Abstract
Exposure of neutrophils to LPS (lipopolysaccharide) triggers their oxidative response. However, the relationship between the signalling downstream of TLR4 (Toll-like receptor 4) after LPS stimulation and the activation of the oxidase remains elusive. Phosphorylation of the cytosolic factor p47phox is essential for activation of the NADPH oxidase. In the present study, we examined the hypothesis that IRAK-4 (interleukin-1 receptor-associated kinase-4), the main regulatory kinase downstream of TLR4 activation, regulates the NADPH oxidase through phosphorylation of p47phox. We show that p47phox is a substrate for IRAK-4. Unlike PKC (protein kinase C), IRAK-4 phosphorylates p47phox not only at serine residues, but also at threonine residues. Target residues were identified by tandem MS, revealing a novel threonine-rich regulatory domain. We also show that p47phox is phosphorylated in granulocytes in response to LPS stimulation. LPS-dependent phosphorylation of p47phox was enhanced by the inhibition of p38 MAPK (mitogen-activated protein kinase), confirming that the kinase operates upstream of p38 MAPK. IRAK-4-phosphorylated p47phox activated the NADPH oxidase in a cell-free system, and IRAK-4 overexpression increased NADPH oxidase activity in response to LPS. We have shown that endogenous IRAK-4 interacts with p47phox and they co-localize at the plasma membrane after LPS stimulation, using immunoprecipitation assays and immunofluorescence microscopy respectively. IRAK-4 was activated in neutrophils in response to LPS stimulation. We found that Thr133, Ser288 and Thr356, targets for IRAK-4 phosphorylation in vitro, are also phosphorylated in endogenous p47phox after LPS stimulation. We conclude that IRAK-4 phosphorylates p47phox and regulates NADPH oxidase activation after LPS stimulation.
Keywords: interleukin-1 receptor-associated kinase-4 (IRAK-4), lipopolysaccharide (LPS), myeloid differentiation factor 88 (MyD88), NAPDH oxidase, neutrophil, Toll-like receptor (TLR)
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; DFP, di-isopropyl fluorophosphate; fMLP, N-formylmethionyl-leucylphenylalanine; GST, glutathione S-transferase; GTP[S], guanosine 5′-[γ-thio]triphosphate; IL, interleukin; IRAK, interleukin-1 receptor-associated kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; MS/MS, tandem MS; MyD88, myeloid differentiation factor 88; PKC, protein kinase C; ROS, reactive oxygen species; SH3, Src homology 3 domain; TIR, Toll/IL-1 receptor; TLR4, Toll-like receptor 4; TNF, tumour necrosis factor; TRAF, TNF receptor-associated factor; TRD, threonine-rich domain; TRIF, TIR domain-containing adaptor protein inducing interferon β.
INTRODUCTION
Human neutrophils play a central role in innate immunity by combating bacterial and fungal infections. In neutrophils, the production of ROS (reactive oxygen species) relies on the NADPH oxidase, a multisubunit enzymatic complex that is responsible for the monoelectronic reduction of oxygen to produce superoxide anion (O2−) [1]. The production of O2− is directly related to the microbicidal capacity of these cells, since patients with CGD (chronic granulomatous disease), whose NADPH oxidase is inactive, suffer recurrent bacterial and fungal infections [2]. The NADPH oxidase consists of the cytosolic factors p47phox, p67phox and p40phox, the membrane-associated cytochrome b558 and the accessory proteins Rac2 and Rap1a. In resting neutrophils, the oxidase remains unassembled and therefore inactive. In response to adequate stimuli, the cytosolic factor p47phox is phosphorylated on serine residues located at its C-terminus, known as the activation domain. The phosphorylation of these residues is a central event during NADPH oxidase activation. This unmasks the p47phox SH3 (Src homology 3) domains [3] and the phox homology domain [4], allowing p47phox to bind to the cytosolic domain of its target p22phox and to phosphoinositides respectively, thus switching the NADPH oxidase to its active form.
During microbial infections, neutrophils are exposed, often simultaneously, to a variety of soluble and particulate stimuli that can differentially modulate the activity of the NADPH oxidase and the microbicidal capacity of these granulocytes. It has been known for some time that neutrophils exposed to the Gram-negative bacterial cell wall component LPS (lipopolysaccharide) have an increased oxidative response to fMLP (N-formylmethionyl-leucylphenylalanine) when compared with those exposed to fMLP alone, a process usually referred to as priming [5]. Neutrophils also respond to LPS, producing a relatively weak, but consistent, oxidative response [6]. The LPS-dependent activation of the neutrophil NADPH oxidase has been the subject of many studies, and the results of those studies support two possible mechanisms that are not mutually exclusive. The first one involves the translocation of cytochrome b558 from intracellular storage organelles to the plasma membrane in response to LPS. This has been proposed to increase the number of NADPH oxidase subunits available for activation in response to a subsequent stimulus [7]. The second mechanism involves the phosphorylation of p47phox as a consequence of LPS stimulation [6]. Although many aspects of the signalling mechanisms involved in the activation of the neutrophil NADPH oxidase by LPS remain obscure, the receptor for LPS has been identified [8] and the signalling pathways downstream of TLR4 (Toll-like receptor 4) activation after LPS stimulation are starting to be elucidated. It is now known that, in response to LPS binding to TLR4, many proteins are recruited to the cytoplasmic domain of the receptor {the TIR [Toll/IL (interleukin)-1 receptor] domain}. This includes the adaptor protein MyD88 (myeloid differentiation factor 88). It associates with the TIR domain of TLRs and recruits IRAKs (IL-1 receptor-associated kinases) upon activation. One of these kinases, IRAK-4, plays a central role in TLR signalling by phosphorylating another kinase from the same family, IRAK-1. For a complete description of the MyD88-dependent and -independent signalling pathways, see [9]. That IRAK-4 and IRAK-1 are essential components of the MyD88-dependent signalling downstream of TLR4 activation by LPS has been demonstrated in respective knockout mice models [10,11]. On the basis of mutagenesis analysis, it has been shown that IRAK-4, but not IRAK-1, kinase activity is essential during IL-1-mediated NF-κB (nuclear factor κB) activation [12]. Therefore IRAK-4 is considered to be a central TIR signalling mediator in innate immunity [13] and has been proposed to be the only true kinase of the IRAK family [14]. The importance of IRAK-4 in innate immunity is highlighted by the finding that its deficiency leads to a human immunodeficiency characterized by susceptibility to pyogenic bacterial infections [15].
In the present study, we show that the NADPH oxidase is up-regulated as a result of the phosphorylation of p47phox by IRAK-4 and have identified the residues of p47phox that are targets of IRAK-4 phosphorylation using MS analysis.
EXPERIMENTAL
Cloning
The steps in the cloning of the constructs used in the present study were performed using standard techniques. All constructs were verified by sequencing using an automated fluorescent dyeterminator sequencer. The cloning of the full-length p47phox, the full-length Rac2 and the p67phox truncation corresponding to residues 1–210 into the pGEX 6P-1 vector has been described previously [16]. The full-length IRAK-4 cDNA was amplified from human liver cDNA (Clontech) with Pfu polymerase (Stratagene), the 5′ primer, 5′-CCCGAATTCATGAACAAACCCATAACACCATCAA-3′, and the 3′ primer, 5′-CCCCTCGAGTTAAGAAGCTGTCATCTCTTGCAGC-3′, containing EcoRI I and XhoI sites (underlined). The fragment was purified and ligated into the pGEX-6P-1 vector (Amersham Biosciences) and the pCMV-Tag2 (FLAG) expression vector (Stratagene) pre-digested with EcoRI and XhoI.
