Abstract
The dopamine transporter (DAT) is a target of amphetamine (AMPH) and cocaine. These psychostimulants attenuate DAT clearance efficiency, thereby increasing synaptic dopamine (DA) levels. Re-uptake rate is determined by the number of functional transporters at the cell surface as well as by their turnover rate. Here, we present evidence that DAT substrates, including AMPH and DA, cause internalization of human DAT, thereby reducing transport capacity. Acute treatment with AMPH reduced the maximal rate of [3H]DA uptake, decreased AMPH-induced currents, and significantly redistributed the immunofluorescence of an epitope-tagged DAT from the plasma membrane to the cytosol in human embryonic kidney 293 cells. Conversely, DAT inhibitors, such as cocaine, mazindol, and nomifensine, when administered with AMPH, blocked the reduction in [3H]DA uptake and the redistribution of DAT immunofluorescence to the cytosol. The reductions of [3H]DA uptake and AMPH-induced DAT internalization also were inhibited by coexpression of a dominant negative mutant of dynamin I (K44A), indicating that endocytosis modulates transport capacity, likely through a clathrin-mediated pathway. With this mechanism of regulation, acute application of AMPH would reduce DA uptake not only by direct competition for uptake, but also by reducing the available cell-surface DAT. Moreover, AMPH-induced internalization might diminish the amount of DAT available for DA efflux, thereby modulating the cytotoxic effects of elevated extracellular DA.
Dopamine (DA) signaling in the central nervous system mediates a wide variety of physiologic functions such as movement, motivational control of voluntary behavior, and lactation (1, 2). The magnitude and duration of DA signaling is defined by the amount of vesicular release, the sensitivity of the DA receptors, and the efficiency of DA clearance. The DA transporter (DAT) is largely responsible for regulating DA clearance (3).
Psychostimulants, such as cocaine and amphetamine (AMPH), induce DA overflow into the synaptic cleft by acting on the DAT, thereby enhancing dopaminergic transmission (4). Cocaine acts by inhibiting the re-uptake of released DA (5, 6). AMPH-like drugs, however, are thought to promote the release of the transmitter (carrier-mediated efflux) as well as to inhibit its uptake (7, 8). Repeated administration of AMPH has been shown to sensitize monoaminergic synapses to subsequent psychostimulant challenge (9). Furthermore, administration of a single, high dose of AMPH acutely (1 h) decreased DAT function in vivo as assessed in striatal synaptosomes prepared from drug-treated rats (10). In contrast, administration of a high dose of cocaine had no effect on subsequent transporter activity (10).
To explore the mechanism for the differential effects of AMPH and cocaine on the homeostatic uptake capacity of the human DAT (hDAT), we stably expressed a FLAG-tagged hDAT in EM4 cells (see Materials and Methods). The use of the FLAG fusion protein has provided the opportunity for confocal microscopy analysis of trafficking of the transporter in cells. Here, we report that AMPH caused the hDAT to redistribute intracellularly in a dynamin-dependent manner, consequently reducing subsequent DA transport capacity. These results provide a mechanism for the AMPH-induced elevation of synaptic DA mediated through a reduction of the number of transporters on the cell surface.
Materials and Methods
Cell Culture.
We created a synthetic hDAT gene, which was tagged at the amino terminus with a FLAG epitope. The gene encodes a protein with an amino acid sequence identical to that of wild-type hDAT with the Met at position 1 replaced by MDYKDDDDKA, but the nucleotide sequence was altered to increase the number of unique restriction sites and to optimize codon utilization. The nucleotide sequence of this construct and its creation will be described elsewhere. The FLAG-tagged syntheticDAT was subcloned into a bicistronic expression vector that expresses the syntheticDAT from a cytomegalovirus promoter and the hygromycin resistance gene from an internal ribosomal entry site. This vector, pCIHyg, was constructed by replacing the NsiI to XhoI fragment from pCIN4 (11) (kindly provided by S. Rees, Glaxo Wellcome) with the NsiI to XhoI fragment from pIREShygro (CLONTECH). The final construct is referred to as FLAG-hDAT, whereas the non-FLAG-tagged hDAT construct is referred to as hDAT. (Some biotinylation experiments also were done in a FLAG-hemagglutinin hDAT construct, in which the first 22 residues of the hDAT sequence in FLAG-hDAT were replaced by a hemagglutinin tag.)
EM4 cells, human embryonic kidney (HEK) 293 cells stably transfected with macrophage scavenger to increase their adherence to tissue culture plastic (12), were kindly provided by R. Horlick (Pharmacopeia, Cranbury, NJ). EM4 cells were stably transfected with the FLAG-hDAT with Lipofectamine (GIBCO/BRL) as described (13), and a stably transfected pool was selected in hygromycin (250 μg/ml). Cells were grown in DMEM supplemented with 10% FBS at 37°C and 5% CO2. In addition, in some experiments, HEK 293 cells stably transfected with the wild-type hDAT cDNA in pCIN4 as described (13) were used.
Uptake of [3H]DA.
