Abstract
Exercise increases AMPK (AMP-activated protein kinase) activity in human and rat adipocytes, but the underlying molecular mechanisms and functional consequences of this activation are not known. Since adrenaline (epinephrine) concentrations increase with exercise, in the present study we hypothesized that adrenaline activates AMPK in adipocytes. We show that a single bout of exercise increases AMPKα1 and α2 activities and ACC (acetyl-CoA carboxylase) Ser79 phosphorylation in rat adipocytes. Similarly to exercise, adrenaline treatment in vivo increased AMPK activities and ACC phosphorylation. Pre-treatment of rats with the β-blocker propranolol fully blocked exercise-induced AMPK activation. Increased AMPK activity with exercise and adrenaline treatment in vivo was accompanied by an increased AMP/ATP ratio. Adrenaline incubation of isolated adipocytes also increased the AMP/ATP ratio and AMPK activities, an effect blocked by propranolol. Adrenaline incubation increased lipolysis in isolated adipocytes, and Compound C, an AMPK inhibitor, attenuated this effect. Finally, a potential role for AMPK in the decreased adiposity associated with chronic exercise was suggested by marked increases in AMPKα1 and α2 activities in adipocytes from rats trained for 6 weeks. In conclusion, both acute and chronic exercise are significant regulators of AMPK activity in rat adipocytes. Our findings suggest that adrenaline plays a critical role in exercise-stimulated AMPKα1 and α2 activities in adipocytes, and that AMPK can function in the regulation of lipolysis.
Keywords: adrenaline, AMP-activated protein kinase (AMPK), cAMP-dependent protein kinase (PKA), exercise, fatty acid synthesis, lipolysis
Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-amino-4-imidazolecarboxamide riboside; AMPK, AMP-activated protein kinase; CaMKK, Ca2+/calmodulin-dependent protein kinase kinase; CREB, cAMP-response-element-binding protein; HRP, horseradish peroxidase; HSL, hormone-sensitive lipase; KRB, Krebs–Ringer bicarbonate; PKA, cAMP-dependent protein kinase
INTRODUCTION
It is widely accepted that physical exercise is an efficient way to reduce visceral fat, the fat depot that is thought to contribute to obesity-associated complications such as Type 2 diabetes and cardiovascular disease. A single bout of exercise decreases fatty acid synthesis and increases lipolysis in adipocytes [1,2], suggesting that regular exercise training reduces adiposity by repeated activation of the lipolytic process and the reduction of fatty acid synthesis [3]. The underlying molecular mechanisms by which an acute bout of exercise decreases fatty acid synthesis and increases lipolysis in adipocytes are not fully understood.
Physical exercise increases the concentration of several blood hormones, neurotransmitters and other circulating factors, including adrenaline (epinephrine). Adrenaline binds to and acts through the adrenergic receptor pathway, increasing lipolysis via a mechanism that involves activation of PKA (cAMP-dependent protein kinase). PKA phosphorylates HSL (hormone-sensitive lipase) at Ser563, Ser659 and Ser660 [4], two of which are critical sites for HSL activity [4]. Adrenaline also inhibits fatty acid synthesis via inhibition of ACC (acetyl-CoA carboxylase), the rate-limiting enzyme in fatty acid synthesis, although the mechanism by which adrenaline regulates ACC activity has not been elucidated.
AMPK (AMP-activated protein kinase) is a member of an energy-sensing serine/threonine protein kinase family. AMPK is activated by an increase in the AMP/ATP ratio, via a complex mechanism that involves allosteric modification, phosphorylation by one or more AMPK kinases and a decrease in phosphatase activities [5]. There have been relatively few reports of AMPK function in adipocyte metabolism, and the reports that do exist have been somewhat inconsistent. Activation of AMPK by AICAR (5-amino-4-imidazolecarboxamide riboside) in isolated fat cells has been shown to inhibit isoprenaline (isoproterenol)-induced lipolysis [6,7], suggesting that AMPK may be a negative regulator of lipolytic activity. On the other hand, AMPK activation in 3T3-L1 cells did not inhibit adrenaline-stimulated HSL activity or glycerol release [8]. Overexpression of a dominant-inhibitory mutant of AMPK in 3T3-L1 cells impaired isoprenaline-induced lipolysis, suggesting that AMPK activity is a positive regulator of lipolysis in adipocytes [9]. Furthermore, compared with wild-type mice, AMPKα2 whole-body knockout mice have greater increases in adiposity and adipocyte hypertrophy in response to high-fat feeding [10].
Previous studies have demonstrated that exercise increases AMPK in rat [11] and human [8] adipocytes. In addition, two studies have indicated that adrenergic receptor activation can stimulate AMPK in L6 cells [12] and brown adipocytes [13]. Since adrenaline is a hormone induced by exercise and acts through β-adrenergic receptors, we hypothesized that adrenaline is a mediator of exercise-induced AMPK activation in adipocytes.
In the present study, we investigated the effects of both acute exercise and chronic exercise training on AMPK activities in rat adipocytes isolated from epididymal fat pads. We also tested the hypothesis that exercise-induced increases in circulating adrenaline are responsible for increased AMPK activity in adipocytes. To study further the effects of adrenaline on adipocyte metabolism, we incubated isolated rat adipocytes in vitro and assessed the regulation of AMPK, ACC, the AMP/ATP ratio and lipolysis. Our results demonstrate that both a single bout of exercise and chronic exercise training are major regulators of AMPK activity in adipocytes, and that adrenaline plays an important role in the effects of acute exercise to activate AMPK in rat adipocytes in vivo. Furthermore, AMPK activity is necessary for maximal activation of adrenaline-induced lipolysis in rat adipocytes.
MATERIALS AND METHODS
Animals
Sprague–Dawley rats were obtained from Taconic. Rats were provided with standard rodent chow and water ad libitum and were housed under normal laboratory conditions (12 h light/12 h dark cycle). All experimental procedures were conducted in accordance with NIH (National Institutes of Health) guidelines and approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center.
Acute exercise experiments
Male and female rats weighing ∼150 g were used for the acute exercise studies. Rats were familiarized with the treadmill (Quinton model 42) by running at a slow pace for 5–10 min on the 2 days before the experiment. The exercised groups performed steady-state treadmill running at 20 m/min up a 12% gradient for 15, 30 or 60 min, and additional animals were exercised for 60 min and were killed 60 min later (post-exercise group). Control rats participated in treadmill familiarization, but were not exercised on the day of the experiment. Rats were killed via decapitation and exsanguinated. Epididymal (male) or parametrial (female) fat pads were separated rapidly, removed and snap-frozen in liquid nitrogen until subsequent processing and analyses (see below).