Purification of recombinant proteins
Recombinant fusion proteins composed of an upstream GST (glutathione S-transferase) linked to a downstream p67phox truncated form (residues 1–210), p47phox or Rac2 were purified by affinity chromatography on glutathione–agarose beads (Amersham Biosciences) as described in [16,17]. In some cases, recombinant proteins were cleaved from GST using PreScission protease (Amersham Biosciences). Concentrations of all proteins (95–99% pure) were determined by the Bradford method using a protein assay kit (Bio-Rad) and BSA as a standard. GST–IRAK-4 was purified from BL21 transformed cells after induction with 0.1 mM IPTG (isopropyl β-D-thiogalactoside) for 8 h at 16 °C. Excess glutathione was removed from the purified recombinant proteins using PD-10 desalting columns (Amersham Biosciences) equilibrated in PBS. In some experiments, we used recombinant GST–IRAK-4 acquired from Cell Signaling Technology.
Isolation and fractionation of neutrophils
Neutrophils were prepared from normal subjects by dextran sedimentation and Ficoll–Hypaque fractionation of freshly drawn citrate-anticoagulated blood. The neutrophils were treated with DFP (di-isopropyl fluorophosphate) and resuspended at 108 cells/ml in a modified relaxation buffer (0.1 M KCl, 3 mM NaCl, 3.5 mM MgCl2 and 10 mM Pipes buffer, pH 7.3). Neutrophil cytosol and membranes were prepared as described in [18] and stored at −70 °C until used.
Flavocytochrome b558
The process for the purification, relipidation and reflavination of cytochrome b558 was described previously [19]. Flavocytochrome b558 was a gift from Dr Andrew R. Cross (Department of Chemistry, King Edward VI School, Southampton, U.K.).
In vitro phosphorylation
Phosphorylation of p47phox by recombinant GST–IRAK-4 or by the catalytic subunit of PKC (protein kinase C) (Calbiochem) was performed by incubating a reaction mixture containing 20 pmol of recombinant p47phox and the indicated amount of GST–IRAK-4 or PKC in a kinase buffer containing 50 mm Hepes, pH 7.4, 10 mM MgCl2, 1 mM ATP and 5 μCi of [γ-32P]ATP. The reactions were carried out for 30 min at 37 °C in a total volume of 30 μl and were terminated by the addition of 10 μl of 4× sample buffer. The samples were then resolved by gel electrophoresis, the proteins were visualized by Coomassie Blue staining and the radiolabelled proteins were detected by autoradiography. Where indicated, radiolabelled ATP was omitted, proteins were transferred on to nitrocellulose membranes, and the phosphorylated proteins were detected using a mouse monoclonal anti-phosphothreonine antibody (Cell Signaling Technology). In some experiments, the p38 MAPK (mitogen-activated protein kinase) inhibitor SB203580 (Calbiochem) was included in the kinase reactions at the indicated concentrations.
Two-dimensional electrophoresis of phosphoamino acids
The phosphoamino acid analysis of p47phox after phosphorylation with IRAK-4 or PKC was performed exactly as described in [20]. Briefly, 32P-phosphorylated p47phox (5 μg) was resolved by gel electrophoresis, transferred on to PVDF membranes by Western blot and visualized by autoradiography. The strip of membrane containing p47phox was excised and membrane-bound 32P-labelled p47phox was hydrolysed by incubation in 5 M HCl for 1 h at 110 °C. The hydrolytic product was transferred to a clean tube, and the HCl was evaporated in a centrifugal vacuum concentrator. The samples were dissolved in 10 μl of electrophoresis buffer containing 2.2% (w/v) methanoic (formic) acid and 1.35 M ethanoic (acetic) acid, pH 1.9, containing 1 mg/ml unlabelled phosphoamino acid standards (Sigma) and loaded on to cellulose thin-layer plates (Sigma). Phosphoamino acids were separated by high-voltage electrophoresis in two dimensions (at pH 1.9 and 3.5). 32P-labelled phosphoamino acids were visualized by autoradiography and were identified by matching the spots to the respective standards visualized using ninhydrin.
Recombinant cell-free activation of the NADPH oxidase using purified cytochrome b558
Phosphorylation of recombinant tag-free p47phox was carried out as described above, omitting the radioactive ATP and using 25 μg of p47phox in a final volume of 15 μl. The protein was incubated for 30 min at 37 °C with 0.025 unit of PKC and/or 2 pmol of GST–IRAK-4 in kinase buffer. In some experiments, the phosphorylated protein was separated from ATP using a Microcon centrifugal filter device (Millipore) or was separated from the GST–IRAK-4 by the addition of pre-washed 50% slurry of glutathione–Sepharose as described in [21]. To evaluate the role of p47phox phosphorylation in the activation of the NADPH oxidase, we adapted a previously described cell-free system that utilizes purified cytochrome b558 [22]. In these experiments, the NADPH oxidase activity is independent of membrane-associated inhibitory factors known to interfere with the production of superoxide anion [22]. The NADPH oxidase activity was measured directly by following the superoxide dismutase-inhibitable reduction of cytochrome c at 550 nm in 96-well microtitre plates in a SpectraMax 190 spectrophotometer (Molecular Devices). The reaction mixtures contained 10 μM GTP[S] (guanosine 5′-[γ-thio]triphosphate), 100 nM FAD, 100 μM cytochrome c, 5 pmol of phosphorylated or unphosphorylated p47phox, 10 pmol of p67phox, 4 pmol of Rac2 and 0.3 pmol of cytochrome b558 in relaxation buffer. SDS and p40phox were omitted so that the activity of the oxidase was evaluated based exclusively on activation through phosphorylated p47phox. Reaction mixtures were incubated for 5 min at room temperature (21 °C), and reactions were started by the addition of 160 μM NADPH to reach a final volume of 250 μl. Reactions were carried out in duplicate or triplicate.
Determination of p47phox phosphorylation sites by MS
IRAK-4-dependent phosphorylated residues in p47phox were detected using microcapillary reverse-phase HPLC–nanoelectrospray tandem MS (MS/MS). For this purpose, IRAK-4-phosphorylated recombinant p47phox or endogenous p47phox immunoprecipitated from LPS (Escherichia coli serotype 515; Alexis)-stimulated human neutrophils was resolved by gel electrophoresis in 10% Bis-Tris NuPAGE gels, and protein was visualized with Simply Blue Coomassie Blue staining (Invitrogen). The p47phox sequence was evaluated using an in-house program, Enzyme Optimizer, for a dual-enzyme strategy that would optimize for coverage of serine/threonine/tyrosine residues. The band corresponding to p47phox was then divided in half for separate in-gel AspN and chymotryptic digestions after reduction and carboxyamidomethylation. The resultant digests were pooled just before HPLC–MS/MS injection. Phosphorylated peptide sequences were determined using a 75-μm reverse-phase micro-column terminating in a custom nanoelectrospray source directly coupled to a LCQ DECA XP Plus quadrupole ion trap mass spectrometer (ThermoFinnigan). The flow rate was nominally 200 nl/min. The ion trap repetitively surveyed the m/z range 395–1600, executing data-dependent MS/MS for peptide sequence information on the four most abundant ions in each survey scan. MS/MS spectra were acquired with relative collision energy of 30% and an isolation width of 2.5 Da. Recurring ions were dynamically excluded. After database correlation with the algorithm SEQUEST [23], phosphorylated peptides were confirmed by manual de novo interpretation of the MS/MS spectra using FuzzyIons [24].