EM4 cells expressing FLAG-hDAT were seeded in 96- or 24-well plates ≈48 h before the experiments and grown to confluence (approximately 25,000 cells per well in 96-well plates, 100,000 cells per well for 24-well plates). The FLAG-hDAT cells were preincubated with or without 2 μM d-AMPH (Sigma) in serum-free DMEM (GIBCO) for 1 h at 37°C, unless stated otherwise. One millimolar tropolone (Sigma) was added during the last 10 min of the incubation. The plate of cells was placed on ice and washed three times with 200 μl ice-cold uptake buffer (130 mM NaCl/1.3 mM KCl/10 mM Hepes/1.2 mM MgSO4/1.2 mM KH2PO4/2.2 mM CaCl2/10 mM glucose, pH 7.4). After the washes, the cells were placed on a 37°C plate warmer, and [3H]DA (NEN) uptake was performed immediately. In triplicate wells, 7 nM [3H]DA together with 3 μM DA was added in a final volume of 50 μl unless otherwise stated. All reagents were diluted in uptake buffer prewarmed to 37°C. The mixture was incubated at 37°C for 1 min and then aspirated to terminate uptake. After three washes with ice-cold buffer, cells were permeabilized with 50 μl 0.1% Triton X-100. Radioactivity was measured in a Trilux scintillation counter with OptiPhase Supermix mixture (Wallac, Gaithersburg, MD). Specific uptake was defined as total uptake less nonspecific in the presence of 5 μM mazindol (MZ).
The experimental determination of Km and Vmax after treatment with AMPH or vehicle was done as described above with several modifications. Cells were treated for 4 h with 40 μg/ml cycloheximide at 37°C in serum-free DMEM to block the potential contribution of newly synthesized transporter coming to the cell surface during the uptake assay. To minimize internalization during the assessment of uptake, the uptake experiments were done at 18°C for 2 min. [3H]DA (100 nM) was used with nine concentrations of unlabeled DA between 0.03 μM and 60 μM, and the data were fit by homologous competition by nonlinear regression (prism, GraphPad, San Diego).
Transient Expression of Dominant Negative Dynamin.
The stable FLAG-hDAT expressing EM4 cells were transiently transfected with the human wild-type and K44A mutant dynamin I in pCB1 by using the calcium phosphate transfection method with overnight incubation in the presence of 1 μg of DNA per 35-mm dish (immunofluorescence assays) and 0.25 μg per well of a 24-well plate (uptake assays). Forty-eight hours after transfection, the cells were treated with either AMPH or vehicle for 1 h at 37°C in uptake buffer, and uptake was determined as described above except at 10°C for 2 min in a final volume of 250 μl of uptake buffer containing 50 nM [3H]DA, 100 μM ascorbic acid, and 100 μM pargyline. The transfection efficiency was 70–80% as assessed by fluorescence microscopy of green fluorescent protein (GFP) in parallel transient transfection with pGREEN LANTERNTM-1 (GIBCO/BRL) (data not shown).
Immunofluorescence Analysis.
To examine the localization and trafficking of the hDAT in the FLAG-hDAT cell line, cells were treated with AMPH (2 μM) at 37°C for 1 h unless otherwise stated. Preincubations of 20 min were done for experiments involving Con A (250 μg/ml), MZ (3 μM), nomifensine (2 μM), and cocaine (3 μM), followed by the addition of AMPH. The immunostaining steps were conducted at room temperature while rocking the cells on a rotating platform. The coverslips containing the cells (50–70% confluent) were washed twice with PBS (154 mM NaCl/11 mM Na2HPO4/2.7 mM KCl/1 mM MgCl2/0.1 mM CaCl2, pH 7.4). They then were fixed for 25 min with 4% paraformaldehyde made up in PBS. The cells were rinsed twice with PBS and blocked with 5% normal goat serum diluted in 0.05% Triton X-100/PBS (PBST) for 1 h. The blocking solution then was aspirated and the cells were rinsed once with PBST. The coverslips were incubated with the primary antibody (anti-FLAG M-2 mAb, Sigma #F3165) at a dilution of 1:3,000 in PBST for 1 h. The primary antibody was aspirated, and the cells were washed three times with PBST for 5 min per incubation. After washing, the cells were incubated with secondary antibody [goat anti-mouse IgG (H&L) TriTC, Kirkegarrd & Perry Laboratories #03–18-06] diluted 1:200 in PBST for 1 h. The secondary antibody was removed and the cells were washed three times with PBST (5-min incubations each), and once with PBS. Coverslips then were mounted onto slides with Crystal Mount (Biomedia, Foster City, CA) and allowed to dry. Confocal microscopy was performed by using a Bio-Rad MRC1024 confocal imaging system operated via a Nikon Diaphot inverted microscope. Sample illumination was via a krypton-argon laser with 568-nm excitation and a 598 ± 40-nm emission filter. lasersharp acquisition software (Bio-Rad) allowed for z-resolution imaging at 1-μm increments.
Biochemical Analysis of Transporter Endocytosis Using Cleavable Biotin.