Adrenaline treatment in vivo
Male rats weighing ∼150 g were used for the adrenaline studies. At 15 min after the injection of either saline or propranolol (2 mg/100 g of body mass), rats were injected intraperitoneally with either vehicle (1 mg/ml of ascorbic acid) or adrenaline (25 μg/100 g of body mass). At 15 min after the injection of the hormones, animals were killed by decapitation, and epididymal fat pads were removed immediately and frozen in liquid nitrogen.
Exercise training
Female rats initially weighing 75–100 g were purchased from Taconic. Female rats were used for these experiments because they maintain body mass better than males when undergoing exercise training protocols (M. F. Hirshman and L. J. Goodyear, unpublished work). The training protocol used in this study has been described previously [14,15]. Briefly, animals were maintained on a 12 h light/12 h dark cycle and were fed on standard laboratory chow and water ad libitum. Rats (n=12) were housed individually in plastic cages and divided into two groups that were counterbalanced by body mass and blood glucose concentrations. Animals were trained on the treadmill twice a day (09:00–10:00 h and 16:00–17:00 h), 6 days/week for 6 weeks. Initial training was at 16 m/min, 15% gradient for 12 min and gradually increased to 32 m/min for 60 min per session twice a day. We separated the training sessions to prevent undue stress on the animals, and because we have found in preliminary studies that this protocol results in optimal training adaptations in skeletal muscle and fat. Food consumption and body mass were measured once a week. In order to differentiate the effects of acute exercise from chronic adaptations to training, rats were killed ∼30 h following the final training session. Parametrial fat pads were snap-frozen in liquid nitrogen and stored at −80 °C until being processed as described below.
Preparation of isolated adipose cells
Male rats weighing ∼120 g were used to prepare isolated adipocytes. Immediately after rats were killed, epididymal adipose tissues were removed and adipose cells were isolated by the method of Rodbell [16] as modified by Cushman [17] using crude collagenase (Worthington Biochemical Corp.). All incubations were carried out in KRB (Krebs–Ringer bicarbonate) buffer, pH 7.4, at 37 °C. Cells from each group were stabilized by incubation for 1 h before treatment. Isolated fat cells were stimulated with 0.1 μg/ml adrenaline for 10 min. To inhibit α- or β-adrenergic receptors, cells were pre-incubated with 100 μM phentolamine (Sigma–Aldrich) or 5 μM propranolol for 15 min respectively.
Lipolysis measurements
Adipose cells were distributed among 20 ml plastic incubation vials to provide a final incubation volume of 1.5 ml of KRB–Hepes buffer containing isolated cells and 1 mg/ml glucose in the absence or presence of 0.1 μg/ml adrenaline (Sigma–Aldrich). To inhibit AMPK, cells were pre-incubated with 50 μM Compound C (Calbiochem) for 15 min before the addition of adrenaline. The final cell concentration was 105 cells/ml. All incubations were carried out in triplicate, and all analyses were corrected for the appropriate blank values obtained from samples of cells incubated for 1 min in an ice bath. At the end of the 30-min incubation, a 0.5 ml sample of cell-free medium was aspirated from each tube and chilled in an ice bath. Glycerol was assayed enzymatically in 0.2 ml of the cell-free medium [18].
Isoform-specific AMPK activity
Fat pads obtained from rats studied in vivo and adipocytes incubated in vitro were homogenized as described previously [19]. Lysates (100 μg) were immunoprecipitated with specific antibodies against the α1 and α2 catalytic subunits of AMPK [20] and Protein A beads (Pierce Biotechnology). Reactions were carried out using the synthetic SAMS peptide (HMRSAMSGLHLVKRR) as substrate, as described previously [20]. AMPK activity is expressed as ATP incorporation in pmol/min per mg of protein.
Western blot analysis and antibodies
Standard Western blot analyses were used to assess protein and phosphorylation levels of various molecules. Immunoblots were developed using ECL® (enhanced chemiluminescence) reagents (Amersham Biosciences) and quantified using FluorChem version 2.01 (Alpha Innotech). Primary antibodies purchased from commercial sources included those against AMPK-Thr172, CREB (cAMP-response-element-binding protein)-Ser133 and HSL-Ser563 (Cell Signaling Technology), AMPKα1, AMPKα2, ACC and ACC-Ser79 (Upstate), CaMKK (Ca2+/calmodulin-dependent protein kinase kinase; BD Biosciences), and LKB1 (Santa Cruz Biotechnology). Secondary antibodies used were HRP (horseradish peroxidase)-conjugated anti-rabbit (Amersham Biosciences), HRP-conjugated anti-mouse (Upstate) and HRP-conjugated anti-goat (Promega).
Nucleotide extraction and analysis
To measure nucleotide concentrations, isolated adipocytes and epididymal fat pads were frozen, pulverized and homogenized with 0.6 M perchloric acid. After neutralization and centrifugation at 5500 g for 2 min, the supernatant was filtered and used for measuring ATP, ADP and AMP content. Adenine nucleotide content was determined by HPLC (Waters) and normalized to mg of protein [21].
Statistical analysis
Data are means±S.E.M. All data were compared using Student's t test or one-way ANOVA. The differences between groups were considered to be significant when P<0.05.
