Abstract
The HIV-1 gp41 (glycoprotein 41) core plays a critical role in fusion between the viral and target cell membranes. We previously identified a gp41 core-binding motif, HXXNPF, by screening the phage display peptide libraries. In the present study, we elucidated the mechanism of action of HXXNPF motif-containing molecules of different sizes, including the phage clone L7.8 (a selected positive phage clone), L7.8-g3p* (a 10–kDa fragment of the gene 3 protein) and JCH-4 (a peptide containing 13 residues of L7.8-g3p*), regarding their respective binding abilities to the six-helix bundle and inhibition on syncytium formation at different temperatures. We found that all of the HXXNPF motif-containing molecules could bind to the gp41 core, and that their binding sites may be located in the N-helix domain. L7.8-g3p* and JCH-4 effectively inhibited HIV-1 Env (envelope glycoprotein)-mediated syncytium formation at 37 °C, while the phage clone L7.8 showed no inhibition under the same conditions. However, at suboptimal temperature (31.5 °C), all of these HXXNPF motif-containing molecules were capable of inhibiting syncytium formation. These results suggest that these HXXNPF motif-containing molecules mainly bind to the gp41 core and stop the fusion process mediated by the fusion-active core, resulting in inhibition of HIV-1 fusion and entry. The HXXNPF motif-containing molecules may be used as probes for studying the role of the HIV-1 gp41 core in the late stage of the membrane-fusion process.
Keywords: gene 3 protein (g3p), glycoprotein 41 (gp41), HIV-1, membrane fusion, six-helix bundle
Abbreviations: CHO, Chinese-hamster ovary; CHR, C-terminal heptad repeat; DMEM, Dulbecco's modified Eagle's medium; Env, envelope glycoprotein; E/T, effector/target; FBS, foetal bovine serum; g3p, gene 3 protein; GMEM-S, glutamine-deficient minimal essential medium; gp, glycoprotein; IPTG, isopropyl β-D-thiogalactoside; mAb, monoclonal antibody; NHR, N-terminal heptad repeat; Ni-NTA, Ni2+-nitrilotriacetate; OPD, o-phenylenediamine; 6-HB, six-helix bundle; SPR, surface plasmon resonance; TBS, Tris-buffered saline
INTRODUCTION
The HIV-1 Env (envelope glycoprotein) consists of two subunits, gp (glycoprotein) 120 and gp41. The Env surface subunit gp120 binds to the CD4 molecule [1] and then to a chemokine co-receptor, CXCR4 or CCR5, on the target cell [2] initiating a series of conformational changes in gp41 [3]. Three NHR (N-terminal heptad repeat) helices form the central trimeric coiled-coil domain and three CHR (C-terminal heptad repeat) helices pack obliquely in an antiparallel configuration into the highly conserved hydrophobic grooves on the surface of the central coiled-coil domain [4,5]. Subsequently, the NHR and CHR regions associate to form a 6-HB (six-helix bundle) (also known as trimer-of-heterodimers or trimer-of-hairpins), representing the gp41 core. It is widely believed that formation of the 6-HB brings the viral and target cell membranes into close proximity to facilitate their merging [3,6–10]. However, Melikyan and colleagues have observed that the fusion pore forms before completion of 6-HB formation [11]. Therefore the role of the gp41 core in membrane fusion has not been clearly defined.
Peptides derived from the gp41 NHR and CHR regions (designated N- and C-peptides) and 6-HB exhibit inhibitory activity against HIV-1 by binding to the fusion-intermediate conformation of gp41 [6,12–15]. However, most of the antibodies against the N- and C-peptides and 6-HB were unable to inhibit infection under the conventional assay conditions (37 °C) [16–18]. It is possible that the gp41 N- and C-helices, as well as the 6-HB located in a narrow space between the viral and the target cell membranes, may be inaccessible to the antibodies owing to the steric hindrance effect and the unfavourable competition between the kinetics of antibody binding and the E/T (effector/target) cell membrane-fusion process. Indeed, the antibodies targeting gp41 N- and C-peptides and 6-HB exhibited no neutralizing activity under conventional infectivity conditions (37 °C), but became active in blocking HIV-1 fusion at suboptimal temperature (31.5 °C) which slows down the process of 6-HB formation [19].
We previously selected a positive phage clone L7.8 which expresses a peptide containing a motif of HXXNPF and binds to peptide N36(L8)C34. Binding of L7.8 to N36(L8)C34 was blocked by a gp41 core-specific mAb (monoclonal antibody) NC-1. Neither the phage clone L7.8 nor the mAb NC-1 could inhibit HIV-1 Env-induced cell–cell fusion at 37 °C [17,20]. However, under the same conditions, JCH-4, a peptide containing the HXXNPF motif, could effectively inhibit HIV-1 Env-mediated syncytium formation [20].
In the present study, we constructed and expressed a HXXNPF motif-containing molecule, L7.8-g3p*, which is a truncated version of g3p (gene 3 protein) of the phage clone L7.8. Then, we compared the activities of the phage clone L7.8, the recombinant protein L7.8-g3p* and a synthetic 13-mer peptide JCH-4 during binding with the HIV-1 gp41 core, and inhibiting of HIV-1 Envmediated syncytium formation, in order to determine the mechanism of action of the HXXNPF motif-containing molecules.