Cell surface biotinylation with cleavable sulfo-N-hydroxysuccinimide-S-S-biotin (0.25 mg/ml) (Pierce) and glutathione strip to cleave all surface-localized biotinylated transporter were performed on stably transfected EM4 cells expressing FLAG-DAT (or FLAG-hemagglutinin-DAT) as described (14). After biotinylation but before the strip, cells were incubated for 1 h at 37°C in DMEM supplemented or not with 2 μM d-AMPH. Cells were scraped into PBS-PI buffer (PBS supplemented with 1 μg/ml leupeptin, 1 μg/ml pepstatin, 2 μg/ml aprotinin, 2 μg/ml pefablock, and 10 mM N-ethylmaleimide). Cells were pelleted at 4°C and incubated in PBS-PI supplemented with 0.2% digitonin at 4°C for 20 min. Cells were pelleted and then incubated in lysis buffer (PBS-PI containing 1% Triton X-100) at 4°C for 45 min. The extract was centrifuged at 4°C (14,000 × g) for 30 min. An aliquot of the extract was removed for determination of total DAT. The remaining extract was incubated with 50 μl neutrAvidin Plus beads (Pierce) for 1 h at room temperature. The beads were washed twice with lysis buffer and then eluted with SDS sample buffer containing 100 mM DTT. The crude extracts and the eluted proteins were resolved by SDS/PAGE, transferred to poly(vinylidene difluoride) membranes (Millipore), and blocked for 1 h in 5% dry milk, 1% BSA, 0.1% Tween-20 in Tris-buffered saline. FLAG-DAT (or FLAG-hemagglutinin-DAT) was detected by anti-FLAG M-2 primary antibody and anti-mouse-horseradish peroxidase secondary antibody (Santa Cruz Biotechnology), with ECL-Plus (Amersham Pharmacia) and fluorescence detection and quantitation on a Storm 840 imager (Molecular Dynamics).
Electrophysiology.
Parental or stably transfected cells were plated at a density of 105 per 35-mm culture dish. Before electrical recording (performed at 25°C) attached cells were washed three times with bath solution at room temperature. The bath contained: 130 mM NaCl, 1.3 mM KCl, 1.3 mM KH2PO4, 0.5 mM MgSO2, 1.5 mM CaCl2, 10 mM Hepes, and 34 mM dextrose. The solution was adjusted to pH 7.35 and 300 mOsm with 1 M NaOH and dextrose. Pipette solutions for the whole-cell recording contained: 130 mM KCl, 0.1 mM CaCl2, 2 mM MgCl2, 1.1 mM EGTA, 10 mM Hepes, and 30 mM dextrose adjusted to pH 7.35 and 270 mOsm. Free Ca2+ in the pipette was calculated as 0.1 μM. Electrodes were pulled with a programmable puller (Sutter Instruments, Novato, CA, p-2000). Series conductance was 0.1 μS or greater, and cell capacitance was 25 to 80 pF. Voltage steps ranged from −140 to +60 mV and lasted 500 msec. Between test pulses membrane potential was held at −40 mV for 4 sec. Values for steady-state currents were taken between 400 and 500 msec after the step. An Axopatch 200B amplifier band-limited at 5,000 Hz was used to measure current. Data were stored digitally on VCR and analyzed on a Nicolet Integra oscilloscope and a Pentium computer, using instrumentation and programs written by W.N. Goolsby (Emory University, Atlanta, available on request).
Results and Discussion
DA uptake was not observed in EM4 cells not transfected with the hDAT. Moreover, neither 2 μM AMPH, 100 μM DA, nor 2 μM AMPH together with 3 μM MZ produced whole-cell inward currents in untransfected cells. Thus, the EM4 cells provided a suitable null background in which to study the function and trafficking of hDAT stimulated by AMPH.
Characterization of the FLAG-hDAT Cell Line.
The regulation of DAT by intracellular signaling, and in particular by protein kinase C, has been described (15–18). Using biochemical and electrophysiological approaches, those authors demonstrated that trafficking of DAT can play a role in the function of the transporter. The ability to use a FLAG-hDAT fusion protein for immunocytochemistry in parallel with uptake and electrophysiological strategies offers a means to understand whether hDAT cell surface distribution is regulated by substrates and by inhibitors of the carrier.
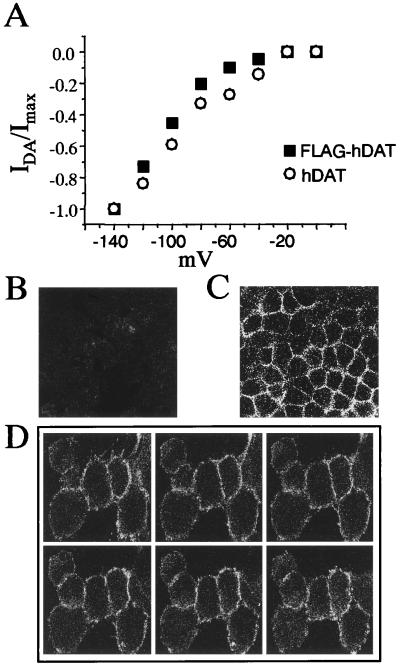
Addition of the N-terminal FLAG tag did not significantly alter [3H]DA uptake by the transporter, which had a Km of 1.2 ± 0.3 μM and a Vmax of 239 ± 42 fmol/min per well (n = 3). The function of the transporter was further assessed electrophysiologically. As seen in Fig. 1A, the FLAG tag did not perturb the ability of the transporter to produce substrate-induced currents. In the whole-cell configuration, the membrane potential of the cell was held at −40 mV and then the voltage was stepped to a new potential between −140 mV and 0 mV in 20-mV increments. The application of DA or AMPH induced a substrate-mediated current (19, 20) in both the hDAT and the FLAG-hDAT cells. The substrate-induced current was defined as the current recorded at a particular voltage in the presence of substrate (in these experiments, 100 μM DA) at steady state, minus the current recorded at the same potential in the presence of the substrate and 10 μM MZ. The substrate-induced current had a similar steady-state voltage dependence in the hDAT and the FLAG-hDAT cell line.
