Abstract
Populations native to the Tibetan and Andean Plateaus are descended from colonizers who arrived perhaps 25,000 and 11,000 years ago, respectively. Both have been exposed to the opportunity for natural selection for traits that offset the unavoidable environmental stress of severe lifelong high-altitude hypoxia. This paper presents evidence that Tibetan and Andean high-altitude natives have adapted differently, as indicated by large quantitative differences in numerous physiological traits comprising the oxygen delivery process. These findings suggest the hypothesis that evolutionary processes have tinkered differently on the two founding populations and their descendents, with the result that the two followed different routes to the same functional outcome of successful oxygen delivery, long-term persistence and high function. Assessed on the basis of basal and maximal oxygen consumption, both populations avail themselves of essentially the full range of oxygen-using metabolism as populations at sea level, in contrast with the curtailed range available to visitors at high altitudes. Efforts to identify the genetic bases of these traits have included quantitative genetics, genetic admixture, and candidate gene approaches. These reveal generally more genetic variance in the Tibetan population and more potential for natural selection. There is evidence that natural selection is ongoing in the Tibetan population, where women estimated to have genotypes for high oxygen saturation of hemoglobin (and less physiological stress) have higher offspring survival. Identifying the genetic bases of these traits is crucial to discovering the steps along the Tibetan and Andean routes to functional adaptation.
Keywords: hypoxia, natural selection, human
People have occupied many different habitats since leaving Africa, probably during the past 100,000 years (1). Behavioral buffering and biological adaptability have enabled human occupation of environments spanning large ranges in features such as temperature, UV radiation, and diet. However, only biological adaptability has contributed to our success in occupying high-altitude lands (to ≈5,400 m), because traditional technology could not buffer us from the unavoidable environmental stress of high-altitude hypoxia (less than the normal amount of oxygen in the air because of reduced atmospheric pressure).
Indigenous human populations on the Tibetan and Andean Plateaus are descendents of colonizers who arrived at most ≈25,000 and 11,000 years ago, respectively (2). Abundant evidence documents the reduced physical function of low-altitude natives visiting high altitudes who engage many homeostatic responses yet do not restore preexposure function (3). The two high-altitude populations can be viewed as the current outcome of separate replications of a natural experiment in which an ancestral founding population moved from low to high altitude, and its descendents have been exposed for millennia to the opportunity for natural selection to improve function under high-altitude hypoxia. Both experiments have been successful, as indicated by the rise of great civilizations, long-term persistence, and population growth. However, the experiments have proceeded differently, as indicated by large quantitative differences in physiological traits related to offsetting the stress of high-altitude hypoxia. Evolutionary theory suggests that features of physiology or metabolism that are distinctive as compared with ancestral conditions or other populations represent functional adaptations. The purpose of this paper is to present evidence for Tibetan–Andean contrasts in functional adaptations that offset the stress of hypoxia and to consider the evidence for a genetic basis for these differences between Tibetan and Andean high-altitude natives.
The environmental stress of high altitude is hypoxia that, in turn, creates the conditions for physiological hypoxia (less than the normal amount of oxygen in the organism). The severity of high-altitude hypobaric hypoxia is illustrated in Fig. 1 by the regular decrease in the partial pressure of oxygen in the atmosphere with increasing altitude. Studies of adaptation to high-altitude hypoxia usually focus on populations living at ≥2,500 m, where physiological effects become more easily detectable with more severe stress. Many studies report about populations living in the range of 3,500–4,500 m, because many people live in that altitude range on both plateaus, and because those residents must deal with severe stress and may be most likely to exhibit adaptive responses. At 4,000-m elevation, every breath of air contains only ≈60% of the oxygen molecules in the same breath at sea level. This is a constant feature of the ambient environment to which every person at a given altitude is inexorably exposed. Less oxygen in inspired air results in less oxygen to diffuse into the bloodstream to be carried to the cells for oxygen-requiring energy-producing metabolism in the mitochondria. Humans do not store oxygen, because it reacts so rapidly and destructively with other molecules. Therefore, oxygen must be supplied, without interruption, to the mitochondria and to the ≥1,000 oxygen-requiring enzymatic reactions in various cells and tissues (4).
Fig. 1.
