Abstract
Adiponectin is intimately involved in the regulation of insulin sensitivity, carbohydrate and lipid metabolism, and cardiovascular functions. The circulating concentration of adiponectin is decreased in obesity and Type 2 diabetes. The present study attempts to elucidate the mechanisms underlying the regulation of adiponectin secretion and expression in rat primary adipocytes. The β-agonist, isoprenaline, decreased adiponectin secretion and expression in a dose-dependent manner in primary adipocytes. Importantly, such an inhibitory effect could be blocked by insulin. The opposing effects of isoprenaline and insulin could be explained by differential regulation of intracellular cAMP levels, since cAMP analogues suppressed adiponectin secretion and expression in a fashion similar to isoprenaline, and insulin blocked the inhibitory effects of the cAMP analogue hydrolysable by PDE (phosphodiesterase). A specific PDE3 inhibitor, milrinone, and PI3K (phosphoinositide 3-kinase) inhibitors abolished the effects of insulin on adiponectin secretion and expression. In the same studies, leptin secretion and expression displayed a similar pattern of regulation to adiponectin. We conclude that insulin and β-agonists act directly at the adipocytes in opposing fashions to regulate the production of adiponectin and leptin, and that a PI3K-PDE3B-cAMP pathway mediates the effects of insulin to restore β-agonist/cAMP-suppressed secretion and expression of these two adipokines.
Keywords: adiponectin, cAMP, leptin, milrinone, phosphodiesterase 3B (PDE3B), wortmannin
Abbreviations: ALBP, adipocyte lipid-binding protein; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; PDE, phosphodiesterase; PI3K, phosphoinositide 3-kinase
INTRODUCTION
Adipocytes secrete several functionally distinct factors collectively termed adipokines (for review see [1]). One member of this group, adiponectin, has recently been shown to increase hepatic insulin sensitivity [2], to stimulate fatty acid oxidation in liver and skeletal muscle, and to have protective effects on cardiovascular functions [3,4]. In obesity, adiponectin levels are reduced [5], suggesting that altered adiponectin homoeostasis, possibly caused by suppression of adiponectin secretion and/or gene expression, may play a role in the development of obesity-related insulin resistance.
The cellular mechanisms underlying adiponectin homoeostasis are still being defined. Previous studies have consistently reported the inhibitory effects of β-agonists/cAMP on adiponectin expression [6]. The role of insulin itself in adiponectin expression and secretion is somewhat controversial with some reporting inhibitory effects in differentiated 3T3-L1 adipocytes [7]. However, other studies have shown stimulatory effects of insulin on adiponectin secretion in 3T3-L1 adipocytes and in human primary adipocytes [8–10]. In the case of human adipocytes, the modest stimulatory effect of insulin also seems to be depot-specific [10]. Compared with our knowledge about the regulation of adiponectin, leptin homoeostasis is better studied and may provide insight into the regulation of adiponectin. For example, insulin and β-adrenergic mechanisms have been proposed as important positive and negative regulators respectively, of leptin secretion and expression [11,12], but the molecular mechanisms underlying these physiological actions are not completely clear. The effects of insulin on leptin expression appear to be partially mediated by the stimulation of glucose metabolism in adipocytes [13]. Due to the suppressive nature of β-adrenergic mechanisms on leptin levels, the stimulatory effects of insulin on leptin may potentially entail the reduction of cAMP levels. Indeed, reduction of cAMP through PI3K (phosphoinositide 3-kinase)-dependent activation of PDE3B (phosphodiesterase 3B) mediates at least in part the anti-lipolytic action of insulin in the adipose tissue [14]. In the present study, we attempt to explore the possibility that insulin and β-agonists have opposing actions in regulating adiponectin secretion and expression in rat primary adipocytes, and that activation of PDE3B may mediate the effects of insulin on the production of adiponectin.
MATERIALS AND METHODS
Primary adipocyte studies
Primary adipocytes were prepared from epididymal fat pads from male rats (Sprague–Dawley) with body mass between 200–250 g as described previously [15]. The animals were anaesthetized with Nembutal (at 100 mg/Kg) before the dissection of the adipose tissues. All procedures were approved by the Institutional Animal Care and Usage Committee (IACUC) at the University of Pittsburgh. After digestion with collagenase (1 μg/ml), isolated fat cells were washed with KRH buffer [Krebs-Ringer-Hepes buffer (pH=7.4); 25 mM Hepes, 2 mM glucose and 1% BSA (w/v)]. The fat cells were further incubated with adenosine deaminase (1 unit/ml) and the adenosine antagonist, PIA [N6-(2-phenylisopropyl)adenosine; 1 μM] for 1 h before being washed again with KRH buffer. The prepared adipocytes [1 ml, 10–12% (v/v)] were incubated in DMEM (Dulbecco's modified Eagle's medium) containing 25 mM glucose (no serum) and 3% BSA at 37 °C with gentle shaking in the presence of various hormones and inhibitors. Incubations continued for 4, 6, 8, 12, 16 or 24 h before medium was collected for measurements of adiponectin and leptin concentrations and the adipocytes were dissolved in TRIzol® reagent (Invitrogen) for the extraction of RNA.
