, traumatic, ischemic, hypoxic, or metabolic causes; from endocarditis or pericarditis; or from aberrant parasite migration (1,5). In the case described, the diffuse and mature myocardial fibrosis was considered to be consistent with myocardial infarction or ischemia, the cause of which was not determined. Coronary thrombosis has been suspected as the cause of diffuse or regional infarction (5). Myocardial fibrosis can be found on postmortem examination of horses without previously reported cardiovascular signs. Wide myocardial fibrosis has also been reported to induce sudden death (12) or sustained ventricular tachycardia (4). In this case, no abnormal clinical signs were noticed on the routine preanesthetic examination or in the case history (exercise intolerance, abnormal tiredness), which emphasizes the difficulty in detecting myocardial injury clinically and in preventing the development of arrhythmia under general anesthesia. Cardiac auscultation and electrocardiography, during and after exercise, echocardiography, or both, could have detected significant dysrhythmias or myocardial disease, but these procedures are not routinely performed during a preanesthetic evaluation. CVJ
References
- 1.McGuirk SM, Muir WW. Diagnosis and treatment of cardiac arrhythmias. Vet Clin North Am Equine Pract. 1985;1:353–370. doi: 10.1016/s0749-0739(17)30760-5. [DOI] [PubMed] [Google Scholar]
- 2.Muir WW. Anaesthetic complications and cardiopulmonary resuscitation in the horse. In: Reinhardt RW, Steube M, eds. Equine Anaesthesia: Monitoring and Emergency Therapy. St Louis: Mosby-Year Book, 1991: 461–484.
- 3.Reimer JM, Reef VB, Sweeney RW. Ventricular arrhythmias in horses: 21 cases (1984–1989) J Am Vet Med Assoc. 1992;201:1237–1243. [PubMed] [Google Scholar]
- 4.Traub-Dargatz JL, Schlipf JW, Boon J, et al. Ventricular tachycardia and myocardial dysfunction in a horse. J Am Vet Med Assoc. 1994;205:1569–1573. [PubMed] [Google Scholar]
- 5.Schwarzwald CC, Hardy J, Buccellato M. High cardiac troponin I serum concentration in a horse with multiform ventricular tachycardia and myocardial necrosis. J Vet Intern Med. 2003;17:364–368. [PubMed] [Google Scholar]
- 6.Atwood S, Miller MS, Williams M. Ventricular tachycardia: A case report. J Equine Vet Sci. 1985;6:102–104. [Google Scholar]
- 7.Sponseller BT, Ware WA. ECG of the month. J Am Vet Med Assoc. 2002;221:196–197. doi: 10.2460/javma.2002.221.196. [DOI] [PubMed] [Google Scholar]
- 8.Kellager REB, Watney GCG. Cardiac arrest during anaesthesia in two horses. Vet Rec. 1986;119:347–349. doi: 10.1136/vr.119.14.347. [DOI] [PubMed] [Google Scholar]
- 9.Lees P, Tavernor W. Influence of halothane and catecholamines on heart rate and rhythm in the horse. Br J Pharmacol. 1970;39:149–159. doi: 10.1111/j.1476-5381.1970.tb09564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garber JL, Reef VB, Reimer JM, Evans LH. Postsurgical ventricular tachycardia in a horse. J Am Vet Med Assoc. 1992;201:1038–1039. [PubMed] [Google Scholar]
- 11.Nielsen I. Ventricular tachycardia in a thoroughbred racehorse. Aust Vet J. 1990;67:140–142. doi: 10.1111/j.1751-0813.1990.tb07732.x. [DOI] [PubMed] [Google Scholar]
- 12.Schiff P, Knottenbelt DC. Sudden death in an 11-year-old Thoroughbred stallion. Equine Vet Educ. 1990;2:8–10. [Google Scholar]
- 13.Bonagura JD, Muir WW. The cardiovascular system. In: Reinhardt RW, Steube M, eds. Equine Anaesthesia: Monitoring and Emergency Therapy. St Louis: Mosby-Year Book, 1991:39–104.
- 14.Bonagura JD, Miller MS. Junctionnal and ventricular arrhythmias. J Equine Vet Sci. 1985;5:347–350. [Google Scholar]
- 15.Matthews NS, Hartsfield SM. Arrhythmogenic dose of epinephrine in isoflurane- or sevoflurane-anesthetized horses. J Equine Vet Sci. 2004;24:110–114. [Google Scholar]
- 16.Light GS, Hellyer PW, Swanson CR. Parasympathetic influence on the arrhythmogenicity of graded dobutamine infusions in halothane-anaesthezied horses. Am J Vet Res. 1992;53:1154–1160. [PubMed] [Google Scholar]
- 17.Donaldson LL. Restrospective assessment of dobutamine therapy for hypotension in anaesthetized horses. Vet Surg. 1998;17:53–57. doi: 10.1111/j.1532-950x.1988.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 18.Doherty TJ, Frazier DL. Effect of intravenous lidocaine on halothane minimum alveolar concentration in ponies. Equine Vet J. 1998;30:300–303. doi: 10.1111/j.2042-3306.1998.tb04101.x. [DOI] [PubMed] [Google Scholar]