EXPERIMENTAL
Cell lines
3T3 cells stably transduced with MLV MX-CD4 and MX-CXCR4 vectors (3T3.T4.CXCR4) were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS (foetal bovine serum), 100–IU/ml penicillin and 100–IU/ml streptomycin (Invitrogen). CHO (Chinese-hamster ovary) cells stably transfected with the HIV-1HXB2 Env-expressing vector pEE14 (CHO-WT) or control pEE14 vector (CHO-EE) were cultured in GMEM-S (glutamine-deficient minimal essential medium) containing 400–μM methionine sulfoximine (Sigma).
Expression and purification of L7.8-g3p*
For expression of L7.8-g3p* (with a Pro-Ser-Gly-Leu-Glu-His6 tail), the gene fragments coding for the N-terminal domains of the wild-type g3p were amplified by PCR from the corresponding single-stranded phage DNA with the primers 5′-CCATGGGCAAAAAAT TATTATTCGCAATTCC-3′ (forward) and 5′-CTCGAGCCCGGACGGAGCATTGACAGGAGG-3′ (reverse) [21]. The fragments were cloned into the expression plasmid pET-28b via its NcoI and XhoI restriction sites and the proteins were overproduced in Escherichia coli strain BL21. The cells were lysed using lysis buffer (50–mM Tris/HCl, 50–mM NaCl and 10–mM EDTA, pH–8.0) and sonication. After centrifugation at 12000 g for 10 min, the supernatants containing the g3p* were collected. The g3p* was then purified by immobilized metal-affinity chromatography on a Ni-NTA (Ni2+-nitrilotriacetate) column through elution with imidazole (200 mM).
SPR (surface plasmon resonance) assay
The kinetics of the binding affinity of the polypeptide L7.8-g3p* to N36(L8)C34 was determined by SPR at 25 °C using the Biacore 2000 system. N36(L8)C34 (1 μM) was immobilized on to the CM5 sensorchip according to the amine coupling protocol, and the unreacted sites were blocked with 1 M Tris/HCl (pH 8.5). The association reaction was initiated by injecting L7.8-g3p* at a flow rate of 5 μl/min. The dissociation reaction was done by washing with PBS. M13-g3p* was used as a control. At the end of the cycle, the sensorchip surface was regenerated with 0.1 M glycine/HCl (pH 2.5) for 30 s.
ELISA
To determine the activity of the phage clone L7.8 binding to peptides N36, C34 and N36(L8)C34 respectively, wells of microplates were coated with 50 μl of N36, C34 or N36(L8)C34 (2.5 μM) in 0.1 M NaHCO3 buffer (pH 8.6) overnight. The coated wells were blocked with TBS (Tris-buffered saline), pH 7.5, containing 0.25% gelatin. After three washes with TBS containing 0.1% Tween 20 (TBS-T), phages in TBS at 5-fold serial dilutions (starting from 1011 particles/ml) were added to the wells, followed by incubation at room temperature for 1.5 h with agitation. After extensive washes, the amount of bound phage was detected by addition of peroxidase-conjugated anti-M13 phage antibody and substrate, OPD (o-phenylenediamine), sequentially. The absorbance at 450 nm was recorded.
To determine the binding activity of L7.8-g3p* and JCH-4 to N36, C34 and N36(L8)C34, wells of microplates were coated with L7.8-g3p* or JCH-4 (10 μg/ml). After blocking and washes, N36, C34 or N36(L8)C34, at 4-fold serial dilutions (starting from 5 μM), was added. After incubation at room temperature (25 °C) for 1.5 h and extensive washes, the bound N36, C34 or N36(L8)C34 was detected by addition of 50 μl of rabbit anti-N36/C34 IgG (3.3 μg/ml) and horseradish-peroxidase-conjugated goat anti-rabbit IgG and the substrate OPD, sequentially. The absorbance at 450 nm was measured.
To determine whether the phage clones L7.8, L7.8-g3p* and JCH-4 peptide block the gp41 6-HB formation, a microplate was coated with 100 μl of rabbit anti-N36/C34 IgG (4 μg/ml in 0.1 M Tris/HCl, pH 8.8) at 4 °C overnight. After blocking with 1% milk at 37 °C for 1 h, 50 μl of phage clone L7.8 (starting from 5×1010 particles/ml), L7.8-g3p* (starting from 100 μg/ml) or JCH-4 (starting from 200 μg/ml) at 4-fold serial dilutions was mixed with 25 μl of N36 (4 μM). After incubation at 37 °C for 30 min, 25 μl of C34 (4 μM) was added and incubated at 37 °C for an additional 30 min. ADS-J1, a small-molecule HIV-1 fusion inhibitor that blocks the gp41 6-HB formation [22], was included as a control. The mixture was added to the coated wells, followed by incubation at 37 °C for 1 h. After extensive washes, the 6-HB formed by N36 and C34 was detected by adding 100 μl of mAb NC-1 (5 μg/ml), and horseradish-peroxidase-conjugated anti-mouse IgG and substrate OPD, sequentially.