Quantitative analysis of gene expression levels
The total RNA samples were treated with DNase I (Ambion) and reverse-transcribed with a Superscript kit (Invitrogen). Real-time PCR reactions were performed on an ABI 7700 Taqman machine (Applied Biosystems) with the following cycles: 1 cycle at 50 °C for 2 min; 1 cycle at 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 60 °C for 1 min. rRNA (18 S) was used as the internal control for input cDNA quantity. The linearity of each assay was determined based on a co-efficient value of at least 0.99 for the standard curve. The cDNA concentrations of all samples were within the linear range of the standard curve. The design of double-labelled probes and the flanking primers was carried out using Primer-Express software (Applied Biosystems). The probes for rat leptin and 18 S rRNA were labelled at the 5′ end with FAM (6-carboxyfluorescein) and at the 3′ end with TAMARA, whereas the probe for adiponectin, ALBP (adipocyte lipid-binding protein), LPL (lipoprotein lipase) and HSL (hormone-sensitive lipase) was labelled at the 5′ end with FAM and with BHQ-1 at the 3′ end. The probe and primer sequences are as follows. Rat leptin: probe, CAGGCAGAGGGTCACCGGTTTGG; 5′-primer, CACACGCAGTCGGTATCCG; 3′-primer, GTGAAGCCCGGGAATGAAG. Rat adiponectin: probe, AGCCCGGAGAAGCCGCTTACATG; 5′-primer, GCACGAGGCGAGAAAGGA; 3′-primer, CCTACGCTGAATGCTGAGTGAT. Rat ALBP: probe, TGGCCGGTATGGCCAAGCCC; 5′-primer, GGCTTCGCCACCAGGAA; 3′-primer, CCCTTCTACGCTGATGATCAAGT. Rat LPL: probe, CGCTCCACCGCGCTCTAGTCCTC; 5′-primer, TTTAAAGGTTGACTTGCCCCG; 3′-primer, AAAGGGTTGAGCCGGAGG. Rat HSL: probe, CTCAAGCCAGGGCCCAGGAGAGA; 5′-primer, CGCAGAAGACAACATGGCCT; 3′-primer, TGGACAGCCGCCGTG. Rat 18 S rRNA: probe, CGCGCAAATTACCCACTCCCGA; 5′-primer, ACATCCAAGGAAGGCAGCAG; 3′-primer, TTCGTCACTACCTCCCCGG.
Western blot analysis
After the incubation with isoprenaline and/or insulin, whole cell extract samples were prepared from the primary adipocytes and subjected to Western blot analysis. Visualization of protein bands was achieved using a standard enhanced-chemiluminescent reaction. The primary antibody against rat adiponectin was from Chemicon and the antibody against rat leptin was from BioVendor Laboratories.
Hormones and metabolites
Adiponectin and leptin levels were determined by radioimmuno-assay (Linco Research). Glycerol levels in the medium were measured using the Trident kit from Sigma.
Statistical analysis
All comparisons were subject to a two-tailed Student's t test with P<0.05 considered as statistically significant.
RESULTS
The opposing effects of β-agonists and insulin on adiponectin secretion in rat primary adipocytes
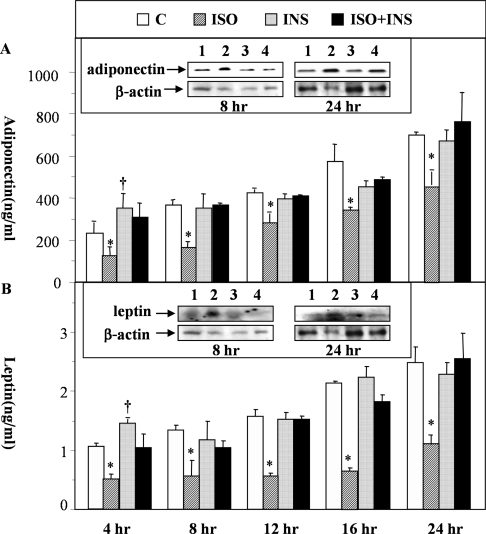
As one of the steps to investigate the roles of insulin and β-agonists on the regulation of adiponectin secretion and expression, we examined the temporal effects of a β-agonist, isoprenaline, on adiponectin secretion and expression in the absence or presence of insulin in rat primary adipocytes. Within the duration of these incubation time frames (4, 8, 12, 16 and 24 h), the primary adipocytes seemed to have retained their characteristics because the expression levels of several adipocyte-specific genes (including adiponectin, leptin, ALBP, LPL and HSL) only showed mild to modest reductions with the HSL gene showing the biggest drop (4-fold) in expression (see Supplementary Table 1 at http://www.BiochemJ.org/bj/403/bj4030519add.htm). The relative expression changes of these genes recorded in our assays were largely consistent with those reported previously [16]. At 200 nM, isoprenaline significantly reduced the secretion of adiponectin and leptin at all selected time points (Figures 1A and 1B). Co-incubation with insulin (20 nM) completely blocked the inhibitory effects of isoprenaline (Figures 1A and 1B). In real-time PCR analysis, we found that the decrease in secretion caused by isoprenaline was not accompanied by significant reductions in gene expression until 12 h (for adiponectin) and 16 h (for leptin) post-addition of isoprenaline to the adipocytes (Table 1). Thus the inhibitory effect of isoprenaline on secretion was due at least in part to its suppression of exocytosis as it induced adiponectin and leptin protein accumulation within the adipocytes (see the inserts within Figures 1A and 1B). In the real-time PCR assays, we further found that, in addition to the mild stimulatory effects on the expression of adiponectin and leptin at early time points (4 and 8 h), insulin could also restore the suppressive effects of isoprenaline on gene expression induced at later time points (12, 16 and 24 h) (Table 1).
