Abstract
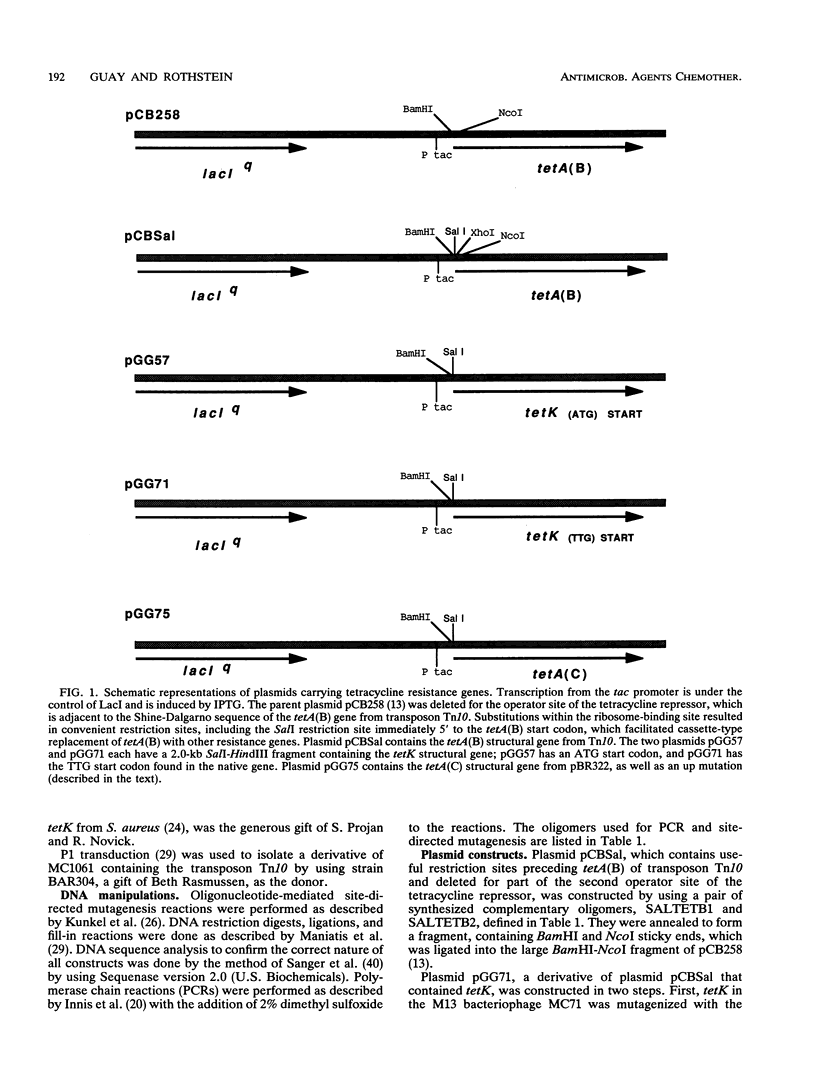
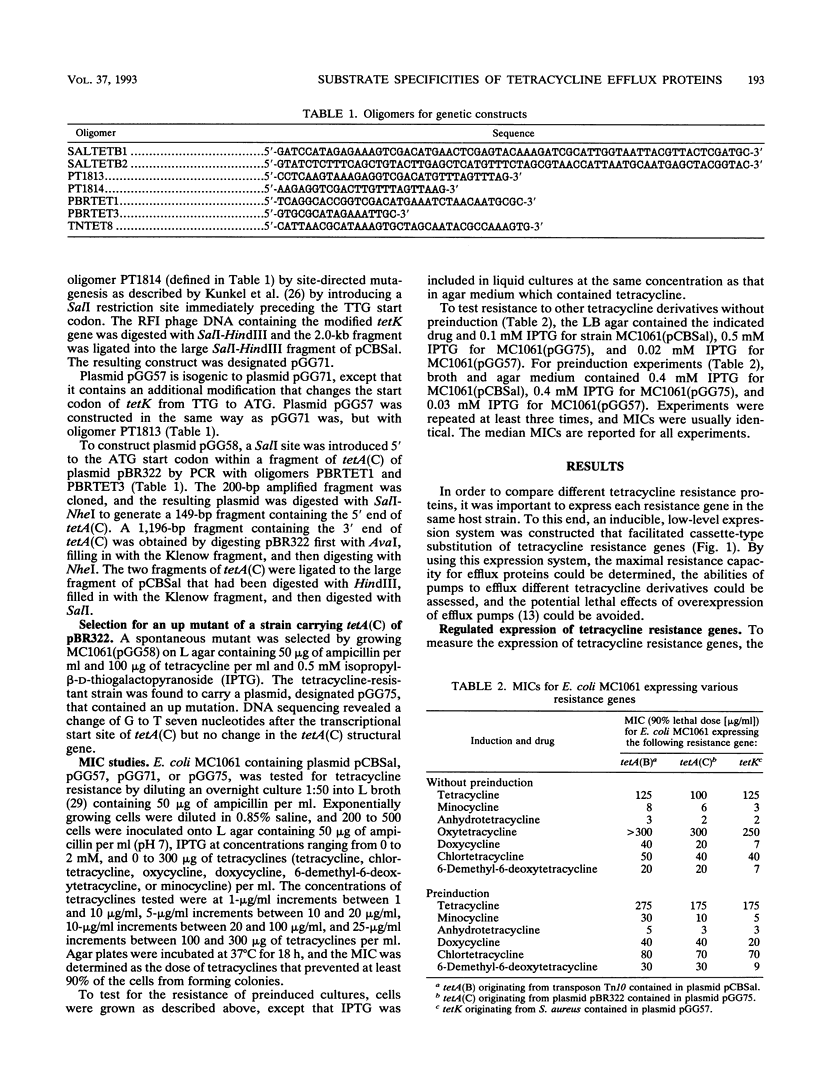
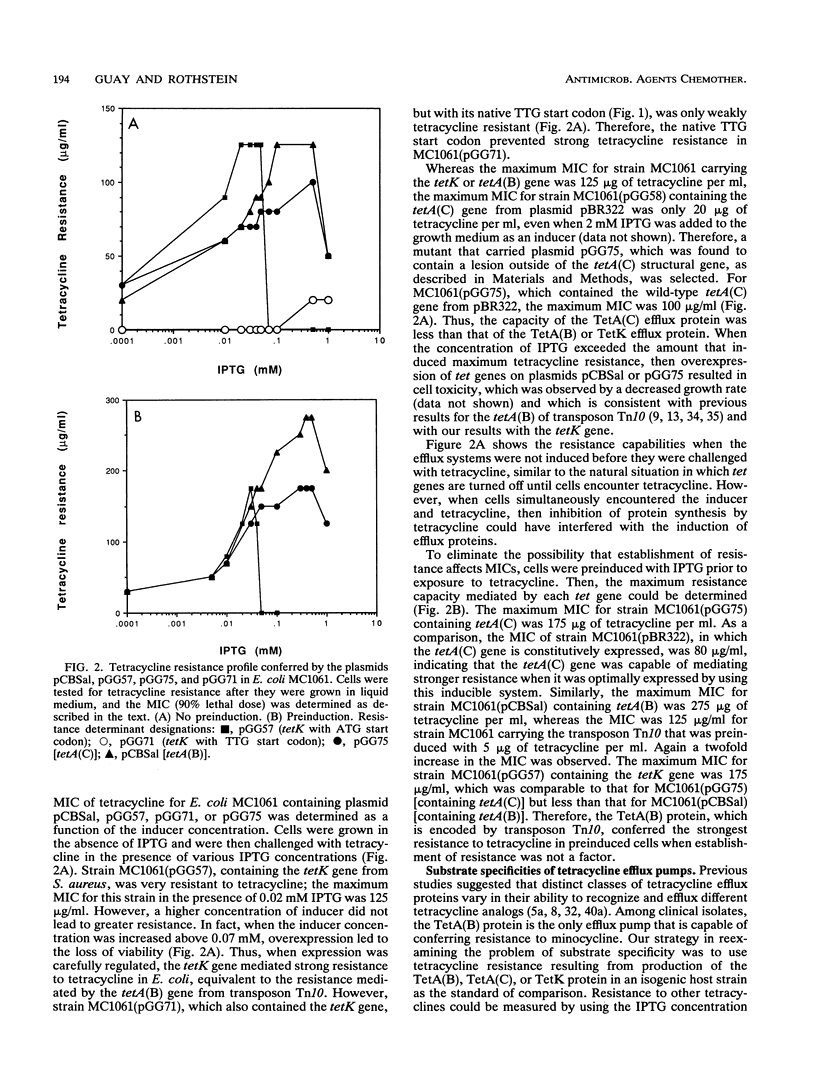
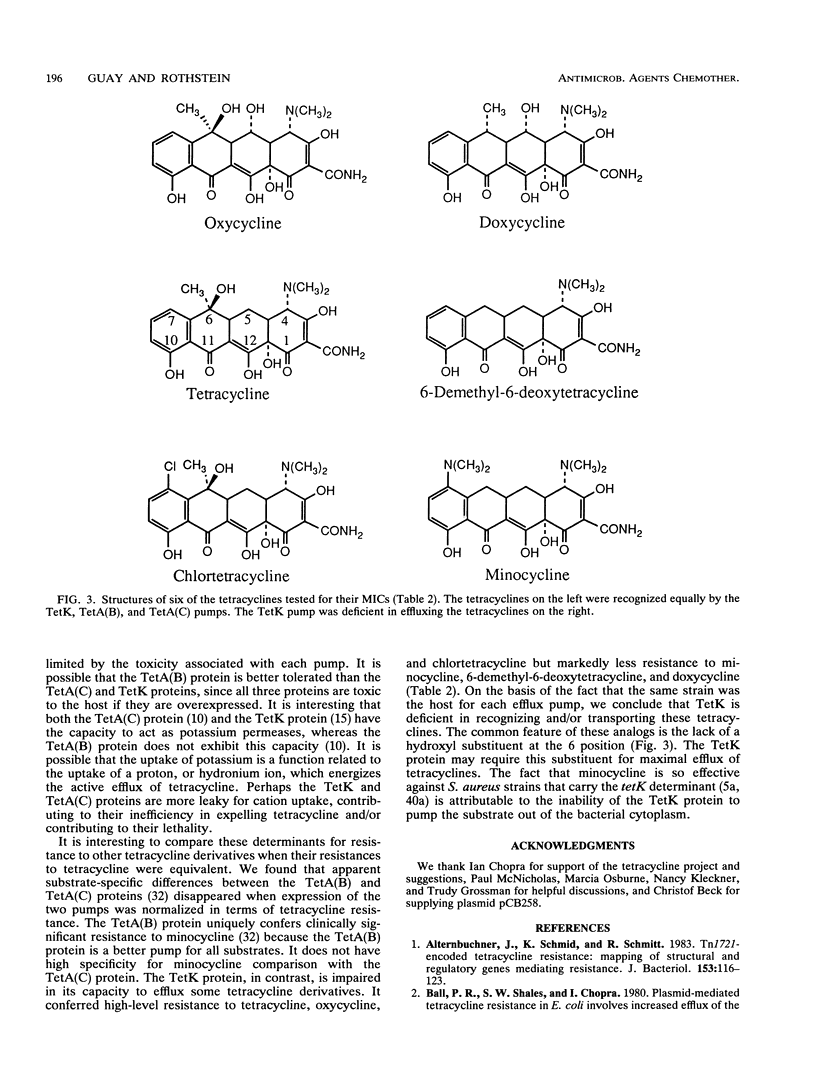
The tetK gene, which encodes a tetracycline efflux pump from Staphylococcus aureus, was expressed in Escherichia coli by using an inducible, low-level expression system. The tetK gene, as well as the tetA(B) gene from the transposon Tn10 and the tetA(C) gene from plasmid pBR322, was subjected to the regulatory control of the lac repressor, and resistance to tetracycline was measured as a function of the isopropyl-beta-D-thiogalactopyranoside concentration. The maximum resistance of the E. coli strain containing the tetK construct was comparable to the maximum resistance of the strain containing the tetA(C) construct but was less than the resistance of the strain containing the tetA(B) construct. Overexpression of the tetK, tetA(B), or tetA(C) genes was toxic. When expression was regulated so that resistance to tetracycline was comparable, then the TetA(B) and TetA(C) proteins conferred very similar levels of resistance to a variety of tetracycline derivatives. In contrast, the TetK protein was less capable of conferring resistance to the tetracycline derivatives minocycline, 6-deoxy-6-demethyltetracycline, and doxycycline. The implications for the recognition of various tetracycline substituents by the TetK protein are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Schmid K., Schmitt R. Tn1721-encoded tetracycline resistance: mapping of structural and regulatory genes mediating resistance. J Bacteriol. 1983 Jan;153(1):116–123. doi: 10.1128/jb.153.1.116-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Beck C. F., Mutzel R., Barbé J., Müller W. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J Bacteriol. 