Abstract
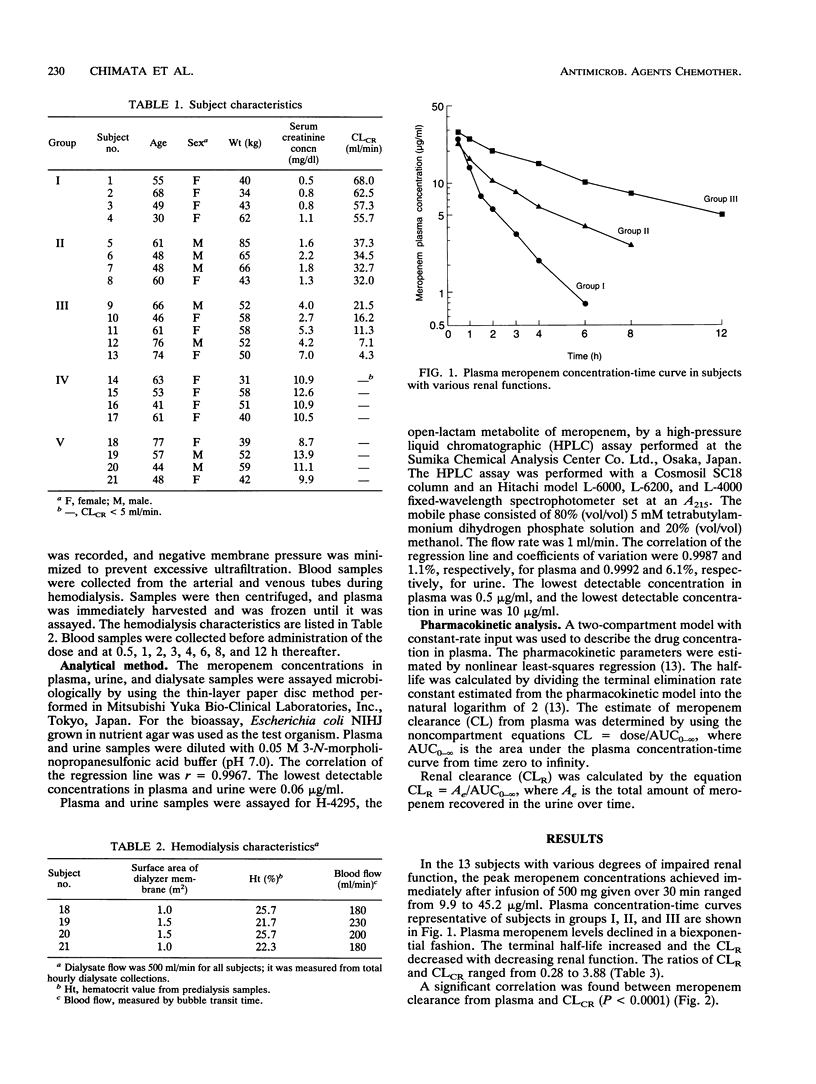
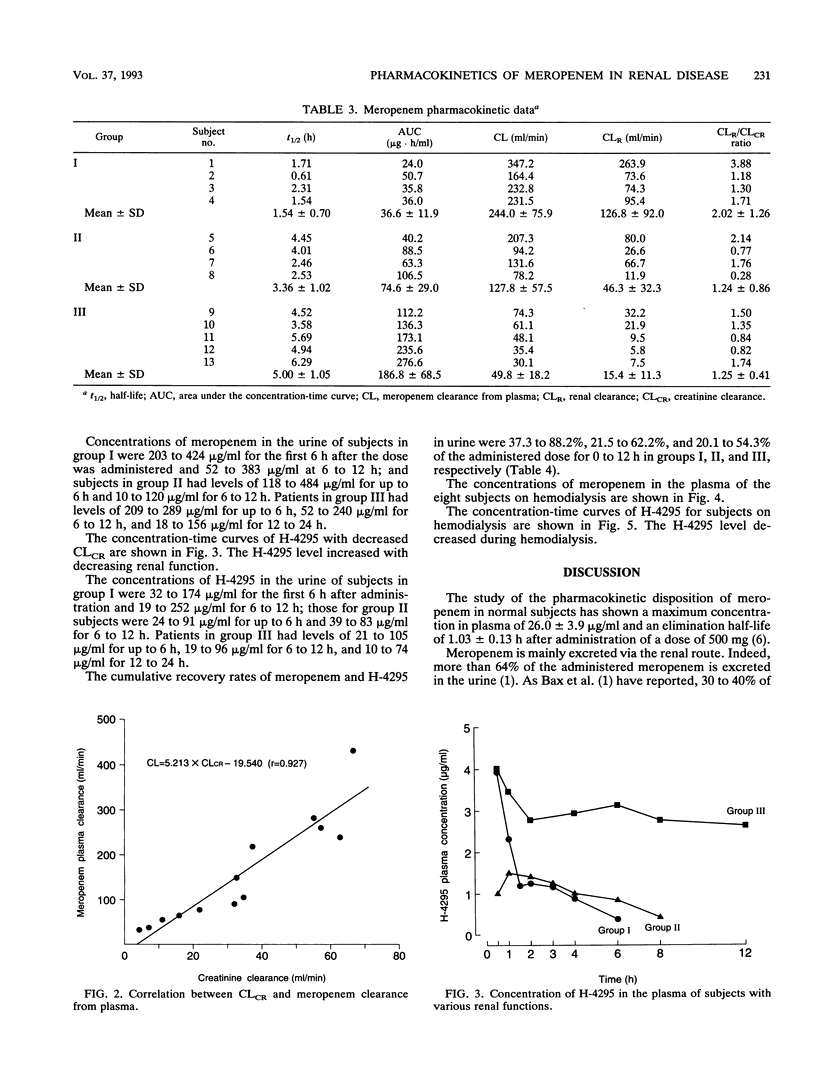
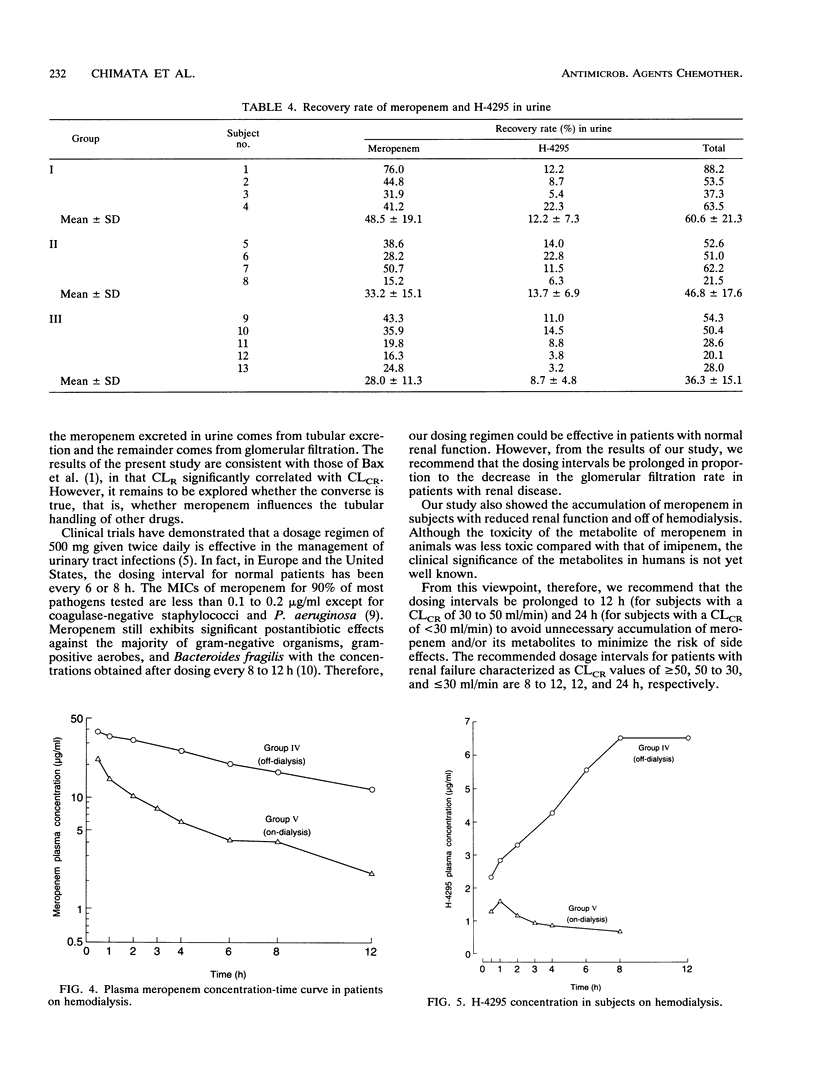
The pharmacokinetics of meropenem were studied after intravenous infusion in 13 patients grouped according to the impairment of their renal function. Creatinine clearance (CLCR) was greater than 50, 50 to 30, and less than 30 ml/min in groups I, II, and III, respectively. Two other groups, groups IV and V, each comprising four patients with end-stage renal disease (CLCR, < 5 ml/min), were also studied, the former on days off of hemodialysis and the latter on days of hemodialysis. The elimination half-lives of meropenem were 1.54 +/- 0.70 h in group I patients, 3.36 +/- 1.02 h in group II patients, and 5.00 +/- 1.05 h in group III patients. Cumulative urinary excretion accounted for 48.5% of the dose in group I patients and decreased progressively with a decline in renal function. Hemodialysis shortened the elimination half-life of meropenem from 7.0 h to 2.9 h. H-4295, the main metabolite of meropenem, had a peak level in plasma of 0.5 to 1.0 h in patients with renal