Figure 1. Temporal effects of isoprenaline on the secretion of adiponectin and leptin in the absence or presence of insulin.
Rat primary white adipocytes were incubated with isoprenaline (ISO; 200 nM) in the presence or absence of insulin (INS; 20 nM) for 4, 8, 12, 16 and 24 h. At the end of the incubation, culture medium was collected for the measurement of adiponectin (A) and leptin (B). *Indicates P<0.05 between indicated groups and untreated controls or the corresponding ISO+INS groups. † Indicates a statistical difference (P<0.05) between indicated groups and untreated controls only. Inserts: evaluation of intracellular adiponectin and leptin with Western blot analysis of whole cell extracts (see the Materials and methods section for details). Lane 1, untreated control; lane 2, ISO treated; lane 3, INS treated; lane 4, ISO+INS treated.
Table 1. Effects of isoprenaline and insulin on gene expression.
The experiments are outlined in the Materials and methods section. The results reflect at least two independent experiments with each sample analysed three times with 4, 8 and 16 ng of template. *Indicates P<0.05 between the indicated groups and untreated controls or the corresponding ISO+INS groups in a two-tailed student's t test. †Indicates P<0.05 when compared to the control (no ISO and INS) groups only. C, untreated control; ISO, 200 nM isoprenaline; INS, 20 nM insulin; ISO+INS, 200 nM isoprenaline+20 nM insulin.
| Adiponectin | Leptin | ALBP | LPL | HSL | ||
|---|---|---|---|---|---|---|
| 4 h | C | 100 | 100 | 100 | 100 | 100 |
| INS | 200±41† | 250±40† | 172±9† | 202±10† | 167±30* | |
| ISO | 129±6 | 145±5 | 151±15 | 130±5 | 166±20 | |
| ISO+INS | 101±12 | 110±18 | 120±21 | 95±9 | 89±18 | |
| 8 h | C | 100 | 100 | 100 | 100 | 100 |
| INS | 101±7 | 135±17 | 92±18 | 92±8 | 92±11 | |
| ISO | 128±8 | 102±13 | 74±1 | 111±6 | 105±6 | |
| ISO+INS | 228±12† | 110±10 | 272±20 | 190±15† | 242±10† | |
| 12 h | C | 100 | 100 | 100 | 100 | 100 |
| INS | 153±20 | 121±20 | 191±2† | 106±6 | 95±8 | |
| ISO | 19±1* | 72±10 | 58±2* | 56±3* | 82±8 | |
| ISO+INS | 151±20 | 174±10 | 275±17† | 224±12† | 232±6† | |
| 16 h | C | 100 | 100 | 100 | 100 | 100 |
| INS | 136±10 | 136±20 | 136±10 | 138±22 | 153±22 | |
| ISO | 18±4* | 38±1* | 41±4* | 30±3* | 81±6 | |
| ISO+INS | 110±12 | 83±10 | 112±22 | 270±6† | 83±9 | |
| 24 h | C | 100 | 100 | 100 | 100 | 100 |
| INS | 182±15 | 124±10 | 105±1 | 109±15 | 105±1 | |
| ISO | 22±4* | 34±4* | 53±12* | 41±2* | 153±7 | |
| ISO+INS | 97±11 | 97±11 | 123±28 | 121±2 | 118±20 |
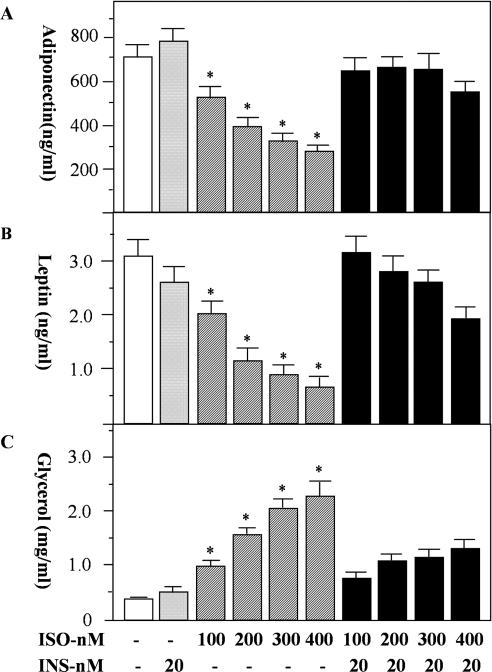
We further evaluated the effects of insulin on the secretion and expression of adiponectin and leptin in the presence of various concentrations of isoprenaline. We selected 24 h for such a study because isoprenaline could maximally suppress the expression of both adiponectin and leptin at this time point (Table 1). Isoprenaline suppressed adiponectin secretion in a dose-dependent manner (Figure 2A). At 400 nM, isoprenaline inhibited the release of adiponectin by approximately 67% (Figure 2A, P<0.05). Similar effects of isoprenaline on leptin secretion were also observed (Figure 2B, P<0.05). By itself, insulin did not increase adiponectin or leptin secretion. However, when co-incubated with isoprenaline, insulin reversed the inhibitory effects of isoprenaline on both adiponectin and leptin secretion (Figures 2A and 2B). As a measure of the opposing effects of β-agonists and insulin on adipocyte metabolism, we determined the effects of isoprenaline and insulin on lipolysis. Glycerol release by adipocytes was used as a surrogate marker of triacylglycerol breakdown. As expected, isoprenaline increased lipolysis in a dose-dependent manner, and insulin substantially reduced the stimulatory effect of isoprenaline (Figure 2C).