RESULTS
Activation of AMPK in rat adipocytes by acute exercise and adrenaline
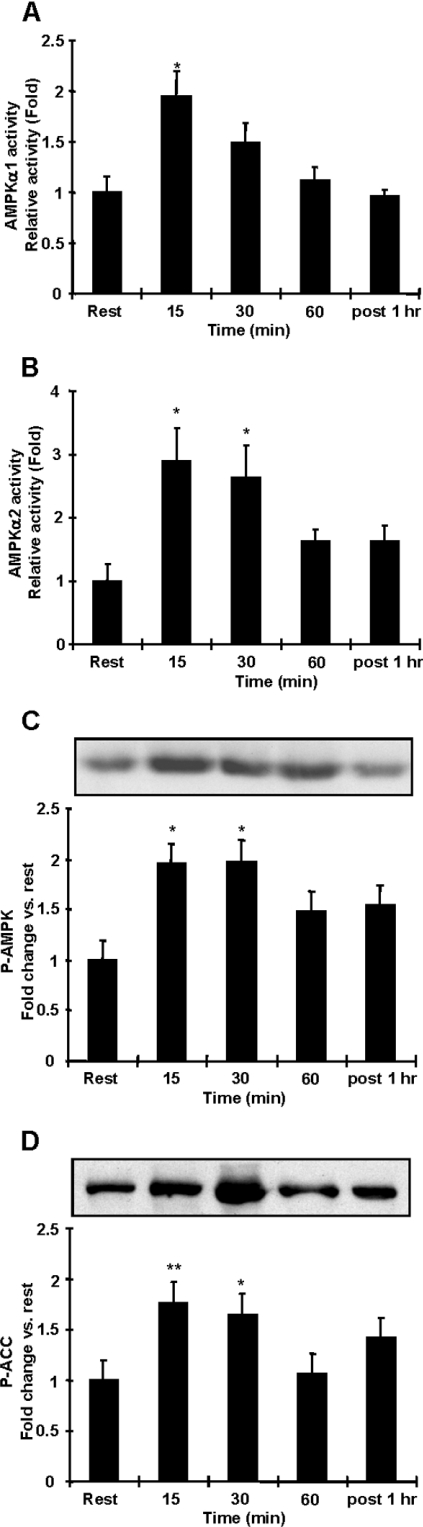
To determine the effects of acute exercise on AMPK activity in adipocytes, rats performed treadmill running exercise at 20 m/min for 15, 30 or 60 min, up a 12% gradient. This short-term exercise had no effect on body mass and epididymal fat pad mass (results not shown). AMPKα1 and α2 activities were increased with 15 and 30 min of exercise 1.5–2-fold and 2.6–2.9-fold respectively (Figures 1A and 1B). After 60 min of exercise, and 1 h after a 60 min exercise bout, AMPKα1 and α2 activities were no longer elevated (Figures 1A and 1B). Consistent with AMPK activity results, AMPK Thr172 phosphorylation was significantly increased after 15 and 30 min of exercise, with no effect at 60 min of exercise (Figure 1C). Downstream of AMPK, phosphorylation of ACC at the inhibitory Ser79 site was also significantly increased with 15 and 30 min of exercise (Figure 1D). These results suggest that a single bout of exercise can increase the activities of both the α1 and α2 catalytic isoforms of AMPK in rat adipocytes, but that this effect is diminished with longer periods of exercise.
Figure 1. Acute exercise increases AMPKα1 and AMPKα2 activities in rat adipocytes.
(A and B) Time course of AMPKα1 (A) and AMPKα2 (B) activity in epididymal fat from rats exercised on a treadmill (22 m/min, 12% gradient) for 15, 30 or 60 min, or exercised for 60 min followed by 60 min of rest. AMPKα1 or AMPKα2 was immunoprecipitated from epididymal fat lysates and used in an in vitro kinase assay with SAMS peptide as substrate. Both AMPKα1 and α2 activities were increased with 15 and 30 min of exercise with no change with 60 min of exercise. (C) Western blot analysis of epididymal fat lysates show that AMPK Thr172 phosphorylation was increased with 15 and 30 min of exercise. (D) There were significant increases of ACC Ser79 phosphorylation with 15 and 30 min exercise. Results are means±S.E.M.; n=5 per group. *P<0.05 and **P<0.01 compared with the resting group.
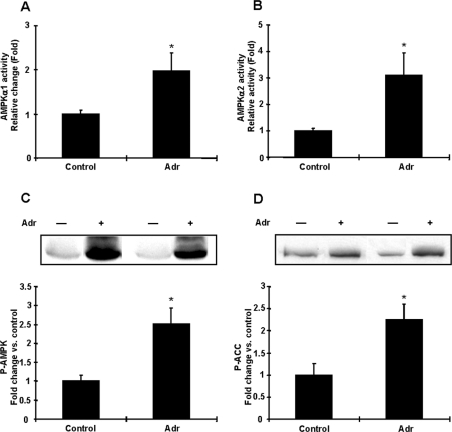
It is well established that adrenaline concentrations in the blood increase in response to exercise [22] and treadmill exercise for 15 and 30 min increases plasma adrenaline in rats [23,24], and that this in turn activates adrenergic receptor signalling. To begin to address the hypothesis that adrenaline may mediate AMPK activation in adipocytes during exercise, we next determined whether adrenaline activates AMPK in vivo, by injecting adrenaline (25 μg/100 g of body mass) and taking fat pads 15 min later. Adrenaline increased AMPK α1 and α2 activities in adipocytes 2–3.1-fold, respectively (Figures 2A and 2B). Likewise, AMPK Thr172 and ACC Ser79 phosphorylation were significantly increased by adrenaline treatment (Figures 2C and 2D). These results demonstrate that adrenaline can also activate AMPK in rat adipocytes in vivo.
Figure 2. Effects of adrenaline on AMPK activity in rat adipocytes in vivo.
Animals were injected with vehicle or adrenaline (Adr) (25 μg/100 g of body mass) and 15 min after injection, epididymal fat pads were removed and assayed for AMPK activity. (A and B) Both AMPKα1 (A) and AMPKα2 (B) activities were increased by an adrenaline injection. (C and D) Adrenaline increased phosphorylation of AMPK (C) and ACC (D) in vivo. Results are means±S.E.M.; n=4–5 per group. *P<0.05 compared with control.
Exercise-induced AMPK activation is mediated by the β-adrenergic receptor
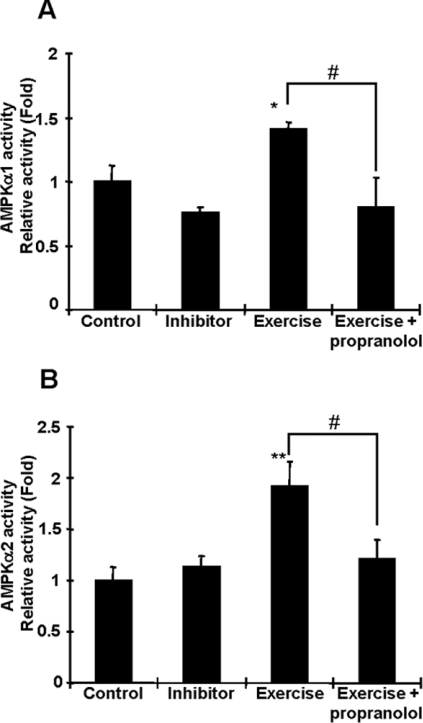
We next tested the hypothesis that β-adrenergic receptors are required for the effects of exercise on AMPK activation in adipocytes. Since the β-adrenergic receptor is predominantly expressed in adipocytes [25], we injected rats with propranolol, a β-adrenergic blocker, or vehicle, 15 min before the start of a 15 min bout of treadmill exercise. Rats treated with propranolol had no difficulty in completing the exercise bout, and the exercise-induced activation of AMPK was completely blunted by this β-adrenergic blockade (Figures 3A and 3B). These results suggest that adrenaline, which is increased by an acute bout of exercise and activates β-adrenergic receptors, could be a critical candidate for exercise-induced AMPK activation.