Assay for HIV-1 Env-mediated syncytium formation
Target (3T3.T4.CXCR4) cells (5×104) resuspended in DMEM containing 10% (v/v) FBS were plated in 48-well plates and incubated overnight at 37 °C, followed by washes with GMEM-S. Then, 3×104 effector (CHO-WT) cells pre-stimulated with 6.5 mM sodium butyrate for approx. 20 h were added in the absence or presence of an inhibitor at graded concentrations. After being co-cultured for 24 h at 37 °C, the syncytia, defined as giant cells with diameters more than four times larger than those of single cells, were counted under a microscope. The percentage inhibition of syncytium formation was calculated using the equation:
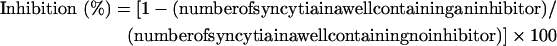 |
To determine the biological functions of the HXXNPF motif-containing molecules, phage clone L7.8 (1010 particles/ml), L7.8-g3p* (starting from 30 μg/ml) or JCH-4 peptide (starting from 200 μg/ml) was pre-incubated with CHO-WT cells at 37 °C for 60 min, followed by addition of the mixture of each peptide and CHO-WT cells to the target cells. The mAbs 2F5 (5 μg/ml) and M13-g3p* (starting from 30 μg/ml) were used as controls respectively. Other steps were the same as above.
To test the biological functions of HXXNPF motif-containing molecules at suboptimal temperature, L7.8 (1010 particles/ml), L7.8-g3p* (30 μg/ml), JCH-4 (100 μg/ml), T20 (2 ng/ml), C34 (20 ng/ml) or NC-1 (starting from 20 μg/ml) was pre-incubated with E/T cells at 31.5 °C for 2 h before transfer to 37 °C. Alternatively, the molecules above were added to the E/T cells after 2 h of pre-incubation at 31.5 °C and then pre-incubated for another 2 h before transfer to 37 °C. Syncytia were scored after 24 h. The IC50 was calculated using Calcusyn software [23].
RESULTS
Expression and characterization of L7.8-g3p*
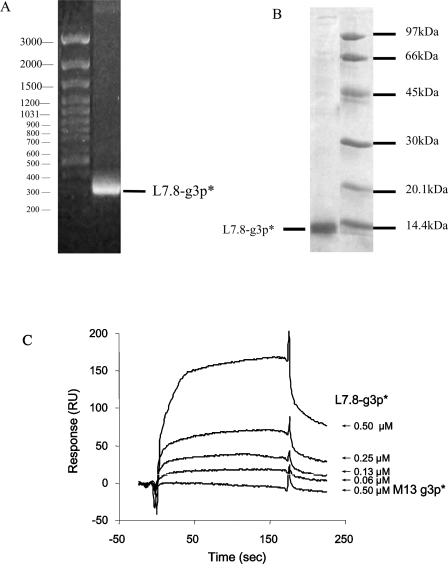
To compare the biological activities of the HXXNPF motif-containing molecules of different sizes, we designed and constructed a fragment of g3p consisting of 89 amino acid residues, including 11 residues of the peptide containing the HXXNPF motif displayed on the phage clone L7.8, and 67 residues of the N1 domain of mature g3p (amino acids 1–67) plus a Pro-Ser-Gly-Leu-Glu-His6 tail (Table 1), designated L7.8-g3p*. It is much smaller (∼10 kDa) than the phage clone L7.8. The L7.8-g3p* coding region was isolated from the phage cDNA and amplified with PCR (Figure 1A). E. coli bearing expression vectors were grown to exponential phase, and fusion-protein expression was induced by addition of IPTG (isopropyl β-D-thiogalactoside). The fusion-protein fragment L7.8-g3p* purified by metal-affinity chromatography from the cell lysates showed a single band with a molecular mass of ∼10 kDa on SDS/PAGE (Figure 1B). M13-g3p*, as a control, was purified using the same procedure.
Table 1. Amino acid sequences of the recombinant fragment L7.8-g3p* and the peptide JCH-4.
The shared sequence is highlighted in italics and the HXXNPF motif is bold and italicized. The underlined sequence is the Pro-Ser-Gly-Leu-Glu-His6 tail.
| Peptide | Sequence |
|---|---|
| L7.8-g3p* | QHLLNPFGGGSAETVESCLAKSHTENSFTNVWKDDKTLDRYANYEGCLWNATGVVVCTGDETQCYGTWVPIGLAIPENPSGLEHHHHHH |
| JCH-4 | SHSQHLLNPFGGC |
Figure 1. Purification and characterization of L7.8-g3p*.
(A) The L7.8-g3p* DNA fragment with a length of 318 bp was isolated from phage cDNA and amplified by PCR. Sizes (in bp) are indicated. (B) SDS/PAGE gel of L7.8-g3p* purified with metal-affinity chromatography. The molecular mass of L7.8-g3p* was approx. 10 kDa. Molecular masses (in kDa) are indicated. (C) Binding of L7.8-g3p* to the gp41 core. The binding of L7.8-g3p* (starting from 0.5 μM) to N36(L8)C34 that was immobilized on the sensorchip CM5 were analysed by SPR. M13-g3p* (0.5 μM) was used as a negative control. RU, response units.
SPR was performed to determine the biological activities of L7.8-g3p*. As shown in Figure 1(C), L7.8-g3p* could significantly bind to N36(L8)C34 in a dose-dependent manner, while the control M13-g3p* did not interact with N36(L8)C34 (Figure 1C). The binding parameters of L7.8-g3p* using a one-site-binding model from the response curves were: kon 1.36×104 M−1·s−1, koff 1.21×10−3 s−1, Ka 1.12×107 M−1 and Kd 8.92×10−8 M. [The association (kon) and dissociation (koff) rate constants were estimated from the response curve and the dissociation equilibrium constant (Kd) was calculated from the equation of Kd=Koff/Kon.] This result suggests that the purified L7.8-g3p* has the same 6-HB-binding ability as L7.8 [20].