1982 May;150(2):633–642. doi: 10.1128/jb.150.2.633-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984 Jun;158(3):910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Bismuth R., Zilhao R., Sakamoto H., Guesdon J. L., Courvalin P. Gene heterogeneity for tetracycline resistance in Staphylococcus spp. Antimicrob Agents Chemother. 1990 Aug;34(8):1611–1614. doi: 10.1128/aac.34.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chopra I. Antibiotic resistance resulting from decreased drug accumulation. Br Med Bull. 1984 Jan;40(1):11–17. doi: 10.1093/oxfordjournals.bmb.a071940. [DOI] [PubMed] [Google Scholar]
- Chopra I., Hawkey P. M., Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992 Mar;29(3):245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S., Ball P. Tetracycline resistance determinants from groups A to D vary in their ability to confer decreased accumulation of tetracycline derivatives by Escherichia coli. J Gen Microbiol. 1982 Apr;128(4):689–692. doi: 10.1099/00221287-128-4-689. [DOI] [PubMed] [Google Scholar]
- Coleman D. C., Foster T. J. Analysis of the reduction in expression of tetracycline resistance determined by transposon Tn10 in the multicopy state. Mol Gen Genet. 1981;182(1):171–177. doi: 10.1007/BF00422786. [DOI] [PubMed] [Google Scholar]
- Dosch D. C., Salvacion F. F., Epstein W. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol. 1984 Dec;160(3):1188–1190. doi: 10.1128/jb.160.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles S., Docherty A., Chopra I., Shales S., Ball P. Tetracycline resistance genes from Bacillus plasmid pAB124 confer decreased accumulation of the antibiotic in Bacillus subtilis but not in Escherichia coli. J Bacteriol. 1981 Mar;145(3):1417–1420. doi: 10.1128/jb.145.3.1417-1420.