Figure 3. Propranolol abolishes exercise-induced AMPK activation in adipocytes.
Rats were injected with propranolol (1 mg/100 g of body mass) or vehicle 15 min before 15 min of treadmill exercise. Exercise-induced AMPKα1 (A) and AMPKα2 (B) activations were completely blunted by β-adrenergic blockade injection. Results are means±S.E.M.; n=4–5 per group. *P<0.05 and **P<0.01 compared with the control. #P<0.05 compared with the exercised group.
Exercise and adrenaline increase AMP concentrations in adipocytes
Increases in AMP concentrations in cells can allosterically activate AMPK, resulting in phosphorylation of the Thr172 domain of the α subunit by upstream kinases, and inhibiting dephosphorylation of Thr172 by protein phosphatases [5]. To address whether exercise- and adrenaline-stimulated AMPK activation are associated with altered AMP content, we measured AMP, ADP and ATP concentrations in adipocytes. As shown in Table 1, treadmill exercise for 15 min, which significantly increased AMPK activity, tended to decrease ATP and increase ADP concentrations, and significantly increased AMP content and the AMP/ATP ratio. An intraperitoneal injection of adrenaline in vivo significantly decreased the concentration of ATP in adipocytes and increased ADP and ATP concentrations 1.6–4.5-fold, resulting in a 6.2-fold increase in the AMP/ATP ratio.
Table 1. Adenine nucleotides in isolated fat cells and rat epididymal fat pads.
Results are means±S.E.M., n=4/group. *P<0.05, **P<0.01 and ***P<0.001 compared with control.
| ATP (nmol/mg of protein) | ADP (nmol/mg of protein) | AMP (nmol/mg of protein) | AMP/ATP | |
|---|---|---|---|---|
| Epididymal fat pads | ||||
| Control | 11.21±0.70 | 2.85±0.17 | 1.20±0.14 | 0.110±0.018 |
| Propranolol | 11.04±0.42 | 3.81±0.64 | 1.33±0.34 | 0.118±0.027 |
| Adrenaline | 07.22±0.59** | 4.67±0.53* | 5.37±1.45* | 0.787±0.230* |
| Adrenaline+ | 11.38±1.03 | 4.79±0.44** | 2.86±0.46* | 0.265±0.060* |
| propranolol | ||||
| Exercise | 10.85±1.44 | 3.59±0.35 | 1.94±0.17* | 0.186±0.024* |
| Isolated fat cells | ||||
| Control | 9.10±0.80 | 1.43±0.30 | 0.38±0.09 | 0.041±0.005 |
| Adrenaline | 5.48±0.26** | 1.94±0.12 | 0.75±0.04** | 0.137±0.004*** |
Adrenaline increases AMPK activity and the AMP/ATP ratio in isolated adipocytes
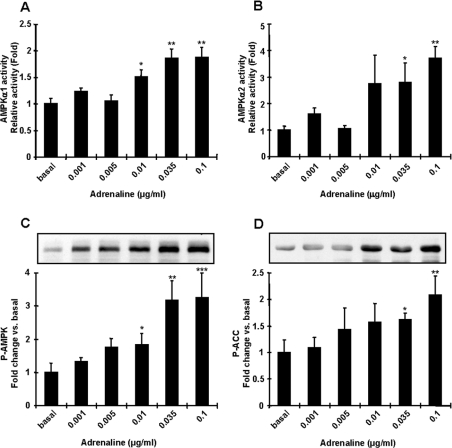
To understand further the mechanisms by which adrenaline regulates AMPK and the metabolic consequences, we next used incubated adipocytes derived from epididymal fat. At lower doses of adrenaline (0.001–0.005 μg/ml), similar to what occurs in the resting state [26–29], adrenaline had no effect on AMPKα1 or α2 activities (Figures 4A and 4B). Adrenaline at high doses, including the physiological range that would occur during in vivo exercise [29,30], increased AMPKα1 and α2 activities up to ∼4-fold above basal (Figures 4A and 4B). The effect of adrenaline on AMPK activation was dose-dependent, with maximum activation at 0.1 μg/ml (Figures 4A and 4B). AMPK Thr172 phosphorylation followed a similar pattern to AMPK activity (Figure 4C), and ACC Ser79 phosphorylation was also significantly increased with adrenaline (Figure 4D).
Figure 4. Adrenaline activates AMPK and ACC phosphorylation in isolated fat cells.
Isolated fat cells from rats were treated with different doses of adrenaline for 10 min. Extracts were prepared and used in an in vitro AMPK activity assay (A and B) or Western blot analyses using anti-AMPK Thr172 and anti-ACC Ser79 antibodies (C and D). Both AMPKα1 (A) and AMPKα2 (B) activities were significantly increased by adrenaline treatment. Phosphorylation of AMPK Thr172 (C) and ACC Ser79 (D) were significantly increased with adrenaline treatment. Results are means±S.E.M.; n=3–6 per group. *P<0.05, **P<0.01 and ***P<0.001 compared with basal.
To determine whether adrenaline-stimulated AMPK activation is associated with altered nucleotide content, we measured concentrations of AMP, ADP and ATP in adipocytes incubated with adrenaline. As shown in Table 1, adrenaline decreased ATP concentrations in the isolated fat cells by 40% and increased the concentration of AMP 2-fold, resulting in a 3.3-fold increase in the AMP/ATP ratio (Table 1). These results suggest that adrenaline and exercise alter adenine nucleotide content in adipocytes, making it likely that this is the mechanism for AMPK activation.