Figure 1.
Transporter function and expression of the FLAG-hDAT cells. (A) Current-voltage relationships of the DA-induced whole-cell currents from either hDAT (○) or FLAG-hDAT (■) cells. The DA-induced current is defined as the whole-cell steady-state current recorded upon DA bath application minus the current obtained in the presence of DA and MZ at a defined membrane voltage. The membrane potential was held at −40 mV, stepping the voltage between −140 and 0 mV for 500 msec, with an interval of 4 sec. Data were normalized at −140 mV. Confocal microscopy images of hDAT cells reveal no significant fluorescence (B), whereas confocal images of the FLAG-hDAT cells show a strong cell surface staining pattern (C). Z sections of the FLAG-hDAT (D), 1 μm in thickness, go from the top to the bottom of the cell; no intracellular immunofluorescence was seen in any of the six focal planes.
Confocal microscopy images of the immunofluorescence of the non-FLAG-tagged hDAT cell showed no staining with the antibody directed against the FLAG epitope, as seen in Fig. 1B. In Fig. 1C, however, a strong peripheral cell surface staining pattern was observed with the FLAG-hDAT cells. This is consistent with the steady-state localization of a GFP-hDAT fusion construct in MDCK cells (18). The immunofluorescence in Fig. 1C is almost exclusively on the cell surface, as evidenced by sectioning through the cells at 1-μm steps (Fig. 1D) in the x-z plane. A very small amount of intracellular fluorescence was observed, however, which might be caused by anterograde and retrograde trafficking of the transporter under steady-state conditions.
These data show that the FLAG-hDAT retained the functional characteristics of the hDAT, including affinity for DA, maximal rate of transport, voltage dependence of the substrate-induced current, and predominant steady-state cell surface localization.
Acute AMPH Treatment Causes a Decrease in [3H]DA Uptake, AMPH-Induced Current, and Cell Surface FLAG-hDAT.
In cells pretreated with 2 μM AMPH for 1 h, the uptake of [3H]DA was significantly decreased (78 ± 5% relative to control) (n = 5; P < 0.01 by paired Student's t test). This decrease in uptake was caused by a decrease in Vmax, without a change in Km [Km was 1.2 ± 0.3 μM for both vehicle and AMPH treatment; Vmax was 239 ± 42 and 189 ± 35 fmol/min per well for vehicle and AMPH treatment, respectively (n = 3; P < 0.025 by paired Student's t test)]. A similar decrease in uptake was observed after preincubation with 10 μM DA (73 ± 4% relative to control) (n = 5; P < 0.01 by paired Student's t test).
To further evaluate the reduction of DAT functional activity upon AMPH treatment, we evaluated the time dependence of AMPH-induced currents. Fig. 2A shows that a bath application of 2 μM AMPH generated an inward current in FLAG-hDAT cells. In the whole-cell configuration, the voltage was stepped from a holding potential of −40 mV to −120 mV for 500 msec while recording a control current (control). After perfusing the cell with 2 μM AMPH, an increase of the steady-state inward current (AMPH) was detected. Both currents were blocked by addition of 3 μM MZ with AMPH still present (AMPH & MZ). MZ by reducing the control current is unmasking the existence of an hDAT leak current that has been described both for the hDAT and other neurotransmitter transporters (19–22). The AMPH-induced current was defined as the current recorded in the presence of AMPH, minus the current recorded after addition of MZ to the bath with AMPH still present, and was analyzed as a function of time (between 0 and 40 min). The AMPH-induced current reached stability between 1 and 2 min after the addition of AMPH, and then decreased in a sigmoidal fashion over time. The time until the onset of this decreasing phase varied from cell to cell [between 5 and 14 min, averaging 8.75 ± 1.93 min (SEM, n = 4) after AMPH application]. To compare the rates of decline between different cells, time 0 was defined as the experimental time point 2 min before the AMPH-induced current began to decline. The current was normalized to the AMPH-induced current recorded at time 0 and plotted over time (Fig. 2B). Because the equilibrium potential of the AMPH-induced current was stable at −7.9 ± 1.7 mV during the duration of the experiments, it is unlikely that the loss of functional hDAT activity estimated by measuring the AMPH-induced current was caused by either a loss of ion gradients or by an intracellular accumulation of AMPH. It is noteworthy that AMPH reduced the substrate-induced currents to a greater extent than it reduced [3H]DA uptake. Thus, AMPH may have additional effects on the ionic movements generated by substrate-induced DAT activity that cannot be detected in uptake assays (see below). Alternatively, the difference in the magnitude of the effect may result from differences between single cells assayed under voltage clamp as compared with determinations of [3H]DA uptake in unclamped populations of cells.
Figure 2.