L7.8, L7.8-g3p* and JCH-4 bind to N36(L8)C34 and N36
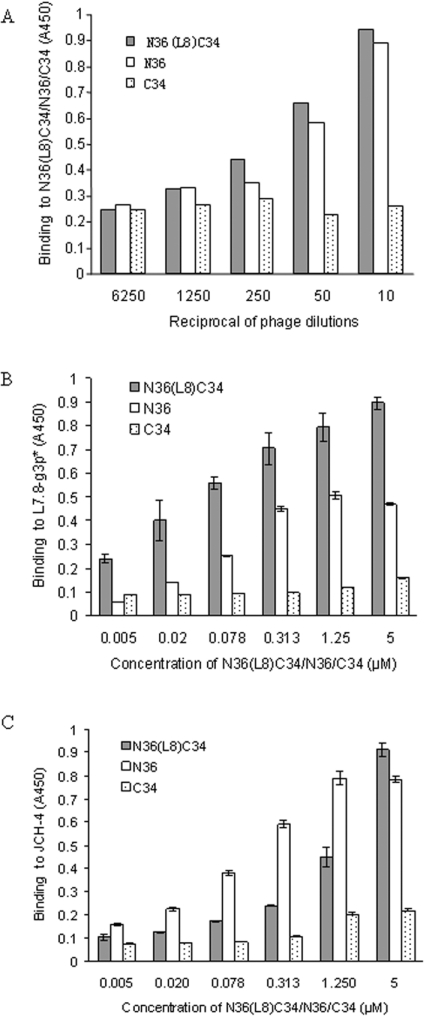
Since L7.8, L7.8-g3p* and JCH-4 all contain the HXXNPF motif, for determination of the functions of these HXXNPF motif-containing molecules, we used ELISA to compare the binding abilities of L7.8 (Figure 2A), L7.8-g3p* (Figure 2B) and peptide JCH-4 (Figure 2C) to the peptides N36, C34 and N36(L8)C34. All of the HXXNPF motif-containing molecules bound to N36 with a similar intensity to that of N36(L8)C34. However, no binding to C34 was seen. These results suggest that all of the HXXNPF motif-containing molecules specifically recognize epitopes in the NHR regions of the gp41 core.
Figure 2. Binding activities of L7.8, L7.8-g3p* and JCH-4.
Binding activities of L7.8 (A) L7.8-g3p* (B) and JCH-4 (C) to N36, C34 and N36(L8)C34 respectively were determined by ELISA.
L7.8, L7.8-g3p* and JCH-4 do not inhibit 6-HB formation
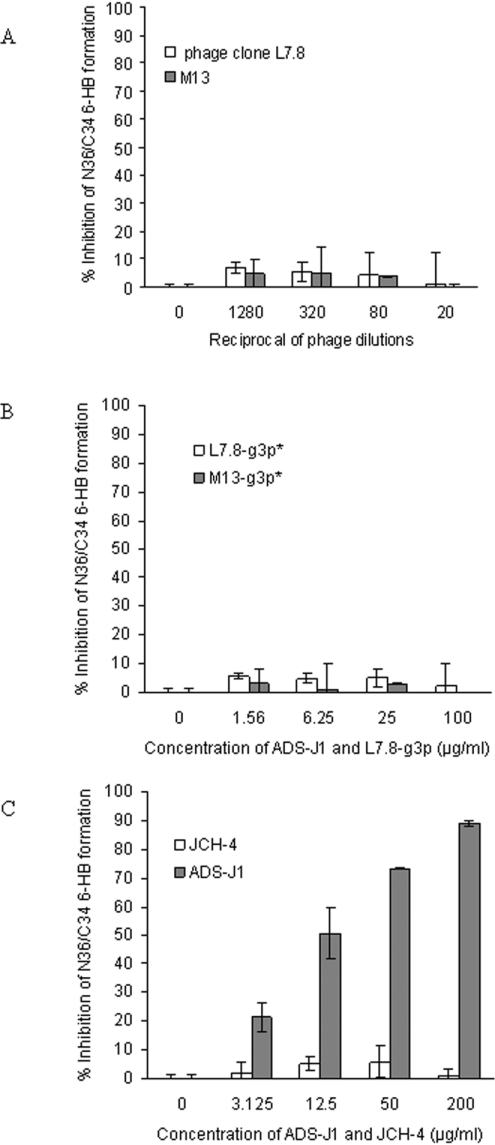
We then tested whether L7.8, L7.8-g3p* and JCH-4 could inhibit the formation of 6-HBs formed by mixing the peptides N36 and C34. ADS-J1, a small-molecule HIV-1 fusion inhibitor that can block gp41 6-HB formation [22], was used as a control. Unlike ADS-J1 (IC50=14.278±0.128 μg/ml) (Figure 3C), L7.8 (Figure 3A), L7.8-g3p* (Figure 3B) and JCH-4 (Figure 3C) could not inhibit 6-HB formation. This finding indicates that the HXXNPF motif may not bind to the residues critical for the formation of 6-HB in NHR regions, such as the e and g positions in the N-helix wheel that retain the interaction with CHR. The HXXNPF motif-containing molecules may react with residues on the exposed N-helix surface, which can be recognized by the mAb NC-1 [24].
Figure 3. Inhibitory activities of L7.8, L7.8-g3p* and JCH-4 on the formation of 6-HBs between N36 and C34.
Inhibitory activities of L7.8 (A), L7.8-g3p* (B) and JCH-4 (C) on the formation of 6-HBs between N36 and C34 respectively were determined by ELISA. ADS-J1 was used as a control.