Figure 2. The effects of isoprenaline and insulin on the secretion of adiponectin and leptin from rat primary adipocytes.
Rat primary white adipocytes were incubated with isoprenaline (ISO) in the presence or absence of insulin (INS) for 24 h. At the end of incubation, the medium was collected for measurement of adiponectin (A) and leptin (B). As a control for the actions of β-agonist and insulin, lipolysis was assessed by measurement of glycerol levels in the same medium (C). Data were obtained from three independent experiments, with quadruple measurements in each experiment. * Indicates a statistical difference at P<0.05 between indicated groups and untreated controls or the corresponding ISO+INS groups.
cAMP analogues mimic the effects of isoprenaline on adiponectin secretion
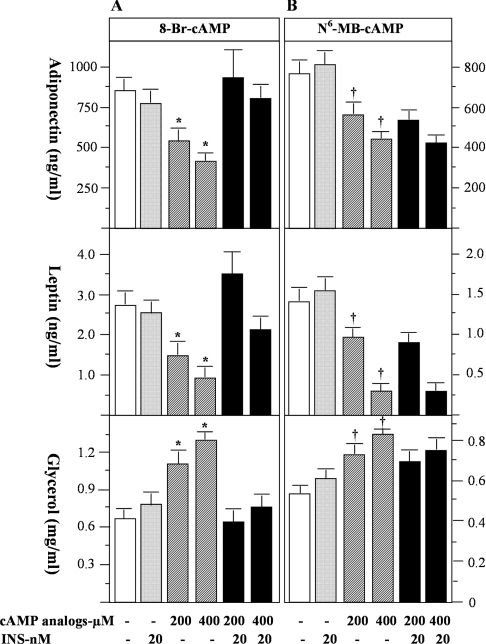
The primary signalling mechanism for β-agonist action is through elevation of intracellular cAMP levels [17]. Insulin can lower cAMP levels by either inhibiting cAMP production through the inhibitory subunit of G proteins (Gi) [18], or increasing cAMP degradation through activation of PDE, notably PDE3B [14,19]. In an attempt to determine the potential role of cAMP and PDE activity in mediating the effects of β-agonists and insulin on the secretion of adiponectin and leptin, we examined the effects of two cAMP-analogues, 8-bromo-cAMP (hydrolysable by PDEs, albeit with lower Vmax than that of cAMP [20]) and N6-monobutyryl-cAMP (resistant to hydrolysis by PDEs [20]) on adipokine secretion in the absence and presence of insulin. Both of these cAMP analogues are PKA (protein kinase A)-activators; however, activation of PDE3B by insulin is not able to reduce the suppressive effects of N6-monobutyryl-cAMP due to its resistance to hydrolysis by PDE [20]. At 400 μM, 8-bromo-cAMP and N6-monobutyryl-cAMP inhibited adiponectin secretion by 53% and 45% (both P<0.05) and leptin secretion by 67% and 57% (both P<0.05) respectively. Insulin blocked the inhibitory effects of 8-bromo-cAMP (Figure 3A), but not the effects of N6-monobutyryl-cAMP, on the secretion of these two adipokines (Figure 3B). As expected, both cAMP analogues stimulated lipolysis (Figures 3A and 3B), while insulin decreased lipolysis stimulated by 8-bromo-cAMP but not by N6-monobutyryl-cAMP (Figure 3B). Taken together, these data confirm the suppressive effects of cAMP on adiponectin and leptin secretion, and suggest that the activation of a PDE by insulin and the subsequent reduction of intracellular cAMP levels play a role in the regulation of secretion of adiponectin and leptin.
Figure 3. The effects of cAMP analogues on the secretion of adiponectin and leptin from rat primary adipocytes.
Rat primary white adipocytes were incubated with cAMP analogues in the presence or absence of insulin (INS) for 24 h. The experimental results are shown in (A) for the effects of 8-bromo-cAMP (8-Br-cAMP) and in (B) for the effects of N6-monobutyryl-cAMP (N6-MB-cAMP). As a control for β-agonist and insulin action, lipolysis was assessed by measurement of glycerol levels. Data were obtained from two independent experiments, with quadruple measurements in each experiment. In (A) * indicates a statistical difference (P<0.05) between indicated groups and untreated controls or the corresponding 8-bromo-cAMP+INS groups. In (B) † indicates a statistical difference (P<0.05) between indicated groups and untreated controls only. There is no statistical significance between N6-monobutyryl-cAMP groups and the corresponding N6-monobutyryl-cAMP+INS groups.