Ambient oxygen levels, measured by the partial pressure of oxygen (solid line) or as a percent of sea-level values (dashed line), decrease with increasing altitude, a situation called high-altitude or hypobaric hypoxia. The atmosphere contains ≈21% oxygen at all altitudes.
The oxygen level is near zero in human mitochondria at all altitudes (5). This condition is described as “primitive,” because it has changed little for the past 2.5 billion years despite wide swings in the amount of atmospheric oxygen (at times it has been 10,000-fold lower; refs. 6 and 7) and “protective” in the sense that it circumvents potentially damaging reactions of oxygen with other molecules (8). Fig. 2 describes the transport of oxygen in humans along a “cascade” of falls in oxygen level from inspired air to the capillaries from which it will diffuse into the mitochondria. The inspired oxygen pressure at the higher altitude of 4,540 m is much lower, so the pressure differences among different stages of oxygen transport are smaller, and the diffusion rate is lower. Fig. 2 identifies several points of potential adaptation with respect to sustaining the process of mitochondrial generation of energy at very low oxygen levels.
Fig. 2.
The oxygen transport cascade at sea level (solid line) and at the high altitude of 4,540 m (dashed line) illustrates the oxygen levels at the major stages of oxygen delivery and suggests potential points of functional adaptation (data from ref. 60).
Potential and Actual Points of Adaptation to Hypoxia
Energy Production.
Lowlanders traveling to high altitude display homeostatic responses to the acute severe hypoxia. The responses are energetically costly, as indicated by an increase in basal metabolic rate (BMR; the minimum amount of energy needed to maintain life with processes such as regulating body temperature, heart rate, and breathing). BMR is increased by ≈17–27% for the first few weeks upon exposure to high altitude and gradually returns toward sea-level baseline (9). In other words, for acutely exposed lowlanders, the fundamental physiological processes required to sustain life at high altitude require more oxygen despite lower oxygen availability. At the other extreme of energy expenditure, a measure of the upper limit to oxygen delivery is the highest oxygen uptake an individual can attain during work. This upper limit is decreased by ≈20–30% during the first weeks and gradually returns toward normal over the course of 1 year (although it does not return to preexposure sea-level baseline) (3, 10–13). The result is a relatively narrow scope for increasing oxygen consumption above the basal requirement for supporting other functions, including growth, reproduction, and physical activity.
In contrast to acutely exposed lowlanders and despite the equally low level of oxygen pressure in the air and lungs, both Andean and Tibetan highlanders display the standard low-altitude range of oxygen delivery from minimal to maximal. Both populations have the normal basal metabolic rate expected for their age, sex, and body weight (14–16), implying that their functional adaptations do not entail increased basal oxygen requirements. Furthermore, Andean and Tibetan highlanders have maximal oxygen uptake expected for their level of physical training (12, 13, 17). For example, a comparative analysis of 17 samples of Tibetan and Andean men living at an average altitude of ≈3,900 m finds estimated maximum oxygen consumptions of 46 and 47 ml/O2 per kilogram, respectively, that are similar to values for untrained men at sea level and ≈10–20% higher than those reported for six low-altitude native samples residing at the same altitudes (17). Thus, they can use at high altitude the same full range of aerobic potential for activities requiring oxygen delivery that others use at low altitude. This represents a functional change from the ancestral acute response to altitude, and it suggests that the high-altitude native populations have adaptations that do not elicit elevated oxygen consumption. Unexpectedly, as described below, the similar functional endpoints are reached differently among Tibetan as compared with Andean high-altitude natives, who differ quantitatively in measures of oxygen delivery along the transport cascade. In turn, this raises questions about the possible mechanisms and evolutionary steps along the two adaptive routes.
Ventilation.