Intracellular phosphorylation of p47phox and co-immunoprecipitation assays
For co-immunoprecipitation assays, human neutrophils were stimulated with LPS (E. coli serotype 026:B6; Sigma) at 100 ng/ml for 30 min and lysed, and then p47phox was immunoprecipitated using a mouse monoclonal specific antibody (clone .33). The immunoprecipitates were resolved by gel electrophoresis, the proteins were transferred on to nitrocellulose, and the membranes were probed for IRAK-4 (Upstate), then stripped with Restore™ (Pierce Biotechnology) and re-probed for the detection of p47phox using our specific polyclonal antibody directed against the C-terminal domain as described previously [25]. For each experiment, LPS was pre-incubated with autologous serum for 5 min. This is necessary to maximize the LPS-dependent priming effect on the neutrophil NADPH oxidase [26,27]. In some experiments, HL-60 cells were differentiated to granulocytes by the addition of 1.3% DMSO for 48 h. The cells were then incubated in the presence of 1 mCi/ml [32P]Pi, treated with the p38 MAPK inhibitor SB203580 (1 μM) or vehicle, and stimulated with 100 ng/ml LPS for 30 min. The cells were lysed and p47phox was immunoprecipitated using our specific polyclonal antibody. The proteins in the immunopellet were resolved by gel electrophoresis and transferred on to nitrocellulose. The membranes were analysed by autoradiography and for the presence of p47phox by Western blot. The signals for p47phox in the Western blot and the signals for phosphorylated p47phox were quantified using Quantity One 4.2.1 software (Bio-Rad), and the phospho-p47phox/p47phox ratio was calculated after densitometry detection.
Detection of ROS production in whole cells
ROS production was measured by luminol-dependent chemiluminescence. Assays using whole cells were carried out as described previously [28] using 106 neutrophils in a final volume of 0.2 ml. The cell suspensions were placed in a 96-well microtitre plate and warmed to 37 °C, and luminol was added to reach a final concentration of 100 μM. The cells were stimulated in the presence of 100 ng/ml LPS (Alexis) for 30 min. In some experiments, the cells were stimulated with 1 μM fMLP. Chemiluminescence was measured at 30 s intervals using a Luminoskan luminometer (Labsystems Research) at 37 °C. In some experiments, HL-60 cells were transfected by nucleofection (Amaxa) with the wild-type IRAK-4 expression vector or with the control vector. The cells were differentiated to granulocytes using 1.3% DMSO, harvested, then stimulated with 100 ng/ml LPS and 1 μM fMLP or left untreated. ROS production was measured by the detection of the luminol-dependent chemiluminescence as described above.
Immunofluorescence
Immunofluorescence analysis in neutrophils was performed as described previously [29]. Briefly, human neutrophils were seeded at 70% confluence in an eight-well chambered coverglass [pre-treated with 0.01% poly(L-lysine) in PBS]. Where indicated, neutrophils were stimulated with 100 ng/ml LPS (Alexis) for 30 min. Cells were fixed with 3.7% (w/v) paraformaldehyde, permeabilized with 0.01% saponin and blocked with a solution of 1% BSA in PBS. In some experiments, autofluorescence was quenched using PBS/glycine (100 mM glycine in PBS). For immunological labelling of endogenous p47phox and IRAK-4, we used a mouse monoclonal specific antibody (clone .33) and a rabbit polyclonal antibody (ProSci) respectively. Samples were incubated with the indicated primary antibodies at a dilution of 1:200 overnight at 4 °C, then the appropriate combinations of Alexa Fluor® 488 or 594-conjugated donkey anti-rabbit and anti-mouse secondary antibodies (Molecular Probes). Controls using normal rabbit or mouse IgG antibodies (Santa Cruz Biotechnology) followed by Alexa Fluor®-conjugated secondary antibodies were always included. In order to stain the nucleus, samples were incubated with DAPI (4′,6-diamidino-2-phenylindole) for 5 min at 21 °C. Samples were stored in Fluoromount-G (Southern Biotechnology) and analysed using a Bio-Rad (Zeiss) 2100 Rainbow Radiance laser-scanning confocal microscope attached to a Nikon TE2000-U microscope at 21 °C with a 60× oil-Plan-Apo-1.4 NA (numerical aperture) objective. For visualization, fluorescence associated with Alexa Fluor® 594-labelled secondary antibody was excited using the 543 nm laser line and collected using a standard Texas Red filter. Fluorescence associated with Alexa Fluor® 488-labelled secondary antibodies was visualized using the 488 nm laser line and collected using a standard FITC filter set. Images were collected using Bio-Rad Laser Sharp 2000 (3.2) software and processed using Image J and Adobe Photoshop CS.
Activity of endogenous IRAK-4
The activity of endogenous IRAK-4 was evaluated as described previously [30]. Briefly, neutrophils were DFP-treated, then resuspended in RPMI 1640 at 107/ml. The cells were stimulated with 100 ng/ml LPS (Alexis) for the indicated time. Cells were spun down, resuspended in 100 μl of lysis buffer (Cell Signaling Technology), incubated in ice for 30 min and briefly sonicated. IRAK-4 was immunoprecipitated from cleared lysates using a rabbit polyclonal antibody (Imgenex). The immunopellets were incubated with 2 μg of histone H1.2 (Calbiochem) in the presence of 4 μCi of [γ-32P]ATP and 250 μM ATP in kinase buffer (Cell Signaling Technology). Reactions were carried out for 1 h at 21 °C with gentle vortex-mixing. Samples were resolved by gel electrophoresis, stained using Simply Blue Coomassie Blue, and radioactive protein was detected by autoradiography, then quantified using Quantity One 4.2.1 software.
Human subjects
All procedures regarding human subjects have been reviewed and approved by the Human Subjects Committee at The Scripps Research Institute and were conducted in accordance with the requirements set forth by the aforementioned Human Subjects Committee.
RESULTS
p47phox is a substrate for IRAK-4
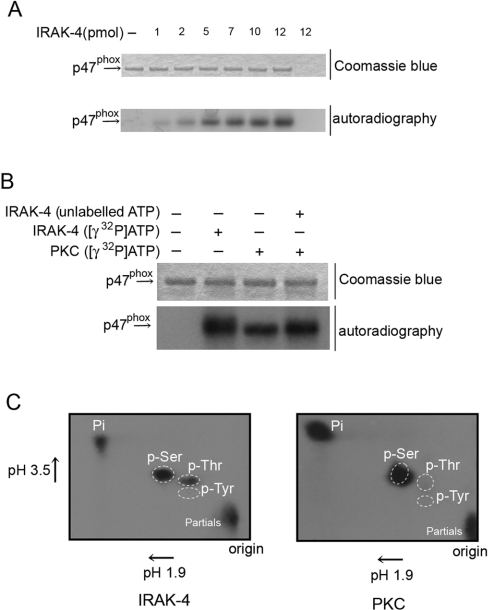
It has been shown previously that p47phox, but not p67phox or Rac2, is translocated to the plasma membrane in response to LPS treatment, that LPS stimulation causes consistent, but limited, phosphorylation of p47phox [6] and that neutrophils from humans with a deficiency in IRAK-4 have a defect in the priming and activation of the oxidative response to LPS [15]. Therefore we decided to explore the hypothesis that IRAK-4 itself may directly regulate the oxidative response of neutrophils to LPS via phosphorylation of p47phox. In Figure 1, we established that IRAK-4 has the ability to phosphorylate p47phox directly in vitro. The idea that phosphorylation is the regulatory mechanism of priming involves the concept of sequential phosphorylation of p47phox via kinases that presumably target different residues [31]. Since PKC has largely been involved as an activator of the NADPH oxidase via p47phox phosphorylation after fMLP treatment in the absence of LPS, we evaluated the ability of PKC to phosphorylate p47phox already phosphorylated by IRAK-4. For these experiments, p47phox was phosphorylated by IRAK-4 in the absence of radiolabelled ATP before its phosphorylation by PKC in the presence of [γ-32P]ATP. In Figure 1(B), we show that the ability of PKC to phosphorylate p47phox was not attenuated by previous phosphorylation by IRAK-4, supporting the idea that these kinases are able to sequentially phosphorylate p47phox. This result also suggests that IRAK-4 and PKC phosphorylate p47phox at different residues. To investigate this, we performed phosphoamino acid analysis of p47phox phosphorylated by PKC or IRAK-4 using two-dimensional electrophoresis (Figure 1C). Our results showed that, while PKC phosphorylates p47phox at serine residues only, IRAK-4 phosphorylates p47phox at serine and threonine residues, confirming our hypothesis and suggesting for the first time that phosphorylation of p47phox at threonine residues may play a regulatory role in the activity of the NADPH oxidase.