Activation of AMPK by adrenaline is mediated by the β-adrenergic receptor
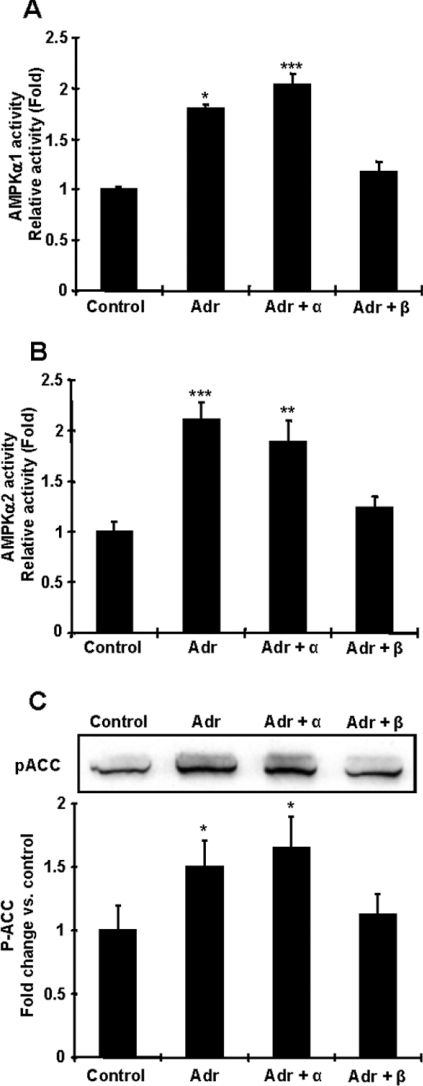
To elucidate whether the effects of adrenaline on AMPK activation are mediated by adrenergic receptor signalling, isolated fat cells were pre-incubated in the presence or absence of α- and β-adrenergic blockades. The α-adrenergic receptor inhibitor had no effect on the ability of adrenaline to stimulate AMPKα1 and α2 activities (Figure 5A), whereas β-adrenergic receptor blockade fully inhibited adrenaline-stimulated AMPKα1 and AMPKα2 activities (Figure 5B). Increases in ACC Ser79 phosphorylation were also completely blunted by β-adrenergic receptor inhibition (Figure 5C).
Figure 5. Adrenaline increases AMPK activity in isolated fat cells through the β-adrenergic receptor pathway.
Isolated fat cells were stimulated with 0.1 μg/ml adrenaline (Adr) for 10 min. To inhibit the α- or β-adrenergic receptor pathway, cells were pre-incubated with 100 μM phentolamine or 5 μM propranolol for 15 min respectively. (A and B) The effect of adrenaline on AMPK activation was abolished by the pre-incubation with propranolol but not phentolamine. (C) The effects of adrenaline on ACC Ser79 phosphorylation were blunted by β-adrenergic blockade, whereas α-adrenergic receptor blockade had no effect. Results are means±S.E.M.; n=3–6 per group. *P<0.05, **P<0.01 and ***P<0.001 compared with control.
AMPK is necessary for maximal activation of lipolysis induced by adrenaline
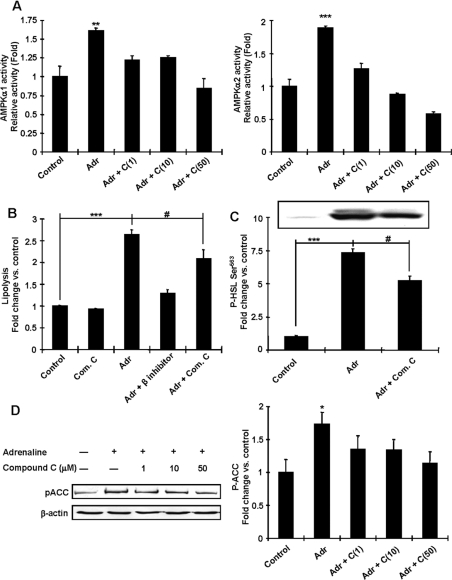
To determine whether AMPK activation mediates adrenaline's effects on lipolysis, we examined lipolytic activity in isolated rat adipocytes. For these studies we used Compound C, which is a potent inhibitor of AMPK [31], and was effective in inhibiting adrenaline-stimulated AMPKα1 and α2 activities in the incubated adipocytes (Figure 6A). Incubation of the control adipocytes with Compound C did not alter lipolysis (Figure 6B). Incubation of isolated adipocytes with adrenaline increased lipolysis 2.6-fold above control, an effect that was completely abolished by propranolol (Figure 6B). Compound C partially inhibited the adrenaline-induced increase in lipolysis (Figure 6B). Phosphorylation of HSL Ser563, which is stimulated by PKA [32], was increased by adrenaline, while compound C treatment blunted this phosphorylation (Figure 6C). The mechanism for this effect is not known, but since CREB phosphorylation at Ser133 (a PKA substrate) was not altered by Compound C pre-treatment (results not shown), it is unlikely to be due to decreases in PKA activity. Compound C was effective in inhibiting adrenaline-stimulated ACC Ser79 phosphorylation, a key enzyme for the regulation of fatty acid synthesis and a downstream substrate of AMPK (Figure 6D). These data suggest that AMPK activation plays an important role in the stimulation of maximal adrenaline-induced lipolysis.
Figure 6. AMPK is necessary for adrenaline-induced lipolysis and inhibition of fatty acid synthesis in isolated adipocytes.
Isolated adipocytes were incubated with adrenaline (Adr) for 30 min in the presence or absence of inhibitors as indicated for 15 min. (A) Adrenaline-induced AMPK activation was blunted by Compound C (C), a known AMPK inhibitor. (B) Released glycerol was measured to assess the rate of lipolysis. Adrenaline increased lipolysis 2.6-fold, but inhibition of AMPK achieved by Compound C (Com. C) treatment (50 μM) blunted this activation. (C) Phosphorylation of HSL Ser563 was increased by adrenaline, whereas pre-treatment with Compound C (Com. C) blunted this phosphorylation. (D) Phosphorylation of ACC Ser79 was increased by adrenaline, whereas co-incubation with Compound C (C) decreased this phosphorylation compared with adrenaline treatment alone. Results are means±S.E.M., and the blots shown are representative of n=3 per group. *P<0.05, **P<0.01 and ***P<0.001 compared with the control. #P<0.05 compared with the indicated control group.