A PI3K-PDE3B mechanism mediates the effects of insulin on adiponectin and leptin secretion in primary adipocytes
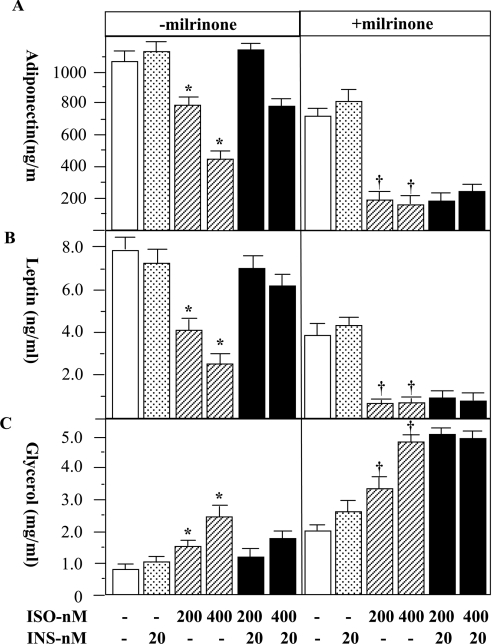
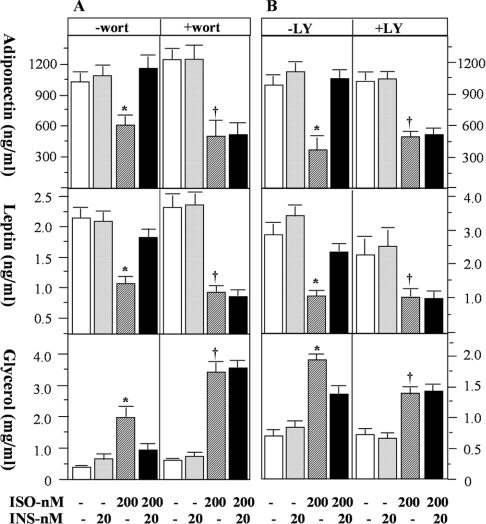
PDE3B represents a major PDE activity in adipocytes and can be rapidly activated by insulin [21]. A primary function of PDE3B in adipocytes is to mediate the anti-lipolytic action of insulin through reduction of cAMP [22]. We postulate that an additional important function of PDE3B in adipocytes may be to mediate the effects of insulin on adiponectin and leptin secretion. To test this concept, we co-incubated a specific inhibitor of type-3 PDE, milrinone [23], with adipocytes in the presence of insulin and/or isoprenaline, and evaluated the effects of this on adiponectin and leptin secretion. Again, the extent of lipolysis was used as the control. The presence of 10 μM milrinone (IC50=3 μM; [23]) completely blocked the effects of insulin to restore isoprenaline-suppressed secretion of adiponectin and leptin (Figures 4A and 4B). As expected, milrinone increased lipolysis and blocked the anti-lipolytic effect of insulin (Figure 4C) [24]. Activation of PDE3B by insulin has been shown to be dependent on the activity of PI3K in a number of studies [14]. To investigate the role of PI3K in insulin-regulated adipokine secretion, we used two inhibitors, wortmannin and LY294002, to block insulin signalling, and subsequently assessed adiponectin secretion. Neither wortmannin (20 nM) nor LY294002 (10 μM) influenced the inhibitory effects of isoprenaline on the secretion of these two adipokines (Figures 5A and 5B). However, these inhibitors completely eliminated the ability of insulin to restore the secretion of adiponectin and leptin (Figures 5A and 5B), and blunted the suppressive effect of insulin on isoprenaline-stimulated lipolysis (Figure 5C). These data suggest that PI3K activation is a component of the regulation of adiponectin and leptin secretion by insulin.
Figure 4. The effects of a specific PDE3 inhibitor, milrinone, on the secretion of adiponectin and leptin from rat primary adipocytes.
Rat primary white adipocytes were incubated in the absence or presence of isoprenaline (ISO), insulin (INS) and a specific PDE3 inhibitor, milrinone (10 μM), for 24 h as indicated. At the end of the incubation, the medium was collected for measurement of adiponectin (A) and leptin (B). As a control for β-agonist and insulin action, lipolysis was assessed by measurement of glycerol levels in the same medium (C). Data were obtained from two independent experiments, with quadruple measurements in each experiment. In groups without milrinone treatment, * indicates a statistical difference (P<0.05) between indicated groups and untreated controls or the corresponding ISO+INS groups. In milrinone-treated groups † indicates P<0.05 when compared with the controls (no ISO and INS) only. In this case, there is no statistical significance between ISO groups and the corresponding ISO+INS groups.
Figure 5. The effects of PI3K inhibitors on the secretion of adiponectin and leptin from rat primary adipocytes.
Rat primary white adipocytes were incubated in the absence or presence of isoprenaline (ISO), insulin (INS) and the PI3K inhibitors wortmannin (wort; 20 nM) and LY294002 (LY; 10 μM) for 24 h. At the end of the incubation, the medium was collected for measurement of adiponectin (A) and leptin (B). As a control for β-agonist and insulin action, lipolysis was assessed by measurement of glycerol levels in the same medium (C). Data were obtained from two independent experiments, with quadruple measurements in each experiment. In groups without PI3K inhibitor treatment, * indicates a statistical difference (P<0.05) when compared with untreated controls (no ISO and INS) or the corresponding ISO+INS groups. In wortmannin- or LY294002-treated groups, † indicates P<0.05 when compared with the controls only, and there is no statistical significance between ISO groups and the corresponding ISO+INS groups.