Figure 1. IRAK-4 phosphorylates p47phox at serine and threonine residues.
(A) In vitro phosphorylation of p47phox. Phosphorylation of p47phox by recombinant IRAK-4 was performed by incubating a reaction mixture containing 20 pmol of recombinant p47phox and the indicated amount of GST–IRAK-4 in a kinase buffer containing 1 mM ATP and 5 μCi of [γ-32P]ATP (for details, see the Experimental section). The reactions were carried out for 30 min at 37 °C in a total volume of 30 μl and were terminated by the addition of sample buffer. Results are representative of three different experiments. (B) Phosphorylation reactions were carried out as above except that, where indicated, p47phox was phosphorylated by the catalytic subunit of PKC or by IRAK-4 in the absence of radiolabelled ATP before phosphorylation by PKC in the presence of [γ-32P]ATP. (C) Two-dimensional electrophoresis of phosphoamino acids. 32P-phosphorylated p47phox (5 μg) was resolved by gel electrophoresis, transferred on to PVDF membranes and visualized by autoradiography. The strip of membrane containing p47phox was excised, hydrolysed and resolved by two-dimensional electrophoresis as described in the Experimental section. Unlabelled phosphoamino acids were used as standards. 32P-labelled phosphoamino acids were visualized by autoradiography and were identified by matching the spots to the respective standards visualized using ninhydrin. p-Ser, phosphoserine; p-Thr, phosphothreonine; p-Tyr, phosphotyrosine; Pi, [32P]Pi.
IRAK-4 phosphorylates p47phox in a TRD (threonine-rich domain) located between residues 129 and 153
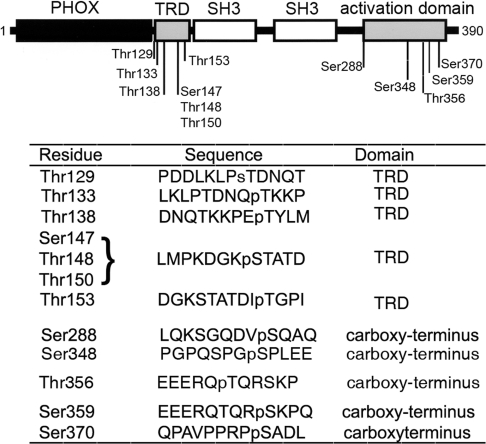
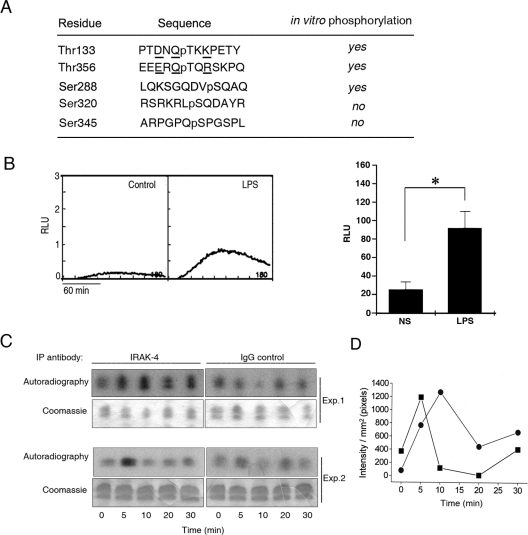
We identified the residues in p47phox that are susceptible to in vitro phosphorylation by recombinant IRAK-4 using microcapillary reverse-phase HPLC–nanoelectrospray MS/MS. The complete MS/MS spectrum is shown in Supplementary Figure S1 (http://www.BiochemJ.org/bj/403/bj4030451add.htm) and is summarized in Figure 2. We show that IRAK-4 phosphorylates p47phox in a TRD located between residues 129 and 153. Three phosphorylation sites were clearly identified by HPLC–MS/MS at Thr133, Thr138 and Thr153. Another possible phosphorylation site was detected at Ser147, although, because of the proximity of Thr148 and Thr150, the precise phosphorylation site could not be resolved. Phosphorylation was also detected at Ser288, Ser359 and Ser370 in the C-terminus activation domain of p47phox. Finally, a relatively weak signature of phosphorylation was detected in Tyr279, Ser348 and Thr356 in the MS/MS spectra.
Figure 2. IRAK-4 dependent phosphorylation sites identified in p47phox by microcapillary HPLC–MS/MS.
IRAK-4-dependent phosphorylated sites in p47phox were detected using microcapillary reversephase HPLC–nanoelectrospray and targeted ion MS/MS as described in the Experimental section. The upper panel shows a schematic representation of p47phox. The phox homology (PHOX) domain is shown in black, the SH3 domains are shown in white, and the phosphorylation domains are in grey. The lower panel shows the sequences surrounding the phosphorylated residues detected by HPLC–MS/MS. Phosphorylated residues are preceded by ‘p’. Owing to the proximity of Ser147, Thr148 and Thr150, the precise phosphorylation site could not be resolved, although a definitive phosphorylation site exists at these residues. For the complete MS/MS spectra, see Supplementary Figure S1 at http://www.BiochemJ.org/bj/403/bj4030451add.htm.
NADPH oxidase is activated by p47phox when phosphorylated by IRAK-4
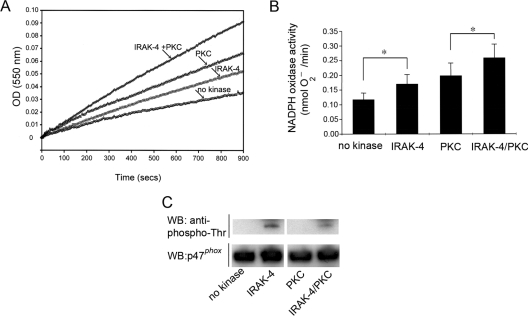
To test the hypothesis that IRAK-4-phosphorylated p47phox has the ability to modulate the activity of the NADPH oxidase, we used a cell-free system. In this system, phosphorylated or unphosphorylated p47phox, p67phox and GTP[S]-pre-loaded Rac2 are incubated with highly purified cytochrome b558 in the absence of amphiphilic molecules, such as SDS. In Figures 3(A) and 3(B), we show that IRAK-4-phosphorylated p47phox activates the NADPH oxidase in this cell-free system. The rate of O2− production using phosphorylated p47phox was significantly different from the unphosphorylated control (P<0.05), supporting the idea that the NADPH oxidase can be activated by direct phosphorylation of p47phox by IRAK-4. Moreover, the level of activity observed with IRAK-4-phosphorylated p47phox was lower than that detected using PKC-phosphorylated p47phox (Figures 3A and 3B). This correlates with the observation that neutrophils treated with LPS alone have a relatively weak oxidative response when compared with that triggered by fMLP [6]. Co-phosphorylation of p47phox by IRAK-4 and PKC significantly enhanced the activity of the NADPH oxidase when compared with PKC phosphorylation alone (P<0.01, Figure 3B), supporting the hypothesis that sequential phosphorylation of p47phox may contribute to the mechanism that mediates the activation of the NADPH oxidase during LPS-dependent priming.
Figure 3. IRAK-4-dependent phosphorylation of p47phox activates the NADPH oxidase in a cell-free system in the absence of amphiphilic molecules.