One potential point of adaptation in oxygen delivery is ventilation, which, if raised, could move a larger overall volume of air and achieve a higher level of oxygen in the alveolar air (Fig. 2) and diffusion of more oxygen. An immediate increase in ventilation is perhaps the most important response of lowlanders acutely exposed to high altitude, although it is not sustained indefinitely and is not found among members of low-altitude populations born and raised at high altitude, such as Europeans or Chinese (3, 18). Tibetan, but not Andean, highlanders have retained this temporary ancestral response, as indicated by elevated resting ventilation, as compared with Andean highlanders and low-altitude populations at low altitude. For example, a comparative analysis summarizing the results of 28 samples of Tibetan and Andean high-altitude natives at an average altitude of ≈3,900 m reported an estimated resting ventilation of 15.0 liters/min among the Tibetan samples as compared with 10.5 liters/min among the Andean samples (19). Fig. 3 illustrates the higher resting ventilation of Tibetans as compared with Andean highlanders evaluated using the same protocol at ≈4,000 m. The mean resting ventilation for Tibetans was >1 SD higher than the mean of the Andean highlanders (20).
Fig. 3.
Boxplots comparing pairs of Tibetan and Andean samples, measured at ≈4,000-m altitude by using the same recruiting and measurement protocols, illustrate the marked quantitative differences in resting ventilation, HVR, hemoglobin concentration, and percent of oxygen saturation (recalculated from data reported in refs. 20 and 27, recalculated from data reported in refs. 28 and 61).
The control of ventilation has been evaluated in the two populations by quantifying the reflexive increase in ventilation induced by exposure to a standardized experimental hypoxic stress, a measure called the hypoxic ventilatory response (HVR). The HVR of low-altitude populations is abruptly and markedly elevated upon acute exposure to high altitude, returns to normal levels after a few days, and falls below normal levels after months or years (21–23). Tibetans express a normal HVR as compared with sea-level populations in their native altitude, whereas Andean highlanders have HVRs generally lower than sea-level values. A comparative analysis summarizing reports on 25 samples of Tibetan and Andean high-altitude natives at an average altitude of ≈3,900 m found that the average HVR of Tibetans was approximately double that of the Andean high-altitude natives (19). The higher HVR of Tibetans is illustrated in Fig. 3 by a comparison of paired samples evaluated at ≈4,000 m (20). The implication is that the Tibetan respiratory physiology has changed from the ancestral functional response of a temporary increase in ventilation and HVR to a pattern of sustaining those responses indefinitely.
Oxygen in the Bloodstream.
The higher ventilation levels among Tibetans that move more oxygen through the lungs, along with the higher HVRs that respond more vigorously to fluctuations in oxygen levels, might be expected to result in more oxygen in the bloodstream. However, the level of oxygen in the arterial blood (Fig. 2) of a sample of Tibetans at ≈3,700 m was lower than that of a sample of Andean high-altitude natives at the same altitude (54 as compared with 57 mmHg; 1 mmHg = 133 Pa) (24, 25). In addition, hemoglobin, the oxygen-carrying molecule in blood, is less saturated with oxygen among Tibetans than among their Andean counterparts (26, 27). Fig. 3 illustrates the lower percent of oxygen saturation of hemoglobin in a sample of Tibetans at ≈4,000 m. The increased breathing of Tibetans does not deliver more oxygen to the hemoglobin in the arteries.
Another potential adaptation in the bloodstream is a higher concentration of hemoglobin itself. However, Tibetans have lower hemoglobin concentrations than their Andean counterparts at the same altitude (e.g., refs. 25 and 28). An analysis summarizing the results of 53 samples of Tibetan and Andean high-altitude native men at an average altitude of ≈3,900 m reported an estimated mean hemoglobin concentration of 16.9 g/dl among Tibetan men as compared with 18.1 g/dl among Andean men (19). Fig. 3 illustrates the markedly lower hemoglobin concentrations in a sample of Tibetan men and women as compared with their Andean counterparts at ≈4,000 m. [The average hemoglobin concentrations were 15.6 and 19.2 g/dl for Tibetan and Andean men, respectively, and 14.2 and 17.8 g/dl for women (28).] Hemoglobin concentration is influenced by many factors, including erythropoietin, a protein that causes differentiation of the precursors that will become hemoglobin-containing red blood cells. Tibetans have slightly lower erythropoietin concentrations than Andean highlanders at the same altitude (25). When matched for volume of red blood cells, a procedure that would effectively compare the highest Tibetan and the lowest Andean values, Andean highlanders have much higher erythropoietin levels, which implies that some sensor is responding as if the stress were more severe, even though the samples were collected at the same altitude of ≈3,700 m.