Exercise training increases AMPK activities in adipocytes
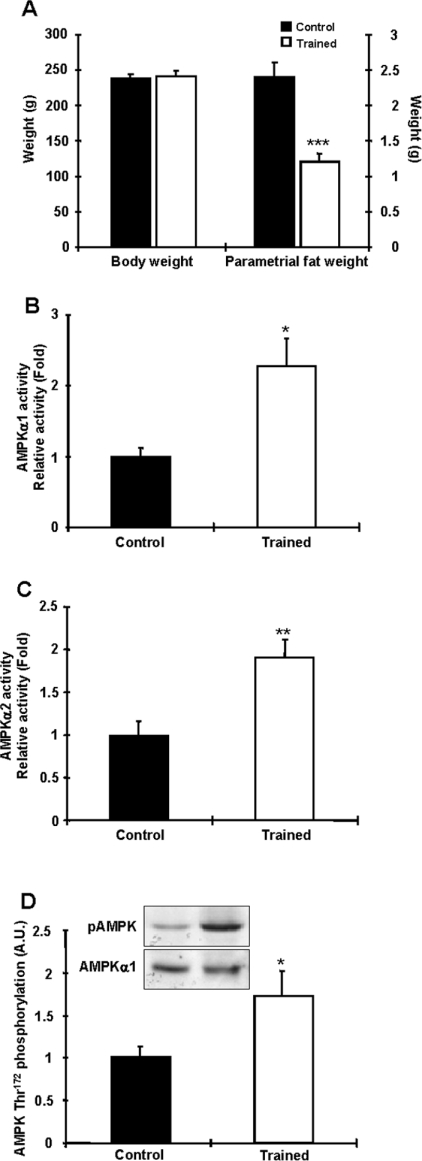
To determine the effects of long-term chronic exercise on AMPK activity and expression in adipocytes, rats were trained using the protocol described in the Materials and methods section. At the end of the 6 weeks, there was no difference in body mass between controls and trained rats, but trained rats had a ∼50% decrease in parametrial fat pad mass (Figure 7A). Food consumption during training was not different between the two groups (data not shown). Training increased AMPKα1 activity 2.3-fold over controls in the adipocytes, with no change in total AMPKα1 expression (Figure 7B). Similarly, AMPKα2 activity was increased 1.9-fold in the trained animals, with no change in AMPKα2 protein (Figure 7C). AMPK Thr172 phosphorylation was also significantly increased in the trained rats (Figure 7D). Thus both short-term and chronic exercise are major regulators of AMPK activity in adipocytes.
Figure 7. Chronic exercise increases AMPKα1 and AMPKα2 activities in rat adipocytes.
(A) Exercise training for 6 weeks efficiently reduced fat mass without altering body mass. (B and C) AMPKα1 (B) and AMPKα2 (C) activities in epididymal fat from rats trained for 6 weeks and allowed to rest for 36 h before study. Both AMPKα1 and AMPKα2 activities were increased in trained rats. (D) AMPK Thr172 phosphorylation was increased in trained rats. Results are means±S.E.M.; n=6 per group. *P<0.05, **P<0.01 and ***P<0.001 compared with the control. A.U., arbitrary units.
DISCUSSION
The effects of chronic exercise training to reduce adiposity in humans results from an overall increase in energy expenditure, due largely to the increased energy demands of the working skeletal muscles during each individual exercise bout. During aerobic exercise, the contracting skeletal muscles require increased carbohydrate and fat, the latter fuel derived almost exclusively from adipocyte lipolysis. Adrenaline has long been known to be an important mediator of exercise-stimulated adipocyte lipolysis, and the results of the present study suggest that AMPK may be an intermediate for this effect. The β-blocker propranolol fully inhibited the increase in AMPK activity and phosphorylation in adipocytes of exercised rats. Furthermore, injection of adrenaline in vivo mimicked the effects of exercise to increase AMPK activity and phosphorylation. Although we cannot fully rule out the possibility that other circulating factors (e.g. hormones and noradrenaline), which are also increased by physical exercise and act through β-adrenergic receptors, are contributing to the effects of exercise on AMPK activity in adipocytes, our data strongly support a primary role for circulating adrenaline.
If adrenaline is mediating the effects of acute exercise to increase AMPK activity, and adrenaline itself increases AMPK activity, what is the mechanism for the adrenaline-induced increases in AMPK activity? Our findings suggest that increases in AMPK activity with exercise and adrenaline in vivo and adrenaline in the isolated adipocytes are due, at least in part, to increases in cellular AMP concentrations. Increases in adrenaline-mediated AMP concentrations are consistent with studies in human adipocytes showing that increases in cAMP and adenine nucleotide catabolism by adrenaline or isoprenaline are associated with an enhanced release of purines in the form of inosine and hypoxanthine [33,34]. Collectively, adrenaline seems to decrease ATP and increase AMP due to increased ATP utilization and adenine nucleotide breakdown, which results in increases in the AMP/ATP ratio, a critical regulator for AMPK activation [35].
Exercise and adrenaline increased Ser79 phosphorylation of ACC (Figures 1D and 2D), which in turn decreased ACC activity, and adrenaline-induced ACC Ser79 phosphorylation was abolished by Compound C treatment, suggesting that adrenaline increased ACC Ser79 phosphorylation by AMPK. It has been reported that PKA, which is activated by adrenaline, also phosphorylates ACC, but this phosphorylation is not necessary for ACC activity [36]. Although we cannot exclude the possibility that ACC phosphorylation by PKA contributes to adrenaline-induced ACC activity, it is apparent that AMPK is a major regulator of ACC activity in adrenaline-induced ACC Ser79 phosphorylation.
A previous study has reported that 30 min of treadmill exercise increases total AMPK activity 1.5-fold in rat adipocytes [11]. Our findings demonstrate that a single bout of exercise increases the activity of both the AMPKα1 and AMPKα2 catalytic subunits, as well as AMPK Thr172 and ACC Ser79 phosphorylation approx. 2–3-fold. We also show that AMPK activation and phosphorylation occur with only 15 min of exercise, but are not maintained with longer periods of exercise or in the post-exercise period. Interestingly, in the skeletal muscle of these same animals, AMPKα2 activity and AMPK Thr172 phosphorylation are increased with 60 min of exercise (H.-J. Koh and L. J. Goodyear, unpublished work). Thus it appears that a single bout of exercise induces a transient activation of AMPK in adipocytes, whereas effects in skeletal muscle are more prolonged. AMPK in adipocytes may be turned on rapidly and transiently because AMPK may function to regulate one or more aspects of the lipolytic machinery in priming fashion. Thus, if AMPK primes adipocytes for lipolysis, i.e. phosphorylates HSL and promotes its translocation into lipid droplets [9], sustained activation of AMPK may not be needed when exercise continues for periods longer than 30 min.
Adipocytes are important organs in which to store excess energy as triacylglycerols and provide energy to skeletal muscles in the form of unbound (‘free’) fatty acids during conditions of increased energy demand such as exercise [37]. The role of AMPK in adipocytes is poorly understood, most notably whether AMPK acts as a pro- or anti-lipolytic signal. Previous reports suggested that the role of AMPK in adipocytes is to function as an anti-lipolytic signal [6,38,39]. In the present study, we found that modulation of AMPK activity by AICAR or Compound C did not affect lipolysis as well as HSL Ser563 phosphorylation (results not shown and Figure 6B). However, inhibition of AMPK by Compound C partially blunted adrenaline-induced lipolysis and HSL phosphorylation (Figures 6B and 6C), suggesting that AMPK is necessary for adrenaline-induced lipolysis. The reasons for the differences in the role of AMPK activation on lipolysis in adipocytes between previous studies and the present study are not clear. However, two studies have recently reached the same conclusion for the function of AMPK as a pro-lipolytic signal [8,9]. Furthermore, the effects of AMPK on HSL activity in a tissue-specific manner have been investigated [8]. HSL activity was reduced in L6 myotubes expressing constitutively active AMPK, while AMPK activation did not prevent HSL activity or glycerol release in 3T3-L1 adipocytes.