(A and B) Recombinant p47phox was phosphorylated by IRAK-4 or PKC and prepared for the assays as described in the Experimental section. The NADPH oxidase activity was measured using a cell-free system that utilizes highly purified cytochrome b558 in the absence of SDS or arachidonic acid. Reaction mixtures containing unphosphorylated or phosphorylated p47phox in addition to cytosolic factors and cytochrome b558 were incubated for 5 min at room temperature, and reactions were started by the addition of NADPH. The NADPH oxidase activity was directly measured by following the superoxide dismutase-inhibitable reduction of cytochrome c at 550 nm. (A) Representative kinetics of the reactions are shown. OD (550 nm), absorbance at 550 nm. (B) Results are means±S.E.M. for four different experiments. *P<0.05. (C) Aliquots of the samples used in the cell-free system reactions were evaluated by Western blot (WB) for their level of p47phox phosphorylation at threonine residues using an anti-phosphothreonine monoclonal antibody (Cell Signaling Technology) (upper panel). The Western blot using an anti-p47phox antibody is shown in the lower panel as control for equal loading.
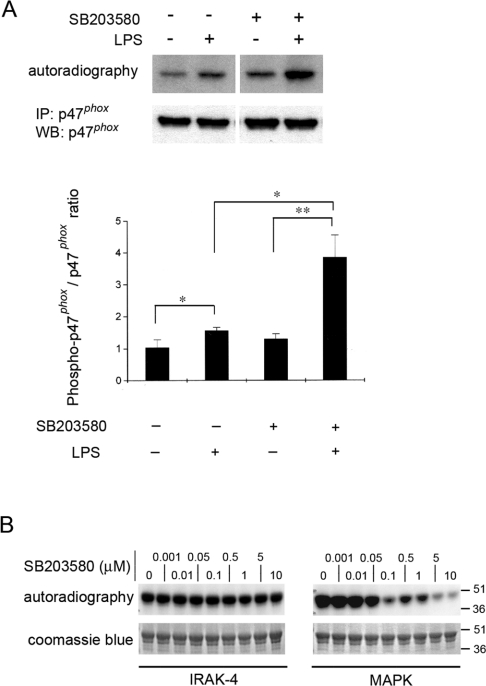
The LPS-dependent phosphorylation of p47phox is up-regulated by inhibition of p38 MAPK
To confirm that endogenous p47phox becomes phosphorylated in response to LPS stimulation, we immunoprecipitated p47phox from LPS-stimulated granulocytes labelled previously with [32P]Pi and evaluated the level of phospho-p47phox. In Figure 4(A), we show that p47phox is significantly phosphorylated under these experimental conditions. This result agrees with a previous work which showed that LPS induces the phosphorylation of p47phox in human neutrophils [6]. Based on the utilization of pharmacological inhibitors, it has been proposed that p38 MAPK-dependent exocytosis plays a central role in LPS-dependent priming of the oxidase [7]. Since p38 MAPK is activated downstream of IRAK-4 after LPS stimulation [9], and given that p47phox has been shown to be a substrate for p38 MAPK [32], it was necessary to analyse whether pharmacological inhibition of p38 MAPK interferes with the phosphorylation event found in the present study. For this purpose, we labelled granulocytes with [32P]Pi and blocked p38 MAPK using the specific pyridinyl imidazole inhibitor SB203580. Strikingly, the phosphorylation of p47phox in response to LPS was increased further after inhibition of p38 MAPK and was significantly different from the level of phosphorylation observed in the absence of p38 MAPK inhibitor (Figure 4A). These results further support a role for a kinase that operates upstream of p38 MAPK in the signalling pathway triggered by TLR4 activation in the phosphorylation of p47phox after LPS stimulation. On the basis of this observation and the data obtained from the in vitro phosphorylation assays, we propose that this kinase is IRAK-4. Importantly, SB203580 does not directly affect IRAK-4-dependent phosphorylation of p47phox (Figure 4B), suggesting that the increase of p47phox phosphorylation observed in the presence of the p38 MAPK inhibitor may be a consequence of the inhibition of a negative regulatory pathway of p38 MAPK over IRAK-4.
Figure 4. Phosphorylation of p47phox is not prevented by p38 MAPK inhibition.
(A) HL-60-differentiated granulocytes were incubated in the presence of 1 mCi/ml [32P]Pi, treated with 1 μM p38 MAPK inhibitor SB203580 (+) or vehicle (−) for 30 min, and stimulated with LPS (100 ng/ml) for 30 min. The cells were lysed and p47phox was immunoprecipitated (IP). The proteins in the immunopellet were resolved by gel electrophoresis and transferred on to nitrocellulose. The membranes were analysed by autoradiography and for the presence of p47phox by Western blot (WB) (upper panel). The signals for p47phox in the Western blot and the signals for phosphorylated p47phox were quantified using Quantity One 4.2.1 software, and the ratio of phosphorylated p47phox/p47phox signal was calculated after densitometry detection (lower panel). Results are means±S.E.M. for three independent experiments. *P<0.05, **P<0.03. (B) In vitro phosphorylation of p47phox by IRAK-4 is not affected by the p38 MAPK inhibitor SB203580. In vitro phosphorylation of recombinant p47phox was performed as described in Figure 1, except that the p38 MAPK inhibitor was included in the reaction medium at the indicated concentrations.
IRAK-4 mediates the activation of the NADPH oxidase in granulocytes
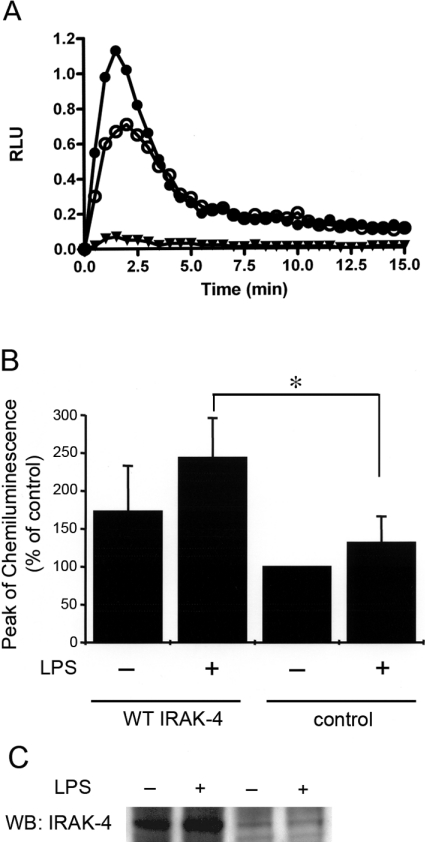
We analysed the ability of IRAK-4 to modulate the NADPH oxidase in intact granulocytes. To maximize the presence of IRAK-4 compared with other endogenous kinases, we transfected HL-60 cells with an expression vector encoding wild-type IRAK-4 or with the control empty vector, then stimulated the cells with LPS and fMLP. As shown in Figure 5, the oxidative response of HL-60 cells to fMLP is monophasic. This is explained by the fact that these granulocytes lack specific and tertiary granules and that most of their cytochrome b558 is located at the plasma membrane [33]. Therefore HL-60 cells are a good model for the analysis of phosphorylation-mediated activation independent of the mechanism involving cytochrome b558 translocation-mediated activation. Moreover, differently from neutrophils, HL-60 cells are easy to transfect, so that the relative abundance of IRAK-4 can be maximized by overexpression. The production of ROS was significantly increased in IRAK-4-overexpressing cells, suggesting that IRAK-4 plays a central role in the regulation of the NADPH oxidase in response to LPS (Figure 5). The oxidative activity was increased in IRAK-4-overexpressing cells even in the absence of LPS-mediated stimulation, suggesting that the phenomenon is IRAK-4-dependent rather than a consequence of the LPS pleiotropic effect. Unfortunately, HL-60 cells transfected with the kinase-inactive IRAK-4 expression vector encoding IRAK-4 (K213A/K214A) were not fully viable, suggesting that IRAK-4 kinase activity is necessary for a survival pathway in these cells (results not shown).
Figure 5. IRAK-4 enhances the NADPH oxidase activity in granulocytes.