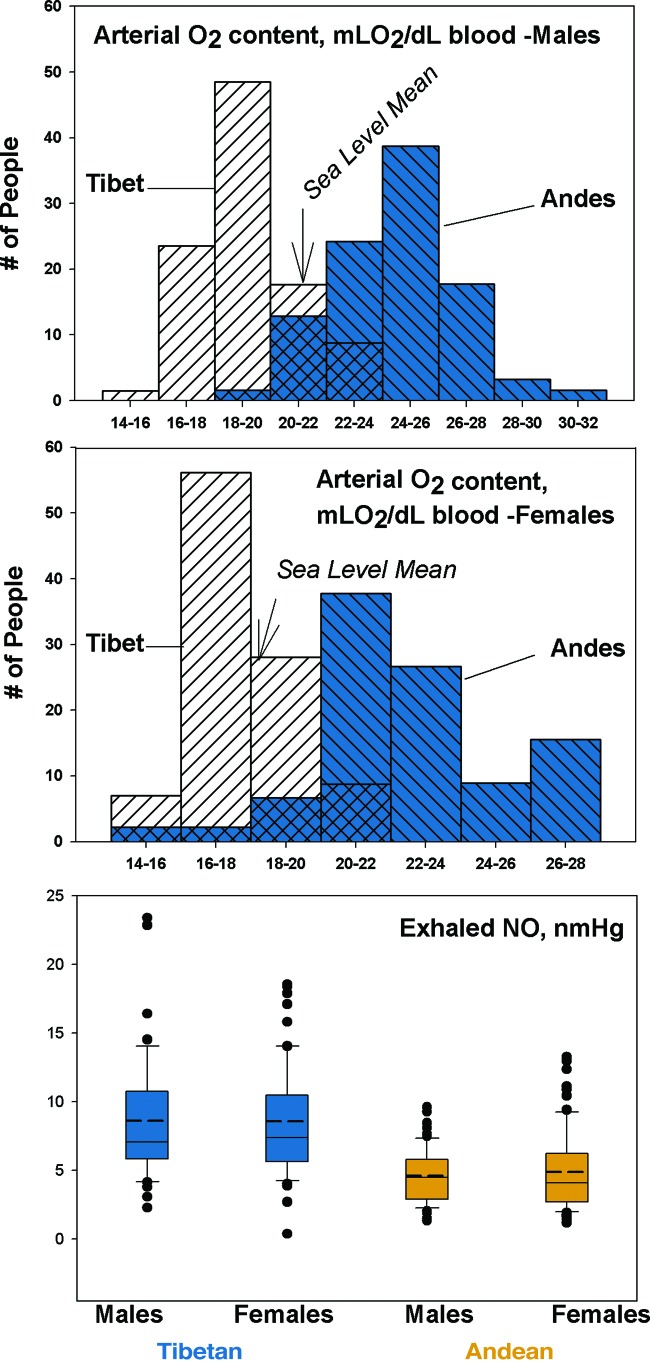
Together, oxygen saturation and hemoglobin concentration determine arterial oxygen content. Fig. 4 illustrates that the calculated arterial oxygen content in a sample of Tibetans is substantially lower than among Andean highlanders, who actually have higher arterial oxygen content than sea-level natives at sea level. On average, neither Andean nor Tibetan highlanders restore the usual sea-level arterial oxygen content. Instead, Andean highlanders have overcompensated for ambient hypoxia according to this measure, whereas Tibetan highlanders have undercompensated. Indeed, Tibetans are profoundly hypoxic and must be engaging other mechanisms or adapting at different points in the oxygen transport cascade to sustain normal aerobic metabolism.
Fig. 4.
The calculated arterial oxygen content of Tibetan men and women is profoundly lower than their Andean counterparts measured at ≈4,000 m (data from ref. 62), whereas the exhaled NO concentration is markedly higher (recalculated from data reported in ref. 34).
Blood Flow and Oxygen Diffusion.
Other potential points of functional adaptation include the rate of flow of oxygen-carrying blood to tissues and the rate of oxygen diffusion from the bloodstream into cells.