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert B., Beck C. F. Overproduction of transposon Tn10-encoded tetracycline resistance protein results in cell death and loss of membrane potential. J Bacteriol. 1989 Jun;171(6):3557–3559. doi: 10.1128/jb.171.6.3557-3559.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert B., Beck C. F. Topology of the transposon Tn10-encoded tetracycline resistance protein within the inner membrane of Escherichia coli. J Biol Chem. 1989 Jul 15;264(20):11663–11670. [PubMed] [Google Scholar]
- Hickman R. K., McMurry L. M., Levy S. B. Overproduction and purification of the Tn10-specified inner membrane tetracycline resistance protein Tet using fusions to beta-galactosidase. Mol Microbiol. 1990 Aug;4(8):1241–1251. doi: 10.1111/j.1365-2958.1990.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives C. L., Bott K. F. Analysis of the tet gene of plasmid pCIS7 isolated from Bacillus subtilis. Gene. 1990 Sep 28;94(1):115–119. doi: 10.1016/0378-1119(90)90476-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Energetics of tetracycline efflux system encoded by Tn10 in Escherichia coli. FEBS Lett. 1985 Dec 2;193(2):194–198. doi: 10.1016/0014-5793(85)80149-6. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Klock G., Unger B., Gatz C., Hillen W., Altenbuchner J., Schmid K., Schmitt R. Heterologous repressor-operator recognition among four classes of tetracycline resistance determinants. J Bacteriol. 1985 Jan;161(1):326–332. doi: 10.1128/jb.161.1.326-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. M., Burdett V., Courvalin P., Hillen W., Roberts M. C., Taylor D. E. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989 Aug;33(8):1373–1374. doi: 10.1128/aac.33.