(A) HL-60 cells were transfected by nucleofection (Amaxa) with the IRAK-4 wild-type expression vector (●) or with the control empty vector (○). The cells were differentiated to granulocytes by incubation with DMSO for 48 h, harvested and incubated with LPS (100 ng/ml) for 30 min before stimulation with 1 μM fMLP. An example of a kinetic reaction in the absence of stimulation is also shown (▼). The production of ROS was measured by the detection of the luminol-dependent chemiluminescence after activation with fMLP at 37 °C as described in the Experimental section. (B) The oxidative response is significantly increased in granulocytes overexpressing IRAK-4. Chemiluminescence response to fMLP is expressed relative to the condition corresponding to cells transfected with the empty vector and in the absence of LPS treatment. Results are means±S.E.M. for three independent experiments. *P=0.05. (C) The cells were recovered and evaluated for the expression of IRAK-4 by Western blot (WB) using a specific anti-IRAK-4 polyclonal antibody (ProSci).
Endogenous IRAK-4 interacts with endogenous p47phox and co-localizes with the cytosolic factor at the plasma membrane after LPS stimulation in human neutrophils
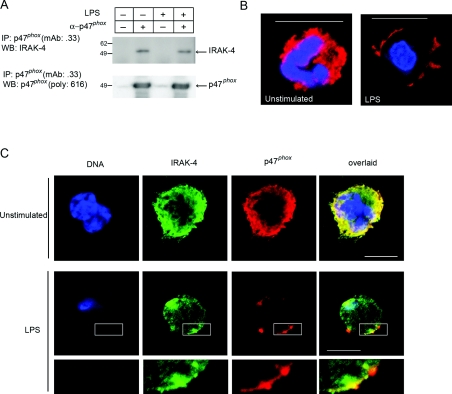
To support further a physiological role for the phosphorylation of p47phox by IRAK-4, we analysed the interaction between endogenous IRAK-4 and p47phox in human neutrophils. In Figure 6(A), we show that endogenous IRAK-4 associates with endogenous p47phox forming a complex that is stable enough to allow co-immunoprecipitation. Our results show that this interaction takes place independently of LPS treatment. Although protein kinases do not usually form stable complexes with their substrates, some substrates have been shown to be able to form detectable complexes with serine/threonine kinases [34]. In particular, IRAK-4 has been shown to co-immunoprecipitate with its substrate IRAK-1, although this interaction only takes place after stimulation [12]. We hypothesize that the interaction between IRAK-4 and p47phox may serve to promote substrate phosphorylation or to anchor the proteins together in a particular cellular compartment. To address the latter, we explored the distribution of endogenous p47phox and IRAK-4 by immunofluorescence and confocal microscopy. First, we found that p47phox, which is homogeneously distributed throughout the cytosol in resting neutrophils, translocates to the plasma membrane in response to LPS (Figure 6B). Then, we confirmed that IRAK-4 and p47phox co-localize in structures identified in close proximity to the plasma membrane after LPS treatment (Figure 6C). These results indicate that p47phox is in close proximity to IRAK-4 in human neutrophils, further supporting a role for IRAK-4 in p47phox phosphorylation after LPS treatment.
Figure 6. Endogenous IRAK-4 interacts with endogenous p47phox in neutrophils.
(A) Human neutrophils were stimulated with LPS (100 ng/ml) for 30 min and lysed, and p47phox was immunoprecipitated (IP) using a specific monoclonal antibody (mAb .33) or mouse IgG as control. The immunoprecipitates were resolved by gel electrophoresis, the proteins were transferred on to nitrocellulose and the membranes were probed against IRAK-4 using a specific polyclonal antibody (Upstate), stripped with Restore™ (Pierce) and re-probed for p47phox using our rabbit polyclonal specific antibody (616). For each experiment, LPS (E. coli; Sigma) was pre-incubated with autologous serum for 5 min before being used to stimulate the cells. WB, Western blot. Endogenous p47phox (B) and endogenous IRAK-4 and p47phox (C) were detected by immunofluorescence in human neutrophils. Cells were seeded on poly(L-lysine)-pre-coated chambered coverglass, stimulated with LPS (100 ng/ml) for 30 min or left untreated and fixed with 3.7% (w/v) paraformaldehyde. Endogenous p47phox (red) and endogenous IRAK-4 (green) were immunolabelled using a mouse monoclonal antibody (clone 0.33) and a rabbit polyclonal antibody (ProSci) respectively, as described in the Experimental section. Parallel experiments included normal mouse or rabbit IgG as negative control (results not shown). For DNA staining, DAPI was used. The samples were analysed by laser-scanning confocal microscopy. The amplified panels (lower panel in C) show areas of co-localization in the proximity of the plasma membrane. Scale bar, 10 μm.
Identification of the phosphorylation sites in endogenous p47phox after LPS stimulation
IRAK-4 and ROS production are activated in human neutrophils in response to LPS stimulation (Figure 7). To identify the residues that become phosphorylated in endogenous p47phox in neutrophils after LPS stimulation, we used microcapillary reverse-phase HPLC–nanoelectrospray MS/MS. To this end, we stimulated human neutrophils with LPS and we purified endogenous p47phox by immunoprecipitation and gel electrophoresis. The complete MS/MS spectrum is shown in Supplementary Figure S2 (http://www.BiochemJ.org/bj/403/bj4030451add.htm) and is summarized in Figure 7. We confirmed that endogenous p47phox is phosphorylated at Thr133, Ser288 and Thr356. All three sites were also detected when recombinant p47phox was phosphorylated directly by IRAK-4 (Figure 2 and Supplementary Figure S1), and they have not been previously shown to be phosphorylated by other kinases. These results strongly support the observation that IRAK-4 is a kinase for p47phox in vivo. We also detected the signature of phosphorylation at Ser320 and Ser345. Ser320, which is located in a PKC consensus region, has been shown to be phosphorylated by various PKC isoforms [25,35] and has been proposed to be phosphorylated during the initial steps of p47phox activation [36]. Phosphorylation of Ser345, which is positioned in a MAPK consensus sequence, has been shown to be phosphorylated by p38 MAPK and ERK1 (extracellular-signal-regulated kinase 1) [37]. These residues were not identified as phosphorylation sites when recombinant IRAK-4 and p47phox were utilized (Figure 2 and Supplementary Figure S1), suggesting that their phosphorylation depends on kinases activated downstream of TLR4 activation other than IRAK-4. Ser359 and Ser370 were detected in recombinant but not in endogenous p47phox (Figures 2 and 7). This is probably explained by the fact that the recovery of peptides from endogenous p47phox containing these sites was relatively low. Therefore IRAK-4-dependent phosphorylation of Ser359 and Ser370 in vivo cannot be ruled out.
Figure 7. Identification of phosphorylated residues in endogenous p47phox following LPS stimulation.
(A) The phosphorylated sites in endogenous p47phox immunoprecipitated from LPS-stimulated neutrophils were detected using microcapillary reverse-phase HPLC–nanoelectrospray and targeted ion MS/MS as described in the Experimental section. The sequences surrounding the phosphorylated residues detected by HPLC–MS/MS in endogenous p47phox are shown. Phosphorylated residues are preceded by ‘p’. (B) ROS production in human neutrophils after LPS stimulation was measured by luminol-dependent chemiluminescence. Kinetics representative of three different experiments (left-hand panel) and means±S.E.M. for three different experiments (right-hand panel) are shown. RLU, relative light units. (C and D) IRAK-4 activity in human neutrophils after LPS stimulation. IRAK-4 was immunoprecipitated (IP) from lysates obtained from neutrophils after stimulation with LPS. IRAK-4 activity was determined by the phosphorylation of the substrate histone H1.2 in the presence of 4 μCi of [γ-32P]ATP. Phosphorylation was analysed by autoradiography. (C) Results are from two different experiments. (D) The signal of phosphorylation for each time point was quantified using Quantity One 4.2.1 software. The background for each time point (IgG control) was subtracted from the IRAK-4 signal. The kinetics for two independent experiments are shown: ●, experiment 1; ■, experiment 2.