Exercise training results in enhanced sensitivity to hormones that control adipocyte lipolysis [40,41]. If AMPK is an important mediator of lipolysis with exercise, then one would predict that AMPK activity would be increased with chronic exercise training. In fact, we found a 2-fold increase in the activity of both AMPK isoforms, as well as a significant increase in AMPK Thr172 phosphorylation in rats trained for 6 weeks. Interestingly, the increase in activity was not accompanied by an increase in the expression of the AMPK catalytic subunits. In addition, training did not result in significant changes in the protein content of LKB1 and CaMKK, known upstream kinases for AMPK ([42–47], and H.-J. Koh and L. J. Goodyear, unpublished work). Determination of the underlying mechanism is currently ongoing in our laboratory. Thus adipocytes adapt to changes in training status by increasing AMPK activity, a factor that may play a role in the effects of training to increase lipolysis. The effect of training to increase adipocyte AMPK activity is different from that which occurs in skeletal muscle, where previous work has shown no change in AMPK activity in response to endurance exercise training [48,49]. Similarly to published reports, AMPK activity in multiple skeletal muscles were not increased in the animals used in the present study (H.-J. Koh and L. J. Goodyear, unpublished work). This demonstrates that adipocytes and skeletal muscle adapt differently to training, and point to differing functions of AMPK in these tissues.
In summary, we show that acute exercise and adrenaline activate AMPK activity in adipocytes. Inhibition of the β-adrenergic receptor abolished the effects of exercise and adrenaline on AMPK activity in adipocytes. We also demonstrate that adrenaline and a single bout of exercise increase the AMP/ATP ratio in adipocytes. Activation of AMPK by adrenaline contributes to maximally activate lipolysis and phosphorylate ACC. All these data suggest that adrenaline is a major molecule for acute exercise-induced AMPK activation, and probably contributes to the benefits of exercise to reduce adiposity. Fully elucidating the molecular signals that mediate the effects of exercise to activate lipolysis has important medical relevance and could provide clues in the development of anti-obesity agents.
Acknowledgments
We thank Dr Naoko Mukai, Jennifer Wu and Lauren E. Peter for assistance with experiments, and Dr Nobuharu Fujii and Dr Richard C. Ho for valuable advice. This work was supported by NIH grants to L.J.G. (AR45670 and DK068626), the DERC (Diabetes and Endocrinology Research Center) at the Joslin Diabetes Center (DK36836), and to J.A.B. (HL 46033). H.-J.K. is supported by a Mary K. Iacocca Fellowship from the Joslin Diabetes Center.
References
- 1.Horowitz J. F., Klein S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000;72:558S–563S. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- 2.Richard D., Trayhurn P. Effect of exercise training on the rates of fatty acid synthesis in mice. Can. J. Physiol. Pharmacol. 1984;62:695–699. doi: 10.1139/y84-114. [DOI] [PubMed] [Google Scholar]
- 3.Sigal R. J., Kenny G. P., Wasserman D. H., Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27:2518–2539. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 4.Holm C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003;31:1120–1124. doi: 10.1042/bst0311120. [DOI] [PubMed] [Google Scholar]
- 5.Kahn B. B., Alquier T., Carling D., Hardie D. G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett. 1994;353:33–36. doi: 10.1016/0014-5793(94)01006-4. [DOI] [PubMed] [Google Scholar]
- 7.Corton J. M., Gillespie J. G., Hawley S. A., Hardie D. G. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 8.Watt M. J., Holmes A. G., Pinnamaneni S. K., Garnham A. P., Steinberg G. R., Kemp B. E., Febbraio M. A. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006;290:E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 9.Yin W., Mu J., Birnbaum M. J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003;278:43074–43080. doi: 10.1074/jbc.M308484200. [DOI] [PubMed] [Google Scholar]
- 10.Villena J. A., Viollet B., Andreelli F., Kahn A., Vaulont S., Sul H. S. Induced adiposity and adipocyte hypertrophy in mice lacking the AMP-activated protein kinase-α2 subunit. Diabetes. 2004;53:2242–2249. doi: 10.2337/diabetes.53.9.2242. [DOI] [PubMed] [Google Scholar]
- 11.Ruderman N. B., Park H., Kaushik V. K., Dean D., Constant S., Prentki M., Saha A. K. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol. Scand. 2003;178:435–442. doi: 10.1046/j.1365-201X.2003.01164.x. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson D. S., Bengtsson T. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by α1-adrenoceptors causing glucose uptake. Diabetes. 2006;55:682–690. doi: 10.2337/diabetes.55.03.06.db05-0901. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson D. S., Chernogubova E., Dallner O. S., Cannon B., Bengtsson T. β-Adrenoceptors, but not α-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 2005;48:2386–2395. doi: 10.1007/s00125-005-1936-7. [DOI] [PubMed] [Google Scholar]
- 14.Goodyear L. J., Hirshman M. F., Knutson S. M., Horton E. D., Horton E. S. Effect of exercise training on glucose homeostasis in normal and insulin-deficient diabetic rats. J. Appl. Physiol. 1988;65:844–851. doi: 10.1152/jappl.1988.65.2.844. [DOI] [PubMed] [Google Scholar]
- 15.Tancrede G., Rousseau-Migneron S., Nadeau A. Beneficial effects of physical training in rats with a mild streptozotocin-induced diabetes mellitus. Diabetes. 1982;31:406–409. doi: 10.2337/diab.31.5.406. [DOI] [PubMed] [Google Scholar]
- 16.Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 17.Cushman S. W. Structure–function relationship in the adipose cell. I. Ultrastructure of the isolated adipose cell. J. Cell Biol. 1970;46:326–341. doi: 10.1083/jcb.46.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wieland O. Glycerol UV-Method/Metabolites: Carbohydrate Metabolism in Methods of Enzymatic Analysis. In: Bergmeyer H. U., editor. New York: Academic Press; 1974. pp. 1448–1453. [Google Scholar]