Effects of isoprenaline, insulin, milrinone and PI3K inhibitors on the gene expression of adiponectin and leptin
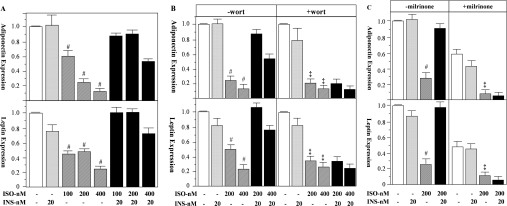
Using real-time PCR assays, we further analysed the effects of isoprenaline, insulin, milrinone and PI3K inhibitors on the expression of adiponectin and leptin genes in rat primary adipocytes. Each of the agents had similar effects on the mRNA levels to those observed in adiponectin and leptin secretion (Figure 6). Accordingly, isoprenaline suppressed leptin and adiponectin mRNA in a dose-dependent manner. At 400 nM, isoprenaline severely suppressed adiponectin and leptin expression to 14.6±0.2% (P<0.01) and 24.8±0.2% (P<0.01) of the control levels respectively. Although insulin by itself did not alter the mRNA levels of adiponectin and leptin (24 h), it restored the expression of these two genes when co-incubated with isoprenaline to that observed in the non-treated controls (Figure 6A). Co-incubation of wortmannin with insulin nullified the effect of insulin on the expression of adiponectin and leptin (Figure 6B). Milrinone further exacerbated the suppressive effects of isoprenaline on the mRNA levels of adiponectin and leptin, and completely blocked the effects of insulin (Figure 6C). Thus the PI3K-PDE3B-cAMP pathway mediates at least in part the effects of insulin in restoring β-agonist/cAMP-suppressed adiponectin and leptin gene expression in rat primary adipocytes.
Figure 6. The effects of isoprenaline, insulin, wortmannin and milrinone on adiponectin and leptin mRNA levels in rat primary adipocytes.
Rat primary white adipocytes were incubated in the absence or presence of combinations of isoprenaline (ISO), insulin (INS), the PI3K inhibitor wortmannin (wort) and a specific PDE3 inhibitor, milrinone for 24 h. Subsequently, mRNA was isolated from adipocytes and adiponectin and leptin mRNA levels were measured using real-time PCR (Taqman). The results of at least two independent experiments, with each sample of each experiment analysed three times with 5, 10 and 20 ng of total RNA, are shown. In (A) and (B), the levels of control or control plus wortmannin were separately set at 1.0; however, in (C), only the level of control (without milrinone) was set at 1.0 to reflect the negative influence of milrinone on the expression of adiponectin and leptin mRNA. In (A) and in (B and C, without the inhibitors), # indicates a statistical difference (P<0.01) when compared with untreated controls or the corresponding ISO+INS treated groups. In the presence of inhibitors (B and C), ‡ indicates P<0.01 when compared with the controls only, and there is no statistical significance between ISO groups and the corresponding ISO+INS groups.
DISCUSSION
In the present study, we have shown that insulin and β-agonists act directly at the adipocytes to regulate adiponectin secretion and expression, and that a PI3K-PDE3B-cAMP-dependent mechanism mediates the effects of insulin to restore β-agonist/cAMP-suppressed secretion and expression of adiponectin and leptin in rat primary adipocytes. Thus a pathway that is traditionally associated with insulin and β-agonist regulation of adipocyte lipolysis [22] is also important for the regulation of leptin and adiponectin secretion and expression. Although a hyperinsulinaemic clamp has been shown to cause a mild inhibition on serum adiponectin levels in humans [25], the physiological implications of this finding are still not clear. In our cellular studies, insulin by itself modestly and transiently elevated the expression and secretion of adiponectin and leptin at some early time points (4 h for secretion and 4 or 8 h for expression), such effects were lost during the longer incubations (Figure 1 and Table 1). The stimulatory effects of insulin on adiponectin and leptin production during a short incubation were consistent with some previous observations made in 3T3-L1 adipocytes and in human primary adipocytes [8–10]. The inhibitory effects of β-agonists and cAMP on the production of adiponectin and leptin have been well demonstrated in several previous studies [6,26–28]. Interestingly, isoprenaline-induced inhibition of adiponectin and leptin secretion was primarily due to the suppression of exocytosis at early time points (such as 8 h; see Figure 1), but was accompanied by the reduction of gene expression at later time points (12 to 24 h) (Table 1). Furthermore, to our knowledge, the counter-regulation of the negative effects of isoprenaline by insulin, as well as the underlying signalling mechanisms, have not yet been reported.
A number of studies have demonstrated that elevated glucose metabolism is an important mechanism mediating the effect of insulin on leptin secretion [13,29], although it remains to be defined whether the same mechanism involving elevated glucose metabolism plays an equally critical role in regulating adiponectin secretion. It is worth noting that these previous studies were performed in the absence of isoprenaline [13], and thus in the presumed presence of low cAMP levels. The negative effect of cAMP on insulin/IGF-1 (insulin-like growth factor 1)-stimulated glucose transport has been well described in the cell lines expressing GLUT4 (glucose transporter 4) [30]. Thus the negative effects of isoprenaline can be partially explained by the suppressed glucose uptake in adipocytes. Interestingly, a recent study [31] has shown that pharmacological inhibition of PDE3B activity in rat primary adipocytes could drastically inhibit insulin-induced glucose uptake. Therefore the reduction of cAMP as a result of PDE3B activation by insulin is consistent with the requirement of elevated glucose metabolism for insulin-regulated leptin production.