failure. The level of H-4295 decreased with hemodialysis. The dosing interval of meropenem should be prolonged in a regular proportion to the decline in CLCR (12 h in group II patients and 24 h in group III patients). In patients receiving hemodialysis, dosing after each hemodialysis session is recommended.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax R. P., Bastain W., Featherstone A., Wilkinson D. M., Hutchison M., Haworth S. J. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):311–320. doi: 10.1093/jac/24.suppl_a.311. [DOI] [PubMed] [Google Scholar]
- Edwards J. R., Turner P. J., Wannop C., Withnell E. S., Grindey A. J., Nairn K. In vitro antibacterial activity of SM-7338, a carbapenem antibiotic with stability to dehydropeptidase I. Antimicrob Agents Chemother. 1989 Feb;33(2):215–222. doi: 10.1128/aac.33.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Thornsberry C. In-vitro studies of meropenem. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):9–29. doi: 10.1093/jac/24.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Thomson K. S., Iaconis J. P. Meropenem: activity against resistant gram-negative bacteria and interactions with beta-lactamases. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):187–196. doi: 10.1093/jac/24.suppl_a.187. [DOI] [PubMed] [Google Scholar]
- Sumita Y., Inoue M., Mitsuhashi S. In vitro antibacterial activity and beta-lactamase stability of the new carbapenem SM-7338. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):908–916. doi: 10.1007/BF01963782. [DOI] [PubMed] [Google Scholar]
- Topham J. C., Murgatroyd L. B., Jones D. V., Goonetilleke U. R., Wright J. Safety evaluation of meropenem in animals: studies on the kidney. J Antimicrob Chemother. 1989 Sep;24 (Suppl A):287–306. doi: 10.1093/jac/24.suppl_a.287. [DOI] [PubMed] [Google Scholar]
- Verpooten G. A., Verbist L., Buntinx A. P., Entwistle L. A., Jones K. H., De Broe M. E. The pharmacokinetics of imipenem (thienamycin-formamidine) and the renal dehydropeptidase inhibitor cilastatin sodium in normal subjects and patients with renal failure. Br J Clin Pharmacol. 1984 Aug;18(2):183–193. doi: 10.1111/j.1365-2125.1984.tb02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]