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- McMurry L. M., Park B. H., Burdett V., Levy S. B. Energy-dependent efflux mediated by class L (tetL) tetracycline resistance determinant from streptococci. Antimicrob Agents Chemother. 1987 Oct;31(10):1648–1650. doi: 10.1128/aac.31.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Mojumdar M., Khan S. A. Characterization of the tetracycline resistance gene of plasmid pT181 of Staphylococcus aureus. J Bacteriol. 1988 Dec;170(12):5522–5528. doi: 10.1128/jb.170.12.5522-5528.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyed H. S., Bertrand K. P. Mutations in multicopy Tn10 tet plasmids that confer resistance to inhibitory effects of inducers of tet gene expression. J Bacteriol. 1983 Aug;155(2):557–564. doi: 10.1128/jb.155.2.557-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyed H. S., Nguyen T. T., Bertrand K. P. Multicopy Tn10 tet plasmids confer sensitivity to induction of tet gene expression. J Bacteriol. 1983 Aug;155(2):549–556. doi: 10.1128/jb.155.2.549-556.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Postle K., Bertrand K. P. Sequence homology between the tetracycline-resistance determinants of Tn10 and pBR322. Gene. 1983 Nov;25(1):83–92. doi: 10.1016/0378-1119(83)90170-1. [DOI] [PubMed] [Google Scholar]
- Reynes J. P., Calmels T., Drocourt D., Tiraby G. Cloning, expression in Escherichia coli and nucleotide sequence of a tetracycline-resistance gene from Streptomyces rimosus. J Gen Microbiol. 1988 Mar;134(3):585–598. doi: 10.1099/00221287-134-3-585. [DOI] [PubMed] [Google Scholar]