AMPH-induced loss of hDAT function and hDAT cell surface expression. (A) Whole-cell currents recorded from FLAG-hDAT cells. In this representative experiment, the cell was held at −40 mV and then the voltage was stepped to −120 mV for 500 msec. The labels refer to: control current before addition of substrate (control), current after addition of 2 μM AMPH to the bath (AMPH), and inhibited current after addition of 5 μM MZ to the bath (AMPH & MZ) with AMPH still present. (B) AMPH induced a loss of hDAT whole-cell current over time. Cells were held at −40 mV and then the voltage was stepped to −120 mV for 500 msec every 1–2 min. Upon AMPH bath application, the hDAT inward current increased, reaching the maximum value over a time of 1–2 min. After several minutes of stability, the AMPH-induced current began to decrease. From this point, the AMPH-induced current recorded at −120 mV was plotted against time (each symbol type represents a single cell; n = 4). The current recorded during the different experimental time points was normalized to the AMPH-induced current recorded at a virtual time 0 (2 min before the onset of the decreasing phase). (C) Confocal microscopy images in the absence (control) and presence of 2 μM AMPH added for 1 h. The galleries are of 1-μm sections of patches of cells or single cells treated with AMPH showing extensive intracellular immunofluorescence compared with control conditions.
AMPH-induced loss of transporter activity was further investigated by using confocal microscopy. AMPH (2 μM) caused a significant intracellular accumulation of FLAG-hDAT, as seen in Fig. 2C: the cell surface fluorescence intensity became weaker with a significant amount of transporter found in the cytosol. Confocal microscopy images of 1 μm z-sectioning (Fig. 2C) illustrate that the fluorescence shifted substantially from the cell surface to the intracellular compartment, and intracellular vesicles are now clearly visible in the gallery of the 1-μm steps from the top to the bottom of the cells. The loss of cell surface transporter caused by 2 μM AMPH was seen as early as 20 min (data not shown) and was maximal at 1 h, the incubation time chosen for further studies. The AMPH phenomenon was also concentration dependent, as a greater loss of cell surface transporter was seen at 50 μM compared with 2 μM AMPH (data not shown). However, 2 μM AMPH, a value approximating the Km for AMPH (20), was chosen to facilitate the removal of AMPH in subsequent uptake assays. Cell surface redistribution of hDAT was also evident with DA at 100 μM (data not shown). Taken together, these data imply that there is a substrate-induced down-regulation of the hDAT activity, which is likely a consequence of cell surface redistribution of the transporter.
Inhibitors of DAT, Such as Cocaine and MZ, Block the AMPH-Induced Loss of Cell Surface FLAG-hDAT.
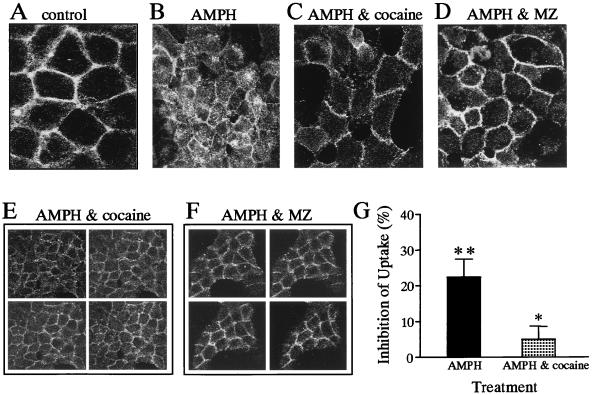
Pretreatment of the FLAG-hDAT cells with the DAT uptake inhibitors cocaine and MZ (Fig. 3C–F) and nomifensine (data not shown) blocked the AMPH-mediated redistribution of FLAG-hDAT. The effect of AMPH alone is shown in Fig. 3B. The lack of significant intracellular fluorescence in the presence of either AMPH plus cocaine or AMPH plus MZ is evident in the 1-μm z-sections (Fig. 3 E and F) from the top to the bottom of the cells. Neither MZ nor cocaine caused internalization when applied in the absence of AMPH (data not shown).
Figure 3.
AMPH-induced loss of cell surface FLAG-hDAT was inhibited by blockers of hDAT activity. Confocal microscopy images of FLAG-hDAT in the presence of vehicle (A), 2 μM AMPH (B), 2 μM AMPH and 3 μM cocaine (C and E), and 2 μM AMPH and 3 μM MZ (D and F). The galleries (E and F) are 1-μm z-sections of patches of FLAG-hDAT from the top to the bottom of the cells. (G) Uptake studies of [3H]DA were done in the absence and presence of 2 μM AMPH and in the presence of 2 μM AMPH and 3 μM cocaine. AMPH significantly inhibited [3H]DA uptake, and this effect was significantly attenuated by coincubation with cocaine (* = P < 0.05; ** = P < 0.01 by paired Student's t test). [3H]DA uptake was expressed as a percentage of inhibition of uptake under control conditions. The data are the mean ± SEM of five experiments performed in triplicate.
Cocaine, which impaired the AMPH-induced trafficking of hDAT, also blocked the AMPH-induced inhibition of [3H]DA uptake (Fig. 3G). Fig. 3G shows that the AMPH-mediated inhibition of [3H]DA uptake (22 ± 5%) (n = 5; P < 0.01 by paired Student's t test) was significantly attenuated when the FLAG-hDAT cells where pretreated (20 min) and coincubated with 3 μM cocaine (5 ± 4%) (n = 5; P < 0.05 by paired Student's t test). Thus, different classes of psychostimulants, such as AMPH and cocaine, appear to have opposite effects on the cell surface distribution and functional activity of hDAT.