L7.8, L7.8-g3p* and JCH-4 exhibit different patterns of inhibition of HIV-1 Env-mediated syncytium formation at different temperatures
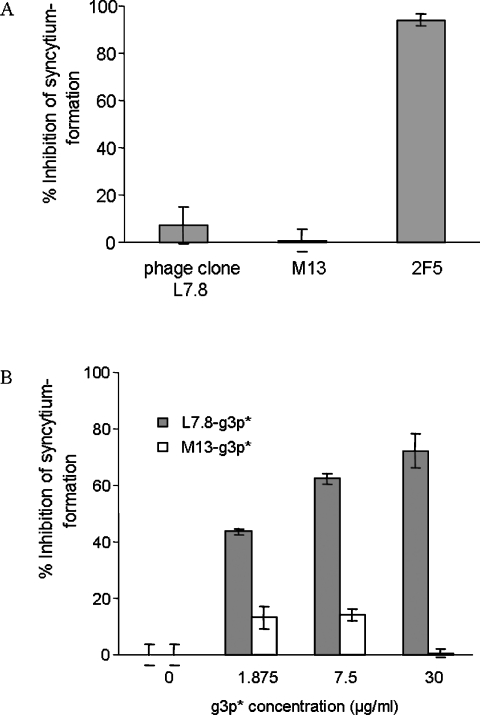
We then investigated whether L7.8, L7.8-g3p* and JCH-4 could inhibit HIV-1 Env-mediated syncytium formation at 37 °C. Both phage clone L7.8 and M13 showed no inhibition, while the control mAb 2F5 had potent inhibitory activity on syncytium formation (Figure 4A). L7.8-g3p* exhibited inhibitory effects on syncytium formation in a dose-dependent manner (IC50=2.975 ±0.005 μg/ml), while the control M13-g3p* did not show any inhibition (Figure 4B). We showed previously that JCH-4 could inhibit syncytium formation (IC50=9.121±1.980 μg/ml) [20]. These results suggest that the large HXXNPF motif-containing molecules, e.g. phage clone L7.8, may not have access to the gp41 core to block membrane fusion owing to steric interference, while the recombinant fragment L7.8-g3p* and synthetic peptide JCH-4 may do so, resulting in inhibition of membrane fusion.
Figure 4. Determination of inhibitory activity of HIV-1 Env-mediated syncytium-formation at 37 °C by phage clone L7.8 (A) and L7.8-g3p* (B).
Phages M13 and M13-g3p* were used as controls. Results are means±S.E.M. for at least two independent measurements for each concentration.
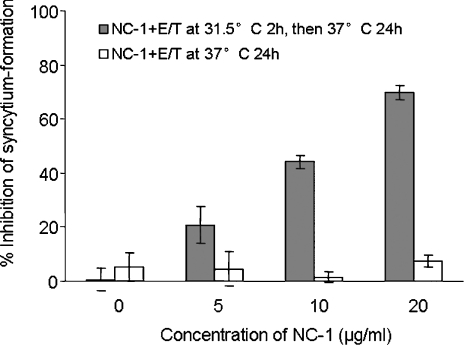
It has been reported that when E/T cells are pre-incubated at suboptimal temperature (31.5 °C) for 1 h to prolong fusion-intermediate states, a significant portion of prehairpin fusion intermediates undergo transition to 6-HBs and remain sensitive to anti-bundle antibodies [19]. We first used NC-1 to see whether it could stop 6-HB formation by CHO-WT and 3T3.T4.CXCR4 at suboptimal temperature. A previous study showed that NC-1 itself had no detectable inhibitory activity on syncytium formation at 37 °C [17] (Figure 5). However, when NC-1 was pre-incubated with E/T cells at 31.5 °C for 2 h before transfer to 37 °C, it efficiently blocked fusion (Figure 5). These results suggest that, at suboptimal temperature, the fusion process is prolonged so that NC-1 can gain access to, and bind with, the gp41 core or fusion-intermediate, resulting in inhibition of syncytium formation.
Figure 5. Comparison of inhibitory activities of NC-1 on HIV-1 Env-mediated syncytium formation at suboptimal temperature (31.5 °C) and conventional temperature (37 °C).