The opposing regulation of insulin and β-agonist/cAMP on the secretion and expression of adiponectin and leptin may have physiological implications in the scheme of acute regulation. In a prolonged fasted state, stimulation of lipolysis is associated with elevation of adipocyte cAMP levels, which is most likely due to the increased β-adrenergic response [32]. The anti-lipolytic action of insulin during re-feeding is mediated at least in part by the reduction of cAMP through activation of PDE3B [22]. Our recent studies found that overnight fasting could significantly lower serum adiponectin and leptin concentrations in rats, an effect that could be reversed by 6 h of re-feeding or by euglycaemic–hyperinsulinaemic clamps (S. R. Commerford, N. Dedousis, A. Z. Zhao and R. M. O'Doherty, unpublished work). From the perspective of insulin, the rapid recovery of blood adiponectin concentrations during re-feeding may help to augment the effects of insulin in liver and possibly in skeletal muscle, since adiponectin has been well established as a hormone to stimulate non-esterified fatty acid oxidation in liver and skeletal muscle [33], and to enhance hepatic insulin action [2,34,35].
Our cellular study may likewise be physiologically relevant to the long-term maintenance of adipokine homoeostasis. The adipocyte expression of PDE3B is reduced in obesity and Type 2 diabetes in humans and rodents [36–38], a condition that potentially contributes to the insulin-resistant state due to chronically elevated lipolysis and hence chronically increased serum non-esterified fatty acid levels. Since the serum adiponectin level is decreased in obesity and Type 2 diabetes [3], it will be important to determine whether the reduction of PDE3B expression is a contributory mechanism underlying the decreased adiponectin levels. However, the decrease in PDE3B expression cannot explain the seemingly paradoxical increases in blood leptin concentrations in obesity, suggesting that the long-term regulation of leptin and adiponectin is very likely dependent on the interactions of multiple signalling pathways. The regulation of PDE3B is very likely to be just one part of a larger story in the long-term regulation of adipokine homoeostasis.
Online data
Acknowledgments
We would like to thank Dr Robert O'Doherty for his critical evaluation of this work. This work was supported in part by a Research Award (7-06-RA-173) from the American Diabetes Association and by a NIH (National Institutes of Health) grant to A. Z. Z. (RO1 DK064383-01).
References
- 1.Saltiel A. R. You are what you secrete. Nat. Med. 2001;7:887–888. doi: 10.1038/90911. [DOI] [PubMed] [Google Scholar]
- 2.Combs T. P., Berg A. H., Obici S., Scherer P. E., Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J. Clin. Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu E., Liang P., Spiegelman B. M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 4.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 5.Weyer C., Funahashi T., Tanaka S., Hotta K., Matsuzawa Y., Pratley R. E., Tataranni P. A. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 6.Delporte M. L., Funahashi T., Takahashi M., Matsuzawa Y., Brichard S. M. Pre- and post-translational negative effect of β-adrenoceptor agonists on adiponectin secretion: in vitro and in vivo studies. Biochem. J. 2002;367:677–685. doi: 10.1042/BJ20020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasshauer M., Klein J., Neumann S., Eszlinger M., Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2002;290:1084–1089. doi: 10.1006/bbrc.2001.6307. [DOI] [PubMed] [Google Scholar]
- 8.Scherer P. E., Williams S., Fogliano M., Baldini G., Lodish H. F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 9.Bogan J. S., Lodish H. F. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J. Cell. Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoshima H., Wu X., Sinha M. K., Hardy V. E., Rosato E. L., Barbot D. J., Rosato F. E., Goldstein B. J. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: effects of insulin and rosiglitazone. J. Clin. Endocrinol. Metab. 2002;87:5662–5667. doi: 10.1210/jc.2002-020635. [DOI] [PubMed] [Google Scholar]
- 11.Barr V. A., Malide D., Zarnowski M. J., Taylor S. I., Cushman S. W. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463–4472. doi: 10.1210/endo.138.10.5451. [DOI] [PubMed] [Google Scholar]
- 12.Gettys T. W., Harkness P. J., Watson P. M. The β3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology. 1996;137:4054–4057. doi: 10.1210/endo.137.9.8756584. [DOI] [PubMed] [Google Scholar]
- 13.Mueller W. M., Gregoire F. M., Stanhope K. L., Mobbs C. V., Mizuno T. M., Warden C. H., Stern J. S., Havel P. J. Evidence that glucose metabolism regulates leptin secretion from cultured rat adipocytes. Endocrinology. 1998;139:551–558. doi: 10.1210/endo.139.2.5716. [DOI] [PubMed] [Google Scholar]
- 14.Degerman E., Belfrage P., Manganiello V. C. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3) J. Biol. Chem. 1997;272:6823–6826. doi: 10.1074/jbc.272.11.6823. [DOI] [PubMed] [Google Scholar]