AMPH-Induced Loss of Cell Surface hDAT Is an Internalization Event That Is Dynamin Dependent and Results in Reduced Transporter Capacity.
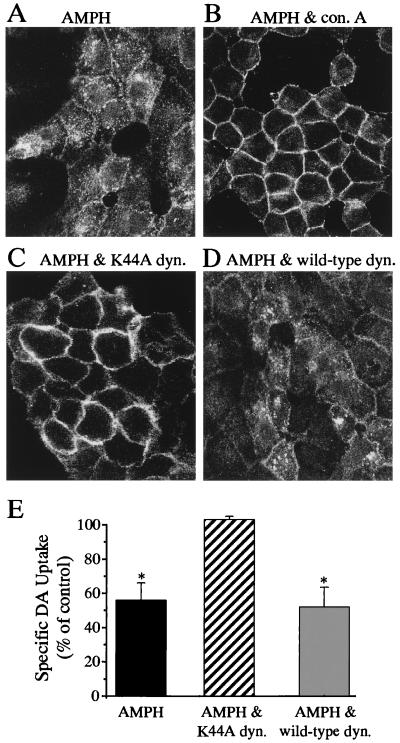
The increase in intracellular FLAG-hDAT caused by AMPH may be caused by sequestration and internalization of the transporter or result from nascent transporter being held up in the cytosol (i.e., hDAT never reached the cell surface). To test this, FLAG-hDAT expressing cells were pretreated with 250 μg/ml of Con A to prevent internalization by stabilizing cell surface integrity caused by tetravalent lectin contacts (23). As seen in Fig. 4, pretreatment with Con A (Fig. 4B) largely prevented the AMPH-mediated loss of cell surface FLAG-hDAT (Fig. 4A). Preincubation with Con A in the absence of AMPH resulted in an immunofluorescence profile similar to that of control (data not shown).
Figure 4.
AMPH-induced internalization of FLAG-hDAT is dynamin dependent. Confocal microscopy images of FLAG-hDAT cells incubated in the presence of either 2 μM AMPH for 1 h (A) or 2 μM AMPH plus 250 μg/ml Con A (B). Confocal microscopy images from FLAG-hDAT cells transiently transfected with either the dominant negative dynamin I mutant, K44A (C), or the wild-type dynamin (D) in the presence of 2 μM AMPH. (E) Uptake of [3H]DA (at 10°C), expressed as a percent of control, after 1 h preincubation with 2 μM AMPH in FLAG-hDAT cells, FLAG-hDAT cells transiently expressing the mutant dynamin, K44A, or the wild-type dynamin. The data are the mean ± SEM of three different experiments performed in triplicate (* = P < 0.05 by paired Student's t test).
Cell-surface biotinylation experiments also demonstrated the presence of AMPH-induced internalization. Incubation with 2 μM AMPH for 1 h resulted in 27.1 ± 7.9% internalization (n = 4). This demonstrates that AMPH produced its effect through hDAT internalization and not by interfering with the delivery of the protein to the cell surface. The extent of internalization was similar to the extent of inhibition of uptake.
To examine the cellular mechanism by which this endocytotic phenomenon occurred, we explored the possible role of clathrin-coated pits by coexpressing the FLAG-hDAT with a dominant negative mutant of dynamin I (K44A) or wild-type dynamin I. Dynamin is a GTPase that catalyzes the pinching off of clathrin-coated pits from the cell surface (24, 25). Coexpression with K44A (Fig. 4C) inhibited the AMPH-mediated internalization of FLAG-hDAT whereas wild-type dynamin (Fig. 4D) did not. Similarly, transient overexpression of K44A has been shown to block internalization of G protein-coupled receptors (26, 27). These data suggest that AMPH-mediated internalization of hDAT may require clathrin-coated pits. Although dynamin also has been suggested to play a role in the budding of caveolae (28, 29) as well as in signal transduction (30, 31), a clathrin-mediated internalization of the hDAT by AMPH seems more likely. A similar block by K44A dynamin of phorbol ester-mediated internalization of GFP-hDAT in MDCK cells has been reported (18), and this was interpreted as a clathrin-mediated mechanism as well.
We examined whether the AMPH-induced decrease in DA uptake (Fig. 3G) was a direct effect of the FLAG-hDAT internalization process itself, rather than a reduction in the maximal rate of transport mediated by enzymatic modification of the transporter, such as phosphorylation. The FLAG-hDAT cell line was transiently transected with either wild-type or K44A dynamin, or neither. After 1 h preincubation with 2 μM AMPH, DA uptake was significantly decreased, both in FLAG-hDAT cells (56 ± 17% relative to control) (n = 3; P < 0.05 by paired Student's t test) and FLAG-hDAT cells transiently transfected with wild-type dynamin 1 (52 ± 20% relative to control) (n = 3; P < 0.05 by paired Student's t test). In contrast, uptake in cells transiently transfected with K44A was unaffected by pretreatment with AMPH (103 ± 3% relative to control) (n = 3). These data further support AMPH-induced internalization of hDAT as being responsible for the decrease of transporter activity observed with acute AMPH treatment.