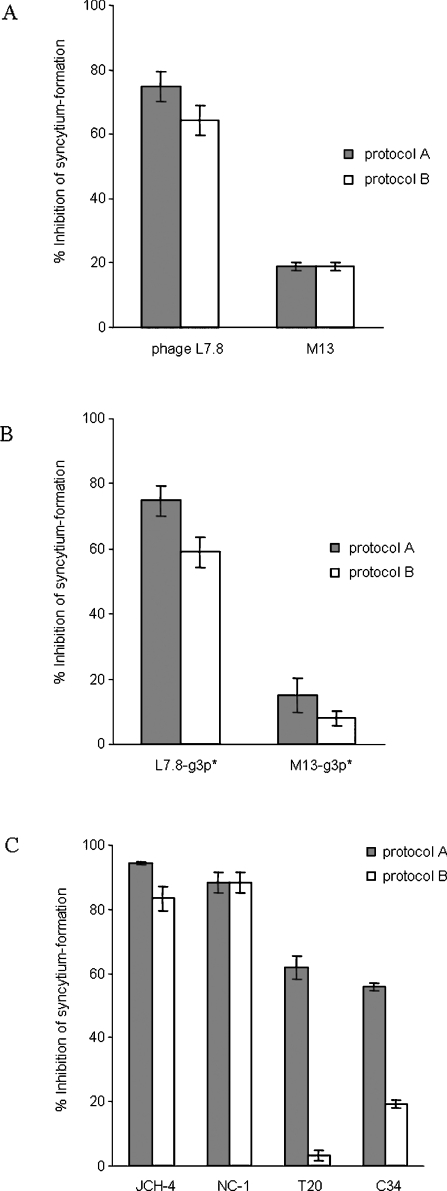
If the HXXNPF motif-containing molecules inhibit fusion by binding to the 6-HB, inhibition after the formation of 6-HB may be seen. However, if they inhibit fusion by binding to the prehairpin fusion intermediates, they may then show no fusion inhibition after 6-HB formation. Two protocols were used to define the biological mechanisms of HXXNPF motif-containing molecules. When L7.8 (Figure 6A), L7.8-g3p* (Figure 6B) or JCH-4 (Figure 6C) was pre-incubated with E/T cells at 31.5 °C for 2 h before transfer to 37 °C (protocol A), like NC-1 (Figure 6C), all of them showed inhibition of syncytium formation, whereas the controls M13 (Figure 6A) and M13-g3p* (Figure 6B) showed no inhibition of fusion. When L7.8 (Figure 6A), L7.8-g3p* (Figure 6B) or JCH-4 (Figure 6C) was added to the E/T cells after 2 h of pre-incubation at 31.5 °C to allow the formation of 6-HB, and then incubated for another 2 h before transfer to 37 °C (protocol B), they still retained inhibitory activities on syncytium formation, while the controls M13 (Figure 6A) and M13-g3p* (Figure 6B) showed no inhibition of fusion. When tested using protocol B, the C-peptides C34 and T20, as controls, exhibited much lower inhibitory activity on syncytium formation than that obtained using protocol A (Figure 6C), consistent with the report of Golding et al. [19]. These results suggest that, unlike the C-peptides, HXXNPF motif-containing molecules mainly interact with the gp41 core to mediate inhibition of membrane fusion.
Figure 6. Inhibitory activity of L7.8 (A), L7.8-g3p* (B) or JCH-4 (C) on HIV-1 Env-mediated syncytium formation.
The inhibitory activities were tested using two protocols. Protocol A: E/T+inhibitor (31.5 °C, 2 h)→37 °C; Protocol B: E/T (31.5 °C, 2 h)+inhibitor (31.5 °C, 2 h)→37 °C. The phage M13, recombinant protein M13-g3p* and peptides C34 and T20 were included as controls.
In the syncytium-formation experiment, 1010 L7.8 phage clone particles/ml exhibited similar inhibitory activity at the suboptimal temperature (31 °C) as 2×1015 L7.8-g3p* molecules/ml and 3.34×1016 JCH-4 molecules/ml (calculated based on the micromolar concentration) did, suggesting that one phage clone particle possesses much stronger inhibitory activity than the smaller molecules L7.8-g3p* and JCH-4. However, at 37 °C, the same amount of phage clone particles showed no inhibition on HIV-1 Env-mediated syncytium formation. This may not be explained by the lack of sufficient inhibitory molecules, since no inhibition was observed when much higher concentrations of L7.8 phage clone particles (1012 particles/ml) were tested (results not shown).
DISCUSSION
Using the single-chain polypeptide N36(L8)C34 as an in vitro model of the gp41 6-HB to screen for gp41 core-binding motif(s) from the 7-mer and 12-mer peptide libraries displayed on the bacteriophage M13, we have identified a common gp41 core-binding motif, HXXNPF. To test whether the HXXNPF-containing molecules of different sizes still retain their biological functions, we constructed and expressed a 10 kDa polypeptide (L7.8-g3p*) that was derived from the N-terminus of g3p of the phage clone L7.8. We also synthesized a short peptide, JCH-4, which corresponds to the partial sequence of L7.8-g3p*. Results suggest that, like L7.8, both L7.8-g3p* and JCH-4 bind to the gp41 core, even though they are different in size.
The HXXNPF motif-containing molecules also bound to N36, but not to C34. This suggests that the binding sites for these HXXNPF motif-containing molecules may be located in the N-helix domain in the gp41 core. Coincidently, the mAb NC-1 was reported to bind with both the gp41 core and the N-helical trimers [24,25], suggesting that NC-1 may bind to the 6-HB through its interaction with the N-helix surface which is exposed between the C-helices in the 6-HB [24]. Since NC-1 and the HXXNPF motif-containing molecules could compete with each other for binding to the 6-HB [20], this suggests that they may share a common binding site in the gp41 core.