- 19.Fujii N., Boppart M. D., Dufresne S. D., Crowley P. F., Jozsi A. C., Sakamoto K., Yu H., Aschenbach W. G., Kim S., Miyazaki H., et al. Overexpression or ablation of JNK in skeletal muscle has no effect on glycogen synthase activity. Am. J. Physiol. Cell. Physiol. 2004;287:C200–C208. doi: 10.1152/ajpcell.00415.2003. [DOI] [PubMed] [Google Scholar]
- 20.Musi N., Fujii N., Hirshman M. F., Ekberg I., Froberg S., Ljungqvist O., Thorell A., Goodyear L. J. AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes. 2001;50:921–927. doi: 10.2337/diabetes.50.5.921. [DOI] [PubMed] [Google Scholar]
- 21.Bak M. I., Ingwall J. S. NMR-invisible ATP in heart: fact or fiction? Am. J. Physiol. 1992;262:E943–E947. doi: 10.1152/ajpendo.1992.262.6.E943. [DOI] [PubMed] [Google Scholar]
- 22.Galbo H. Hormonal and Metabolic Adaptation to Exercise. Thieme, New York; 1982. [Google Scholar]
- 23.Carlson K. I., Marker J. C., Arnall D. A., Terry M. L., Yang H. T., Lindsay L. G., Bracken M. E., Winder W. W. Epinephrine is unessential for stimulation of liver glycogenolysis during exercise. J. Appl. Physiol. 1985;58:544–548. doi: 10.1152/jappl.1985.58.2.544. [DOI] [PubMed] [Google Scholar]
- 24.Winder W. W., Fisher S. R., Gygi S. P., Mitchell J. A., Ojuka E., Weidman D. A. Divergence of muscle and liver fructose 2,6-diphosphate in fasted exercising rats. Am. J. Physiol. 1991;260:E756–E761. doi: 10.1152/ajpendo.1991.260.5.E756. [DOI] [PubMed] [Google Scholar]
- 25.Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific β-adrenergic receptor: molecular cloning and down-regulation in obesity. J. Biol. Chem. 1991;266:24053–24058. [PubMed] [Google Scholar]
- 26.Napoli R., Gibson L., Hirshman M. F., Boppart M. D., Dufresne S. D., Horton E. S., Goodyear L. J. Epinephrine and insulin stimulate different mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Diabetes. 1998;47:1549–1554. doi: 10.2337/diabetes.47.10.1549. [DOI] [PubMed] [Google Scholar]
- 27.Hevener A. L., Bergman R. N., Donovan C. M. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes. 2000;49:8–12. doi: 10.2337/diabetes.49.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Kozyreva T. V., Tkachenko E. Y., Kozaruk V. P., Latysheva T. V., Gilinsky M. A. Effects of slow and rapid cooling on catecholamine concentration in arterial plasma and the skin. Am. J. Physiol. 1999;276:R1668–R1672. doi: 10.1152/ajpregu.1999.276.6.R1668. [DOI] [PubMed] [Google Scholar]
- 29.Miyauchi T., Maeda S., Iemitsu M., Kobayashi T., Kumagai Y., Yamaguchi I., Matsuda M. Exercise causes a tissue-specific change of NO production in the kidney and lung. J. Appl. Physiol. 2003;94:60–68. doi: 10.1152/japplphysiol.00269.2002. [DOI] [PubMed] [Google Scholar]
- 30.Romero S. M., Pereira A. F., Garofalo M. A., Hoffmann A. Effects of exercise on plasma catecholamine levels in the toad, Bufo paracnemis: role of the adrenals and neural control. J. Exp. Zoolog. A Comp. Exp. Biol. 2004;301:911–918. doi: 10.1002/jez.a.91. [DOI] [PubMed] [Google Scholar]
- 31.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degerman E., Smith C. J., Tornqvist H., Vasta V., Belfrage P., Manganiello V. C. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kather H. Purine accumulation in human fat cell suspensions: evidence that human adipocytes release inosine and hypoxanthine rather than adenosine. J. Biol. Chem. 1988;263:8803–8809. [PubMed] [Google Scholar]
- 34.Kather H. β-Adrenergic stimulation of adenine nucleotide catabolism and purine release in human adipocytes. J. Clin. Invest. 1990;85:106–114. doi: 10.1172/JCI114399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aschenbach W. G., Sakamoto K., Goodyear L. J. 5′-Adenosine monophosphate-activated protein kinase, metabolism and exercise. Sports Med. 2004;34:91–103. doi: 10.2165/00007256-200434020-00003. [DOI] [PubMed] [Google Scholar]
- 36.Winder W. W., Wilson H. A., Hardie D. G., Rasmussen C. A., Hutber C. A., Call G. B., Clayton R. D., Conley L. M., Yoon S., Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J. Appl. Physiol. 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- 37.Romijn J. A., Coyle E. F., Sidossis L. S., Gastaldelli A., Horowitz J. F., Endert E., Wolfe R. R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993;265:E380–E391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 38.Garton A. J., Campbell D. G., Carling D., Hardie D. G., Colbran R. J., Yeaman S. J. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase: a possible antilipolytic mechanism. Eur. J. Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- 39.Daval M., Diot-Dupuy F., Bazin R., Hainault I., Viollet B., Vaulont S., Hajduch E., Ferré P., Foufelle F. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J. Biol. Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 40.Enevoldsen L. H., Stallknecht B., Fluckey J. D., Galbo H. Effect of exercise training on in vivo insulin-stimulated glucose uptake in intra-abdominal adipose tissue in rats. Am. J. Physiol. Endocrinol. Metab. 2000;278:E25–E34. doi: 10.1152/ajpendo.2000.278.1.E25. [DOI] [PubMed] [Google Scholar]
- 41.Enevoldsen L. H., Stallknecht B., Langfort J., Petersen L. N., Holm C., Ploug T., Galbo H. The effect of exercise training on hormone-sensitive lipase in rat intra-abdominal adipose tissue and muscle. J. Physiol. 2001;536:871–877. doi: 10.1111/j.1469-7793.2001.t01-1-00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Makela T. P., Alessi D. R., Hardie D. G. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. Calcium (Ca2+)/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 46.Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 48.Hurst D., Taylor E. B., Cline T. D., Greenwood L. J., Compton C. L., Lamb J. D., Winder W. W. AMP-activated protein kinase kinase activity and phosphorylation of AMP-activated protein kinase in contracting muscle of sedentary and endurance trained rats. Am. J. Physiol. Endocrinol. Metab. 2005;289:E710–E715. doi: 10.1152/ajpendo.00155.2005. [DOI] [PubMed] [Google Scholar]
- 49.Durante P. E., Mustard K. J., Park S. H., Winder W. W., Hardie D. G. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am. J. Physiol. Endocrinol. Metab. 2002;283:E178–E186. doi: 10.1152/ajpendo.00404.2001. [DOI] [PubMed] [Google Scholar]