Although it is well known that AMPH increases the concentration of DA at the synaptic cleft, the underlying mechanism has been under considerable debate. Changes in hDAT activity and the extracellular DA concentration caused by AMPH have been ascribed to a counter transport mechanism by the carrier (7, 32). Regulation of transporter surface distribution may represent an additional mechanism by which neurons modulate transport capacity in response to psychostimulants such as AMPH as well as elevated levels of extracellular DA. In fact, our data show that acute exposure to AMPH in FLAG-hDAT cells reduced surface transport activity, as assessed by [3H]DA uptake, AMPH-induced currents (Figs. 2B and 3G), and cell surface localization of FLAG-hDAT as determined with both confocal microscopy (Fig. 2C) and cell surface biotinylation.
Single high doses of AMPH and methamphetamine were found to reduce [3H]DA uptake in synaptosomes prepared from the striatum of rat brains 1 h after drug administration without altering the amount of overall hDAT protein (10, 33). This decrease resulted from a decrease in transporter Vmax that recovered within 24 h. Our in vitro finding of AMPH-induced hDAT internalization may provide a potential mechanism for these observations. Curiously, methamphetamine administration did not reduce uptake in the nucleus accumbens (33), suggesting that this effect may not occur in all brain regions and may be subject to complex regulation.
Because hDAT controls DA clearance from the extracellular fluid, a decrease in the number of transporters on the cell surface because of AMPH exposure would result in an increase in extracellular DA. Therefore, loss of membrane transporter number might contribute to neurotoxicity by decreasing DA clearance, and consequently increasing extracellular DA (34, 35). Conversely, a reduction of cell surface hDAT may be a neuroprotective mechanism in that it could diminish the amount of carriers available for DA efflux upon AMPH stimulation.
Cocaine inhibits the ability of hDAT to traffic in response to AMPH stimulation, indicating that there are different mechanisms of action for these two psychostimulants. Transport blockers, such as MZ and nomifensine, appear to behave like cocaine. Therefore, hDAT ligands capable of inhibiting DAT activity (e.g., cocaine, MZ, and nomifensine) may not only act by preventing the reuptake of substrate, but also by preventing the internalization of transporter itself.
A number of studies have implicated internalization of neurotransmitter transporters as a possible means of modulating transporter activity (15, 36–39). In human embryonic kidney 293 cells, serotonin (5HT) was found to inhibit the phorbol ester-mediated phosphorylation, and the associated internalization, of the serotonin transporter (SERT) (39). A homeostatic mechanism was proposed for this effect, in that elevated synaptic levels of 5HT could prevent the internalization of SERT and thereby maintain effective levels of the transporter at the cell surface where it would help to clear the elevated 5HT. Similarly, a 1-h exposure to γ-aminobutyric acid was shown to slow internalization of GAT1, another member of this gene family (40). Interestingly, the effect we observed in DAT, in the same parental line of cells as the SERT studies, is opposite to that reported with SERT and GAT1, suggesting a markedly different mechanism of regulation of these closely related transporters.
A recent study with a GFP-hDAT fusion protein stably expressed in MDCK cells reported that 10 μM DA did not significantly affect the cellular distribution of GFP fluorescence (18). Whether this discrepancy with our data relates to the concentration of DA, to an impairment in substrate-mediated internalization of GFP-DAT related to the presence of the N-terminal GFP “tag” compared with the much smaller FLAG tag, or from differences in the cell lines used is not yet clear.
It should be noted that AMPH decreased FLAG-hDAT-mediated currents more rapidly and to a greater extent than it reduced DA uptake or produced internalization. Thus, it is possible that AMPH caused a modification of the transporter, such as phosphorylation, which rapidly decreased substrate-induced currents. Indeed, the regulation of DAT by phorbol esters, presumably mediated by protein kinase C, has been described (15–18). Clearly, however, at 1 h AMPH-induced internalization of hDAT and decreased uptake was blocked by dominant negative dynamin I, suggesting that these effects also depend on endocytosis and cannot be fully explained simply by a modification of the transporter. Moreover, we see evidence of internalization by cell surface biotinylation of a magnitude relatively consistent with the observed changes in uptake, but less than the AMPH-induced reduction in current. Thus, the inactivation of hDAT upon AMPH application is largely but perhaps not exclusively controlled by the trafficking of this carrier.
Taken together our data suggest that cell surface redistribution of hDAT is a mechanism that contributes to the enhancement of extracellular DA levels in response to psychostimulants such as AMPH. Greater understanding of this pathway and its mechanistic details may lead to novel cellular targets for substance abuse therapies.
Acknowledgments
We are very grateful to Eileen Grass for her technical support and superb maintenance of the cell lines and to Drs. Alan Frazer, Richard Lamb, William Clarke, Lynette Daws, and Myles Akabas for their helpful comments and review of this manuscript. We also thank Dr. Marc Caron for the kind gift of wild-type dynamin and dynamin (K44A) DNA. This work was supported by a grant from the National Alliance for Research on Schizophrenia and Depression (to A.G.), National Institutes of Health Grant GM41659 (to L.M.F.L.-.L.), and by a Grant-in-Aid from the American Heart Association and National Institutes of Health Grants MH57324, DA11495, and DA12408 (to J.A.J.).