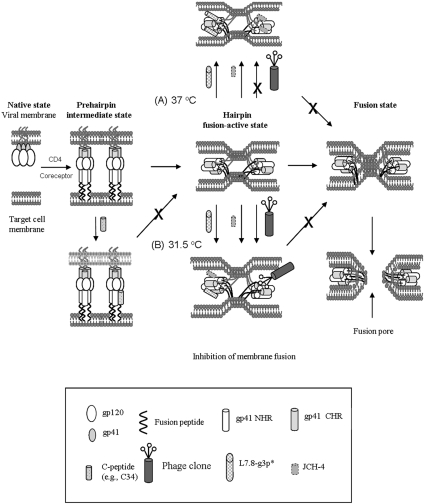
The polypeptide L7.8-g3p* and synthetic peptide JCH-4 could inhibit the gp41-mediated syncytium formation, but could not block the 6-HB formation. This result suggests that these inhibitors may not affect the early stages (e.g. native and intermediate states), but rather act at the late stages of membrane fusion (e.g. fusion-active or pore-formation states). Unlike L7.8-g3p* and JCH-4, the phage clone L7.8 does not have HIV-1 fusion-inhibitory activity at 37 °C (Figure 4A), although they all can interact with the gp41 core. It is possible that the 6-HB, located in a narrow space between the viral and target cell membranes, may be inaccessible to the phage particle L7.8 owing to its large size. It is reported that an inhibitor as large as 41 kDa cannot access the gp41 N-helices during the membrane-fusion process because of the steric blockage effect [26]. In contrast, L7.8-g3p* and JCH-4, protein fragments with a molecular size much smaller than that of L7.8, may be able to access and bind with the 6-HB, perhaps through their interaction with the N-helices in the gp41 core, during the late stages of membrane fusion. Binding of L7.8-g3p* and JCH-4 to the gp41 fusion-active core may hinder the merging of the viral and the target cell membranes or interfere with the fusion pore formation, resulting in the inhibition of HIV-1 Env-induced membrane fusion (Figure 7).
Figure 7. Model showing interaction of the HXXNPF motif-containing molecules with the 6-HB and inhibition of HIV-1 Env-mediated membrane fusion.
After viral gp120 binds to CD4 and a co-receptor on the target cell, gp41 changes its conformation. The fusion peptide inserts into the target cell membrane and the NHR and CHR regions interact with each other to form a 6-HB, bringing the viral and the cellular membranes into close proximity. The gp41 core may play an important role in the late stage of membrane-fusion events, e.g. formation of the fusion pores. Binding of the small molecules L7.8-g3p* and the peptide JCH-4 to the N-helices in the 6-HB at the fusion-active state may prevent the further fusion between the viral and target cell membranes (A and B). The larger molecules, e.g. the phage clone L7.8 and mAb NC-1, though they can bind to the 6-HB formed by the N- and C-peptides in vitro, they cannot access the fusion-active gp41 core formed by the viral gp41 NHR and CHR regions at the conventional temperature (37 °C), resulting in their inability to inhibit HIV fusion (A). However, when suboptimal temperature (31.5 °C) is used to slow down the fusion process and prolong the exposure time of the gp41 coiled-coil structure, the phage clone L7.8 may be able to gain access to the gp41 core and inhibit membrane fusion (B).
To investigate whether the HXXNPF motif-containing molecule-mediated inhibition of syncytium formation occurs before or after the gp41 core formation, we adapted a strategy reported by Golding et al. [19] to slow down the fusion process by using suboptimal temperature (31.5 °C), to dissect steps in cell–cell fusion and to define the potential target sites of the HXXNPF motif-containing molecules. Golding et al. [19] have demonstrated that, after 1 h of co-culture of the E/T cells at 31.5 °C, a significant portion of the prehairpin fusion intermediates have undergone transition to 6-HB, which are resistant to inhibition by C-peptides and antibodies against N- and C-peptides, but become sensitive to 6-HB-specific mAbs, 1281 and 167-D. In agreement with their findings, we show in the present study that the inhibitory activity of the C-peptides C34 and T20 on HIV-1 Env-mediated syncytium formation significantly decreased when added to the E/T cells which were pre-incubated at 31.5 °C for 2 h, while the inhibitory activity of the 6-HB-specific mAb did not show a significant difference when it was tested using the two protocols (Figure 6C). All of the HXXNPF motif-containing molecules retained similar levels of syncytium formation inhibitory activities even they were added to the E/T cells 2 h after these cells co-cultured at 31.5 °C (Figure 6). These results suggest that HXXNPF motif-containing molecules, like the 6-HB-specific mAbs but unlike the C-peptides, inhibit syncytium formation by mainly binding to the fusion-active gp41 core (Figure 7), although we could not exclude the possibility that these molecules may also interact with the gp41 N-helices, which retain the fusion intermediate conformation during the delayed fusion process. Melikyan et al. [27] have proposed that 6-HB may function as a stabilizer of the fusion pore in preventing its collapse and ensuring its growth. Binding to the 6-HB by the HXXNPF motif-containing molecules may block the function of 6-HB, resulting in collapse of the fusion pores and halting of the HIV-1 fusion process.
In conclusion, this work provides new insights into the mechanism of action of HXXNPF motif-containing molecules on HIV-1 Env-mediated syncytium formation. These molecules can be used as probes for studying the possible role of the gp41 core in the late stages of membrane fusion and for identifying new targets in gp41 for rational design of novel anti-HIV-1 therapeutics.
Acknowledgments
We thank Veronica L. Kuhlemann at the New York Blood Center for critical reading of the manuscript. This work was supported by the Emphases Project of NSFC-30530680 and NSFC-30221003 to Y.-H. C. and the NIH (National Institutes of Health) grant of the United States (AI46221) to S. J. The 3T3.T4.CXCR4, CHO-WT and CHO-EE cell lines were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID (National Institute of Allergy and Infectious Diseases), NIH, Bethesda, MD, U.S.A., contributed by Dr Dan R. Littman, Dr Carol Weiss and Dr Judith White respectively.