Because blood flow is a function of the diameter of blood vessels, dilating factors could, in principle, improve the rate of oxygen delivery. Sea-level populations respond to high-altitude hypoxia by narrowing the blood vessels in their lungs, the first point of contact with the circulation. Known as hypoxic pulmonary vasoconstriction, that reflex evolved at sea level to direct blood away from temporarily poorly oxygenated toward better oxygenated parts of the lung. High-altitude hypoxia causes poor oxygenation of the entire lung and general constriction of blood vessels to the degree that it raises pulmonary blood pressure, often to hypertensive levels (3, 29). In contrast, most Tibetans do not have hypoxic pulmonary vasoconstriction or pulmonary hypertension. This is indicated by essentially normal pulmonary blood flow, as measured by normal or only minimally elevated pulmonary artery pressure (29, 30). Although there are no studies of paired Tibetan–Andean samples evaluated by the same investigators, a comparison of a Tibetan sample from 4,200 m and an Andean sample from 3,700 m using the same technology reveals a mean pulmonary artery pressure of 31 mmHg for the Tibetan 28% lower than the mean of 43 mmHg for the Andean (35 mmHg is often considered the upper end of the normal sea-level range) (30, 31). Andean highlanders are consistently reported to have pulmonary hypertension (29). Thus, pulmonary blood flow is another element of oxygen delivery for which Tibetans differ from Andean highlanders in the direction of greater departure from the ancestral response to acute hypoxia.
A probable reason for the normal pulmonary artery pressure among Tibetans is high levels of the vasodilator nitric oxide (NO) gas synthesized in the lining of the blood vessels. Low-altitude populations acutely exposed to high-altitude down-regulate NO synthesis, a response thought to contribute to hypoxic pulmonary vasoconstriction (32, 33). In contrast, NO is substantially elevated in the lungs of Tibetan as compared with Andean highlanders and lowlanders at sea level (Fig. 4) (34). Among Tibetans, higher exhaled NO is associated with higher blood flow through the lungs (30).
Several other lines of evidence highlight the importance of high blood flow for Tibetans. These include greater increase in blood flow after temporary occlusion (35) and higher blood flow to the brain during exercise (36) as compared with lowlanders. Pregnant Tibetans increase blood flow to the uterine arteries, increase oxygen delivery to the uterus and placenta more than acutely exposed lowlanders, and give birth to heavier babies (37). In contrast, pregnant Andean high-altitude natives increase oxygen delivery to the uterus and placenta by increasing ventilation and oxygen saturation, a response that correlated with giving birth to heavier babies (38, 39). These two means for increasing uteroplacental oxygen delivery during pregnancy occur at a point in the life course where natural selection for improving function could be particularly effective. This is because infant birth weight is associated with infant survival and maternal reproductive success. Generally, Tibetans appear to have relatively high blood flow that may contribute significantly to offsetting their low arterial oxygen content.