DISCUSSION
LPS triggers the priming and activation of the NADPH oxidase in human neutrophils. This mechanism is very likely to be important for an accurate neutrophil response during Gram-negative bacterial infections [38]. Despite the physiological significance of the LPS-dependent NADPH oxidase activation, the molecular mechanisms of this process remain relatively unknown. In the present study, we have found evidence that IRAK-4, a kinase that plays a central role in the signalling pathway downstream of TLRs activation by agonists including LPS, phosphorylates p47phox, increases the NADPH oxidase activity in a cell-free system, amplifies the oxidative response of intact granulocytes and interacts with p47phox in vivo. We identified the IRAK-4-dependent phosphorylation sites in p47phox and we confirmed that some of those sites become phosphorylated in endogenous p47phox after LPS stimulation. Our data suggest the molecular basis for the mechanism of LPS-dependent NADPH activation by phosphorylation.
Upon TLR4 activation, MyD88 is recruited to the receptor, a process facilitated by another adaptor protein, MAL (MyD88 adaptor-like) [39]. MyD88 then recruits IRAK-4 and IRAK-1, and phosphorylated IRAK-1 binds to TRAF6 [TNF (tumour necrosis factor) receptor-associated factor 6] which in turn recruits TGF-β (transforming growth factor β)-activated kinase 1 that phosphorylates and activates IKKβ (inhibitory κB kinase β) and MAPKK6 (MAPK kinase 6) and their respective downstream pathways [9]. The binding of LPS to TLR4 also activates a signalling pathway that is independent of MyD88. It involves the TIR domain-containing adaptor proteins TRIF (TIR domain-containing adaptor protein inducing interferon β) [40] and TRAM (TRIF-related adaptor molecule) [41]. The fact that neutrophils from patients with genetic abnormalities in IRAK-4 have a marked impairment in their oxidative response to LPS [15] suggests that the MyD88/IRAK-4-dependent pathway plays a central role in the regulation of this process. In particular, neutrophils from IRAK-4-deficient patients have been shown to lack the ability to produce H2O2 when exposed to LPS and to be unresponsive to LPS as a priming stimulus [15]. Similarly, peritoneal macrophages deficient in the adaptor protein MyD88 have impaired NADPH oxidase activity during phagocytosis [42], supporting further the significance of the MyD88/IRAK-4 signalling pathway in NADPH oxidase activation. Our results suggest that IRAK-4 is involved directly in NADPH oxidase regulation through phosphorylation of p47phox. It was suggested recently that p47phox forms a complex with TRAF6, TRIF and IRAK-1 in addition to TRAF4 and that this interaction is likely to regulate immune cell activation signalling through a novel TRAF6–TRAF4 dimerization-dependent mechanism [43]. In this context, it is possible that the phosphorylation of p47phox by IRAK-4 also plays a significant role in this regulatory mechanism.
IRAK-4-deficient granulocytes have a clear impairment in the oxidative response to LPS [15]. However, the activation of p38 MAPK in response to E. coli-type lipid A is delayed but not abolished in MyD88 deficiency [44]. In principle, these data support a role for IRAK-4 over p38 MAPK in the regulation of the oxidase in response to LPS. Nonetheless, the activation of the oxidase is a complex process, and at least two mechanisms may co-operate during LPS-dependent activation: an exocytotic event that increases the availability of cytochrome b558 subunits at the plasma membrane, and the activation through phosphorylation of p47phox. A role for p38 MAPK during LPS-dependent priming of the oxidase through exocytosis of cytochrome b558-containing granules was described previously [7]. As for the phosphorylation mechanism, our data suggest that IRAK-4 phosphorylates p47phox directly and that this mechanism is sufficient to induce the activation of the oxidase. However, simultaneous phosphorylation of p47phox by IRAK-4 and p38 MAPK may take place in vivo after LPS stimulation. In fact, we show that p47phox is phosphorylated at Ser345 after LPS stimulation, a residue involved in MAPK-dependent priming of the NADPH oxidase after TNFα and GM-CSF (granulocyte/macrophage colony-stimulating factor) stimulation [37]. Since direct phosphorylation of p47phox by IRAK-4 activates the NADPH oxidase, but Ser345 is not phosphorylated directly by IRAK-4, our results support the hypothesis from Dang et al. [37] that Ser345 is induced specifically and has a role in the priming process.
In unstimulated cells, p47phox is known to exist in a self-inhibitory state in which its tandem SH3 domains bind to a polybasic region located in its C-terminus [45]. Biochemical analyses have demonstrated that phosphorylation of p47phox at the polybasic region converts p47phox into its active form [28,46]. Structural studies have confirmed that phosphorylation at Ser303, Ser304 and Ser328 induces structural changes that expose the SH3 domains, allowing direct interaction with the NADPH oxidase membrane-associated subunit p22phox [45]. A previous study indicated that the phosphorylation of p47phox is sequentially regulated and that phosphorylation at Ser359 and Ser370 precedes that at Ser303, Ser304 and Ser328 [28]. We present evidence that IRAK-4 phosphorylates p47phox at Ser359 and Ser370 in vitro, suggesting that the mechanism of LPS-dependent activation may operate, at least in part, through sequential phosphorylation of serine residues at the polybasic region. Although we did not observe direct phosphorylation of p47phox at Ser303, Ser304 or Ser328 by IRAK-4, we show that p47phox was able to activate the NADPH oxidase in a cell-free system when phosphorylated by this kinase. It is possible that this effect was mediated by phosphorylation at other residues, in particular, at Thr133, Ser288 and Thr356, which were shown in our MS/MS analyses to be phosphorylated by IRAK-4 in vitro, and in vivo after LPS stimulation. Thr133 and Thr356 are located in the consensus sequence (D/E)XQTX(K/R) in p47phox. Interestingly, the IRAK-4 substrate IRAK-1 has a threonine residue in a similar consensus sequence in the kinase activation loop (QT383VR). A previous report suggested that Thr387, but not Thr383, is necessary for IRAK-1 autophosphorylation in vitro [47]. However, the amino acid arrangement around Thr383 suggests that it may undergo phosphorylation by IRAK-4 in vivo, and a role for Thr383 phosphorylation in vivo deserves further investigation.
We propose that phosphorylation of p47phox at Thr133, Ser288 and Ser356 may have direct impact on oxidase activation. Thr133 is positioned in a novel TRD located between the phox domain and the proximal SH3 domain of p47phox. It is possible that phosphorylation at the TRD exerts conformational changes in p47phox similar to those described for the phosphorylation at the polybasic region, thus exposing the SH3 domains for interaction with p22phox. On the other hand, an effect of TRD phosphorylation on phox domain-mediated phosphoinositide interaction is unlikely because the IRAK-4-dependent activation of the NADPH oxidase was observed when using a cell-free system with purified cytochrome b558 in the absence of phospholipids. Structural and mutagenic analyses are being conducted in our laboratory to characterize the role of the TRD and Ser288 and Thr356 in the regulation of p47phox functions.
In conclusion, we present evidence that IRAK-4 directly phosphorylates the NADPH oxidase cytosolic factor p47phox, and we suggest that this phenomenon is involved in the mechanism of activation of the oxidase. Owing to the importance of the NADPH oxidase activity in bacterial killing, we suggest that the mechanism described here may have significant consequences for the way microbial infections are resolved by the innate immune system.
Online data
Acknowledgments
This work was supported by U.S. Public Health Service Grant AI-024227 and by the Sam and Rose Stein Endowment Fund. We are thankful to Dr A.R. Cross, who provided us with purified cytochrome b558.