References
- 1.Sattentau Q. J., Moore J. P. The role of CD4 in HIV binding and entry. Philos. Trans. R. Soc. London Ser. B. 1993;342:59–66. doi: 10.1098/rstb.1993.0136. [DOI] [PubMed] [Google Scholar]
- 2.Berger E. A., Murphy P. M., Farber J. M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Chan D. C., Kim P. S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 4.Chan D. C., Fass D., Berger J. M., Kim P. S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 5.Weissenhorn W., Dessen A., Harrison S. C., Skehel J. J., Wiley D. C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–428. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 6.Lu M., Blacklow S. C., Kim P. S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 7.Munoz-Barroso I., Durell S., Sakaguchi K., Appella E., Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu W., Liu J., Ji H., Radigan L., Jiang S., Lu M. Helical interactions in the HIV-1 gp41 core reveal structural basis for the inhibitory activity of gp41 peptides. Biochemistry. 2000;39:1634–1642. doi: 10.1021/bi9921687. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S., Lu H., Liu S., Zhao Q., He Y., Debnath A. K. N-substituted pyrrole derivatives as novel human immunodeficiency virus type 1 entry inhibitors that interfere with the gp41 six-helix bundle formation and block virus fusion. Antimicrob. Agents Chemother. 2004;48:4349–4359. doi: 10.1128/AAC.48.11.4349-4359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S., Lu H., Xu Y., Wu S., Jiang S. Different from the HIV fusion inhibitor C34, the anti-HIV drug Fuzeon (T-20) inhibits HIV-1 entry by targeting multiple sites in gp41 and gp120. J. Biol. Chem. 2005;280:11259–11273. doi: 10.1074/jbc.M411141200. [DOI] [PubMed] [Google Scholar]
- 11.Markosyan R. M., Cohen F. S., Melikyan G. B. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell. 2003;14:926–938. doi: 10.1091/mbc.E02-09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wild C., Oas T., McDanal C., Bolognesi D., Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang S., Lin K., Strick N., Neurath A. R. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 14.Wild C., Dubay J. W., Greenwell T., Baird T., Jr, Oas T. G., McDanal C., Hunter E., Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markosyan R. M., Ma X., Lu M., Cohen F. S., Melikyan G. B. The mechanism of inhibition of HIV-1 env-mediated cell-cell fusion by recombinant cores of gp41 ectodomain. Virology. 2002;302:174–184. doi: 10.1006/viro.2002.1593. [DOI] [PubMed] [Google Scholar]
- 16.Hioe C. E., Xu S., Chigurupati P., Burda S., Williams C., Gorny M. K., Zolla-Pazner S. Neutralization of HIV-1 primary isolates by polyclonal and monoclonal human antibodies. Int. Immunol. 1997;9:1281–1290. doi: 10.1093/intimm/9.9.1281. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S., Lin K., Lu M. A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the HIV-1 envelope glycoprotein. J. Virol. 1998;72:10213–10217. doi: 10.1128/jvi.72.12.10213-10217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C. H., Greenberg M. L., Bolognesi D. P., Matthews T. J. Monoclonal antibodies that bind to the core of fusion-active glycoprotein 41. AIDS Res. Hum. Retroviruses. 2000;16:2037–2041. doi: 10.1089/088922200750054765. [DOI] [PubMed] [Google Scholar]
- 19.Golding H., Zaitseva M., de Rosny E., King L. R., Manischewitz J., Sidorov I., Gorny M. K., Zolla-Pazner S., Dimitrov D. S., Weiss C. D. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 2002;76:6780–6790. doi: 10.1128/JVI.76.13.6780-6790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J. H., Liu Z. Q., Liu S., Jiang S., Chen Y. H. Identification of the HIV-1 gp41 core-binding motif: HXXNPF. FEBS Lett. 2006;580:4807–4814. doi: 10.1016/j.febslet.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 21.Martin A., Schmid F. X. Evolutionary stabilization of the gene-3-protein of phage fd reveals the principles that govern the thermodynamic stability of two-domain proteins. J. Mol. Biol. 2003;328:863–875. doi: 10.1016/s0022-2836(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 22.Jiang S., Lin K., Zhang L., Debnath A. K. A screening assay for antiviral compounds targeted to the HIV-1 gp41 core structure using a conformation-specific monoclonal antibody. J. Virol. Methods. 1999;80:85–96. doi: 10.1016/s0166-0934(99)00041-5. [DOI] [PubMed] [Google Scholar]
- 23.Chou T. C., Talalay P. Quantitative analysis of dose–effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Dimitrov A. S., Louis J. M., Bewley C. A., Clore G. M., Blumenthal R. Conformational changes in HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion and inactivation. Biochemistry. 2005;44:12471–12479. doi: 10.1021/bi051092d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wexler-Cohen Y., Johnson B. T., Puri A., Blumenthal R., Shai Y. Structurally altered peptides reveal an important role for N-terminal heptad repeat binding and stability in the inhibitory action of HIV-1 peptide. Dp178. J Biol. Chem. 2006;281:9005–9010. doi: 10.1074/jbc.M512475200. [DOI] [PubMed] [Google Scholar]
- 26.Hamburger A. E., Kim S., Welch B. D., Kay M. S. Steric accessibility of the HIV-1 gp41 N-trimer region. J. Biol. Chem. 2005;280:12567–12572. doi: 10.1074/jbc.M412770200. [DOI] [PubMed] [Google Scholar]
- 27.Melikyan G. B., Markosyan R. M., Hemmati H., Delmedico M. K., Lambert D. M., Cohen F. S. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 2000;151:413–424. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]