- Sakaguchi R., Shishido K. Molecular cloning of a tetracycline-resistance determinant from Bacillus subtilis chromosomal DNA and its expression in Escherichia coli and B. subtilis. Biochim Biophys Acta. 1988 Jan 25;949(1):49–57. doi: 10.1016/0167-4781(88)90053-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Cardoso M., Wegener H. C. Nucleotide sequence and phylogeny of the tet(L) tetracycline resistance determinant encoded by plasmid pSTE1 from Staphylococcus hyicus. Antimicrob Agents Chemother. 1992 Mar;36(3):580–588. doi: 10.1128/aac.36.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krausz J. Action of 12 tetracyclines on susceptible and resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1973 Sep;4(3):237–247. doi: 10.1128/aac.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar K., Ernst A., Hillen W. Identification and nucleotide sequence of the class E tet regulatory elements and operator and inducer binding of the encoded purified Tet repressor. Mol Gen Genet. 1988 Dec;215(1):76–80. doi: 10.1007/BF00331306. [DOI] [PubMed] [Google Scholar]
- Unger B., Klock G., Hillen W. Nucleotide sequence of the repressor gene of the RA1 tetracycline resistance determinant: structural and functional comparison with three related Tet repressor genes. Nucleic Acids Res. 1984 Oct 25;12(20):7693–7703. doi: 10.1093/nar/12.20.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray L. V., Jr, Jorgensen R. A., Reznikoff W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J Bacteriol. 1981 Aug;147(2):297–304. doi: 10.1128/jb.147.2.297-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Iwasaki-Ohba Y., Ono N., Kaneko-Ohdera M., Sawai T. Stoichiometry of metal-tetracycline/H+ antiport mediated by transposon Tn10-encoded tetracycline resistance protein in Escherichia coli. FEBS Lett. 1991 May 6;282(2):415–418. doi: 10.1016/0014-5793(91)80527-a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Udagawa T., Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990 Mar 25;265(9):4809–4813. [PubMed] [Google Scholar]