References
- 1.Babior B. M. The respiratory burst oxidase and the molecular basis of chronic granulomatous disease. Am. J. Hematol. 1991;37:263–266. doi: 10.1002/ajh.2830370410. [DOI] [PubMed] [Google Scholar]
- 2.Babior B. M., Woodman R. C. Chronic granulomatous disease. Semin. Hematol. 1990;27:247–259. [PubMed] [Google Scholar]
- 3.Ago T., Nunoi H., Ito T., Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox: triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47phox, thereby activating the oxidase. J. Biol. Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 4.Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McPhail L. C., Clayton C. C., Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes: evidence for regulation by multiple signals. J. Biol. Chem. 1984;259:5768–5775. [PubMed] [Google Scholar]
- 6.DeLeo F. R., Renee J., McCormick S., Nakamura M., Apicella M., Weiss J. P., Nauseef W. M. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward R. A., Nakamura M., McLeish K. R. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J. Biol. Chem. 2000;275:36713–36719. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 8.Poltorak A., Smirnova I., He X., Liu M. Y., Van H. C., McNally O., Birdwell D., Alejos E., Silva M., Du X., et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol. Dis. 1998;24:340–355. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 9.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki N., Suzuki S., Duncan G. S., Millar D. G., Wada T., Mirtsos C., Takada H., Wakeham A., Itie A., Li S., et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 11.Swantek J. L., Tsen M. F., Cobb M. H., Thomas J. A. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 2000;164:4301–4306. doi: 10.4049/jimmunol.164.8.4301. [DOI] [PubMed] [Google Scholar]
- 12.Li S., Strelow A., Fontana E. J., Wesche H. IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5567–5572. doi: 10.1073/pnas.082100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki N., Suzuki S., Yeh W. C. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–506. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 14.Janssens S., Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 15.Picard C., Puel A., Bonnet M., Ku C. L., Bustamante J., Yang K., Soudais C., Dupuis S., Feinberg J., Fieschi C., et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 16.Dang P. M., Cross A. R., Quinn M. T., Babior B. M. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67phox and cytochrome b558 II. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyal C. R., Gutierrez A., Young B. M., Catz S. D., Lin J. H., Tsichlis P. N., Babior B. M. Modulation of p47phox activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cross A. R., Erickson R. W., Ellis B. A., Curnutte J. T. Spontaneous activation of NADPH oxidase in a cell-free system: unexpected multiple effects of magnesium ion concentrations. Biochem. J. 1999;338:229–233. [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 21.Park J. W., Hoyal C. R., Benna J. E., Babior B. M. Kinase-dependent activation of the leukocyte NADPH oxidase in a cell-free system: phosphorylation of membranes and p47phox during oxidase activation. J. Biol. Chem. 1997;272:11035–11043. doi: 10.1074/jbc.272.17.11035. [DOI] [PubMed] [Google Scholar]
- 22.Cross A. R., Erickson R. W., Curnutte J. T. The mechanism of activation of NADPH oxidase in the cell-free system: the activation process is primarily catalytic and not through the formation of a stoichiometric complex. Biochem. J. 1999;341:251–255. [PMC free article] [PubMed] [Google Scholar]
- 23.Eng J. K., McCormack A. L., Yates, 3rd J. R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 24.Chittum H. S., Lane W. S., Carlson B. A., Roller P. P., Lung F. D., Lee B. J., Hatfield D. L. Rabbit β-globin is extended beyond its UGA stop codon by multiple suppressions and translational reading gaps. Biochemistry. 1998;37:10866–10870. doi: 10.1021/bi981042r. [DOI] [PubMed] [Google Scholar]
- 25.El Benna J., Faust L. P., Babior B. M. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation: phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 26.Vosbeck K., Tobias P., Mueller H., Allen R. A., Arfors K. E., Ulevitch R. J., Sklar L. A. Priming of polymorphonuclear granulocytes by lipopolysaccharides and its complexes with lipopolysaccharide binding protein and high density lipoprotein. J. Leukocyte Biol. 1990;47:97–104. doi: 10.1002/jlb.47.2.97. [DOI] [PubMed] [Google Scholar]
- 27.Aida Y., Pabst M. J. Priming of neutrophils by lipopolysaccharide for enhanced release of superoxide: requirement for plasma but not for tumor necrosis factor-α. J. Immunol. 1990;145:3017–3025. [PubMed] [Google Scholar]
- 28.Johnson J. L., Park J. W., Benna J. E., Faust L. P., Inanami O., Babior B. M. Activation of p47phox, a cytosolic subunit of the leukocyte NADPH oxidase: phosphorylation of Ser-359 or Ser-370 precedes phosphorylation at other sites and is required for activity. J. Biol. Chem. 1998;273:35147–35152. doi: 10.1074/jbc.273.52.35147. [DOI] [PubMed] [Google Scholar]
- 29.Munafo D. B., Johnson J. L., Ellis B. A., Rutschmann S., Beutler B., Catz S. D. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem. J. 2007;402:229–239. doi: 10.1042/BJ20060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-κB. J. Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 31.Hallett M. B., Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol. Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 32.El Benna J., Han J., Park J. W., Schmid E., Ulevitch R. J., Babior B. M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch. Biochem. Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson A., Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid. Redox Signaling. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 34.Brazil D. P., Park J., Hemmings B. A. PKB binding proteins: getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- 35.Fontayne A., Dang P. M., Gougerot-Pocidalo M. A., El-Benna J. Phosphorylation of p47phox sites by PKCα, βII, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 36.Yuzawa S., Suzuki N. N., Fujioka Y., Ogura K., Sumimoto H., Inagaki F. A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells. 2004;9:443–456. doi: 10.1111/j.1356-9597.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 37.Dang P. M., Stensballe A., Boussetta T., Raad H., Dewas C., Kroviarski Y., Hayem G., Jensen O. N., Gougerot-Pocidalo M. A., El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elbim C., Rajagopalan-Levasseur P., Chollet-Martin S., Gaillard J. L., Fay M., Hakim J., Fischer A., Casanova J. L., Gougerot-Pocidalo M. A. Defective priming of the phagocyte oxidative burst in a child with recurrent intracellular infections. Microbes Infect. 1999;1:581–587. doi: 10.1016/s1286-4579(99)80057-4. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald K. A., Palsson-McDermott E. M., Bowie A. G., Jefferies C. A., Mansell A. S., Brady G., Brint E., Dunne A., Gray P., Harte M. T., et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. 2001;413:78–83. doi: 10.1038/35092578. [DOI] [PubMed] [Google Scholar]
- 40.Hoebe K., Du X., Georgel P., Janssen E., Tabeta K., Kim S. O., Goode J., Lin P., Mann N., Mudd S., et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–748. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 41.Fitzgerald K. A., Rowe D. C., Barnes B. J., Caffrey D. R., Visintin A., Latz E., Monks B., Pitha P. M., Golenbock D. T. LPS–TLR4 signaling to IRF-3/7 and NF-κB involves the toll adapters TRAM and TRIF. J. Exp. Med. 2003;198:1043–1055. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laroux F. S., Romero X., Wetzler L., Engel P., Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of Gram-negative bacteria. J. Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 43.Takeshita F., Ishii K. J., Kobiyama K., Kojima Y., Coban C., Sasaki S., Ishii N., Klinman D. M., Okuda K., Akira S., Suzuki K. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur. J. Immunol. 2005;35:2477–2485. doi: 10.1002/eji.200526151. [DOI] [PubMed] [Google Scholar]
- 44.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 45.Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 46.Inanami O., Johnson J. L., McAdara J. K., Benna J. E., Faust L. R., Newburger P. E., Babior B. M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 47.Kollewe C., Mackensen A. C., Neumann D., Knop J., Cao P., Li S., Wesche H., Martin M. U. Sequential autophosphorylation steps in the interleukin-1 receptor-associated kinase-1 regulate its availability as an adapter in interleukin-1 signaling. J. Biol. Chem. 2004;279:5227–5236. doi: 10.1074/jbc.M309251200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.