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- hDAT
human DAT
- AMPH
amphetamine
- MZ
mazindol
- GFP
green fluorescent protein
- PBST
0.05% Triton X-100/PBS
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110035297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110035297
References
- 1.Iversen L L. Br J Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giros B, Caron M G. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 3.Giros B, Jaber M, Jones S R, Wightman R M, Caron M G. Nature (London) 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 4.Koob G F, Bloom F E. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 5.Harris J E, Baldessarini R J. Neuropharmacology. 1973;12:669–679. doi: 10.1016/0028-3908(73)90120-2. [DOI] [PubMed] [Google Scholar]
- 6.Kuhar M J, Ritz M C, Boja J W. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- 7.Fischer J F, Cho A K. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- 8.Sulzer D, Chen T K, Lau Y Y, Kristensen H, Rayport S, Ewing A. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan A E, Horan B, Dewey S L, Ashby C R., Jr Eur J Pharmacol. 1997;331:R1–R3. doi: 10.1016/s0014-2999(97)01035-2. [DOI] [PubMed] [Google Scholar]
- 10.Fleckenstein A E, Haughey H M, Metzger R R, Kokoshka J M, Riddle E L, Hanson J E, Gibb J W, Hanson G R. Eur J Pharmcol. 1999;382:45–49. doi: 10.1016/s0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- 11.Rees S, Coote J, Stables J, Goodson S, Harris S, Lee M G. BioTechniques. 1996;20:102–104. doi: 10.2144/96201st05. [DOI] [PubMed] [Google Scholar]
- 12.Robbins A K, Horlick R A. BioTechniques. 1998;25:240–244. doi: 10.2144/98252st04. [DOI] [PubMed] [Google Scholar]
- 13.Ferrer J V, Javitch J A. Proc Natl Acad Sci USA. 1998;95:9238–9243. doi: 10.1073/pnas.95.16.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vickery R G, von Zastrow M. J Cell Biol. 1999;144:31–43. doi: 10.1083/jcb.144.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu S J, Kavanaugh M P, Sonders M S, Amara S G, Zahniser N R. J Pharmacol Exp Ther. 1997;282:1358–1365. [PubMed] [Google Scholar]
- 16.Vaughan R A, Huff R A, Uhl G R, Kuhar M J. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- 17.Melikian H E, Buckley K M. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels G M, Amara S G. J Biol Chem. 1999;274:36794–36801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 19.Sonders M S, Zhu S J, Zahniser N R, Kavanaugh M P, Amara S G. J Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sitte H H, Huck S, Reither H, Boehm S, Singer E A, Pifl C. J Neurochem. 1998;71:1289–1297. doi: 10.1046/j.1471-4159.1998.71031289.x. [DOI] [PubMed] [Google Scholar]
- 21.Mager S, Min C, Henry D J, Chavkin C, Hoffman B J, Davidson N, Lester H A. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 22.Galli A, DeFelice L J, Duke B J, Moore K R, Blakely R D. J Exp Biol. 1995;198:2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- 23.Toews M L, Waldo G L, Harden T K, Perkins J P. J Biol Chem. 1984;259:11844–11850. [PubMed] [Google Scholar]
- 24.Herskovits J S, Burgess C C, Obar R A, Vallee R B. J Cell Biol. 1993;122:565–578. doi: 10.1083/jcb.122.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damke H, Baba T, Warnock D E, Schmid S L. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Ferguson S S G, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 27.Vogler O, Bogatkewitsch G S, Wriske C, Krummenerl P, Jakobs K H, van Koppen C J. J Biol Chem. 1998;273:12155–12160. doi: 10.1074/jbc.273.20.12155. [DOI] [PubMed] [Google Scholar]
- 28.Henley J R, Krueger E W, Oswald B J, McNiven M A. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh P, McIntosh D P, Schnitzer J E. J Cell Biol. 1998;141:101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kranenburg O, Verlaan I, Moolenaar W H. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 31.Whistler J L, von Zastrow M. J Biol Chem. 1999;274:24575–24578. doi: 10.1074/jbc.274.35.24575. [DOI] [PubMed] [Google Scholar]
- 32.Jones S R, Gainetdinov R R, Wightman R M, Caron M G. J Neurosci. 1998;18:1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokoshka J M, Vaughan R A, Hanson G R, Fleckenstein A E. Eur J Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- 34.Axt K J, Commins D L, Vosmer G, Seiden L S. Brain Res. 1990;515:269–276. doi: 10.1016/0006-8993(90)90606-c. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Price J O, Graham D G, Montine T J. Biochem Biophys Res Commun. 1998;248:812–816. doi: 10.1006/bbrc.1998.9044. [DOI] [PubMed] [Google Scholar]
- 36.Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice L J, Blakely R D. J Neurosci. 1997;17:45–57. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Coffey L L, Reith M E. Biochem Pharmacol. 1997;53:677–688. doi: 10.1016/s0006-2952(96)00898-2. [DOI] [PubMed] [Google Scholar]
- 38.Apparsundaram S, Galli A, DeFelice L J, Hartzell H C, Blakely R D. J Pharmacol Exp Ther. 1998;287:733–743. [PubMed] [Google Scholar]
- 39.Ramamoorthy S, Blakely R D. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 40.Bernstein E M, Quick M W. J Biol Chem. 1999;274:889–895. doi: 10.1074/jbc.274.2.889. [DOI] [PubMed] [Google Scholar]