- 15.Degerman E., Landstöm T. R., Wijkander J., Holst L. S., Ahmad F., Belfrage P., Manganiello V. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods. 1998;14:43–53. doi: 10.1006/meth.1997.0564. [DOI] [PubMed] [Google Scholar]
- 16.Gesta S., Lolmede K., Daviaud D., Berlan M., Bouloumie A., Lafontan M., Valet P., Saulnier-Blache J. S. Culture of human adipose tissue explants leads to profound alteration of adipocyte gene expression. Horm. Metab. Res. 2003;35:158–163. doi: 10.1055/s-2003-39070. [DOI] [PubMed] [Google Scholar]
- 17.Simonds W. F. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 1999;20:66–73. doi: 10.1016/s0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- 18.Morris A. J., Malbon C. C. Physiological regulation of G protein-linked signaling. Physiol. Rev. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 19.Soderling S. H., Beavo J. A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 20.Beebe S. J., Redmon J. B., Blackmore P. F., Corbin J. D. Discriminative insulin antagonism of stimulatory effects of various cAMP analogs on adipocyte lipolysis and hepatocyte glycogenolysis. J. Biol. Chem. 1985;260:15781–15788. [PubMed] [Google Scholar]
- 21.Degerman E., Smith C. J., Tornqvist H., Vasta V., Belfrage P., Manganiello V. C. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 1990;87:533–537. doi: 10.1073/pnas.87.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manganiello V. C., Degerman E., Smith C. J., Vasta V., Tornqvist H., Belfrage P. Mechanisms for activation of the rat adipocyte particulate cyclic-GMP-inhibited cyclic AMP phosphodiesterase and its importance in the antilipolytic action of insulin. Adv. Second Messenger Phosphoprotein Res. 1992;25:147–164. [PubMed] [Google Scholar]
- 23.Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- 24.Holm C., Langin D., Manganiello V., Belfrage P., Degerman E. Regulation of hormone-sensitive lipase activity in adipose tissue. Methods Enzymol. 1997;286:45–67. doi: 10.1016/s0076-6879(97)86004-1. [DOI] [PubMed] [Google Scholar]
- 25.Yu J. G., Javorschi S., Hevener A. L., Kruszynska Y. T., Norman R. A., Sinha M., Olefsky J. M. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 26.Halleux C. M., Takahashi M., Delporte M. L., Detry R., Funahashi T., Matsuzawa Y., Brichard S. M. Secretion of adiponectin and regulation of apM1 gene expression in human visceral adipose tissue. Biochem. Biophys. Res. Commun. 2001;288:1102–1107. doi: 10.1006/bbrc.2001.5904. [DOI] [PubMed] [Google Scholar]
- 27.Kosaki A., Yamada K., Kuzuya H. Reduced expression of the leptin gene (ob) by catecholamine through a G(S) protein-coupled pathway in 3T3-L1 adipocytes. Diabetes. 1996;45:1744–1749. doi: 10.2337/diab.45.12.1744. [DOI] [PubMed] [Google Scholar]
- 28.Slieker L. J., Sloop K. W., Surface P. L., Kriauciunas A., LaQuier F., Manetta J., Bue-Valleskey J., Stephens T. W. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J. Biol. Chem. 1996;271:5301–5304. doi: 10.1074/jbc.271.10.5301. [DOI] [PubMed] [Google Scholar]
- 29.Havel P. J. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc. Nutr. Soc. 2000;59:359–371. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence J. C., Jr, Piper R. C., Robinson L. J., James D. E. GLUT4 facilitates insulin stimulation and cAMP-mediated inhibition of glucose transport. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3493–3497. doi: 10.1073/pnas.89.8.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zmuda-Trzebiatowska E., Oknianska A., Manganiello V., Degerman E. Role of PDE3B in insulin-induced glucose uptake, GLUT-4 translocation and lipogenesis in primary rat adipocytes. Cell Signalling. 2006;18:382–390. doi: 10.1016/j.cellsig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 32.McKnight G. S., Cummings D. E., Amieux P. S., Sikorski M. A., Brandon E. P., Planas J. V., Motamed K., Idzerda R. L. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog. Horm. Res. 1998;53:139–159. [PubMed] [Google Scholar]
- 33.Fruebis J., Tsao T. S., Javorschi S., Ebbets-Reed D., Erickson M. R., Yen F. T., Bihain B. E., Lodish H. F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg A. H., Combs T. P., Du X., Brownlee M., Scherer P. E. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat. Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 35.Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 36.Engfeldt P., Arner P., Bolinder J., Ostman J. Phosphodiesterase activity in human subcutaneous adipose tissue in insulin- and noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1982;55:983–988. doi: 10.1210/jcem-55-5-983. [DOI] [PubMed] [Google Scholar]
- 37.Solomon S. S., Steiner M. S., Sanders L., Palazzolo M. R. Spontaneous diabetic BB rat: studies of cyclic adenosine 3′,5′- monophosphate phosphodiesterase and calmodulin. Endocrinology. 1986;119:1839–1844. doi: 10.1210/endo-119-4-1839. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y., Osawa H., Onuma H., Nishimiya T., Ochi M., Makino H. Improvement in insulin resistance and the restoration of reduced phosphodiesterase 3B gene expression by pioglitazone in adipose tissue of obese diabetic KKAy mice. Diabetes. 1999;48:1830–1835. doi: 10.2337/diabetes.48.9.1830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.