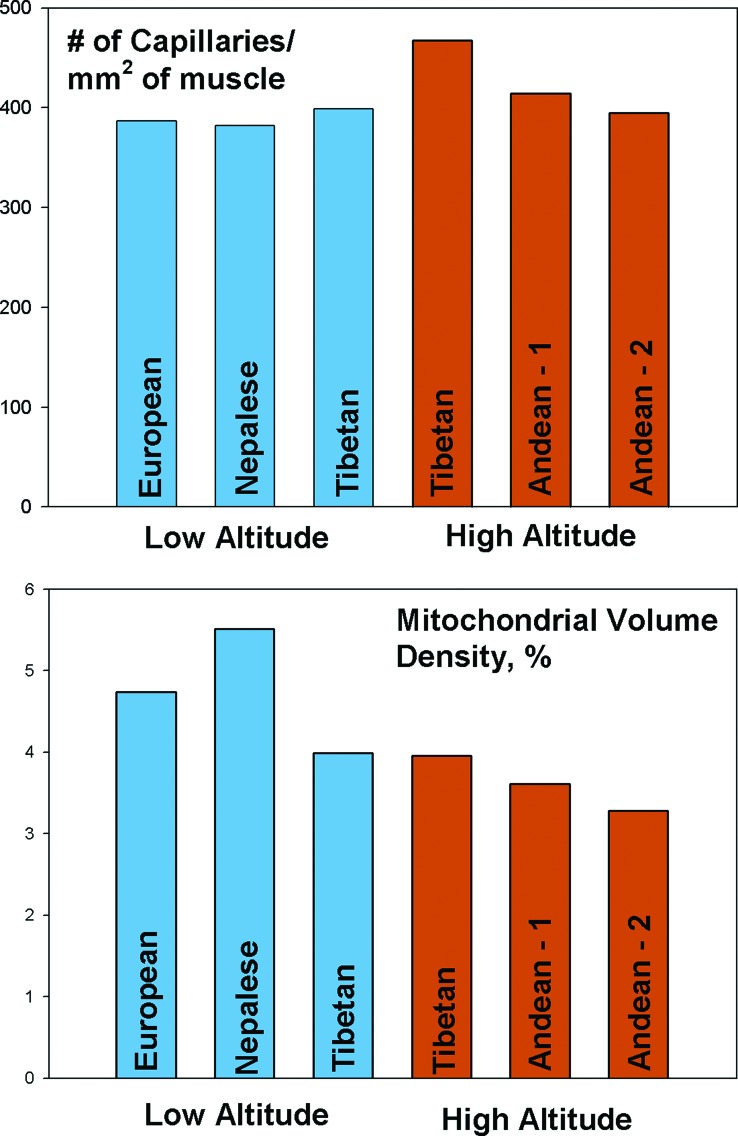
A denser capillary network could potentially improve perfusion and oxygen delivery, because each capillary would supply a smaller area of tissue, and oxygen would diffuse a shorter distance. Tibetans (the study sample were Sherpas, an ethnic group that emigrated from Tibet to Nepal ≈500 years ago) who are born and raised at high altitude have higher capillary density in muscles as compared with Andean high-altitude natives, Tibetans born and raised at low altitude, or lowlanders (Fig. 5) (40). Those findings suggest that another route used particularly by Tibetans to overcome profoundly low arterial oxygen content is a high rate of diffusion. Diffusion could be further enhanced by easier dissociation of oxygen from hemoglobin. However, oxygen dissociation is normal in both Tibetan and Andean populations (25, 41, 42).
Fig. 5.
High-altitude native Tibetans have higher capillary density than their Andean counterparts or populations at low altitude; Tibetan and Andean highlanders both have lower mitochondrial volume than low-altitude populations (data from refs. 40, 44, 63, and 64).
The last potential point of adaptation is at the level of the mitochondrion itself. Acutely exposed lowlanders lose mitochondria in leg muscles during the first 3 weeks at altitude. Similarly, both Tibetan (Sherpas) and Andean high-altitude natives have a lower mitochondrial volume in leg muscle tissue than sea-level natives at sea level (Fig. 5) (40). However, Tibetans born and raised closer to sea level (at 1,200 m) also have few mitochondria, indicating that, for them, expression of this trait does not require exposure to high altitude. The functional implications of fewer mitochondria are unclear, because overall oxygen-requiring metabolism is not lower. Among Tibetans, a smaller mitochondrial volume somehow supports a relatively larger oxygen consumption, perhaps by higher metabolic efficiency (12, 43, 44).
To emphasize the magnitude of these population differences in mean values of healthy Tibetan and Andean high-altitude natives living under the same hypoxic stress, the marked differences at these points in oxygen transport can be quantified further by using a measure of “effect size,” calculated by subtracting the Tibetan mean from the Andean mean and dividing by the pooled variance of the samples (45). An effect size of ≥0.8 is conventionally considered large; it means there is no overlap of ≈48% or more of the observations in the two samples being compared. By this criterion, each of the following contrasts (described above) is large; the higher Tibetan mean for ventilatory traits, the lower Tibetan mean for hematological traits, particularly for hemoglobin for which the mean differences are >2 SD, the higher Tibetan mean for exhaled NO and muscle capillary density, and the lower Tibetan mean values for arterial oxygen level, oxygen saturation, and pulmonary artery pressure (Table 1). Together, these large effects at many points in the oxygen delivery cascade are evidence of two different sets of adaptive responses to millennia of residence at high altitude.
Table 1.
Comparisons of oxygen transport traits for Tibetan and Andean high-altitude natives living at comparable altitudes between 3,500 and 4,500 m (expressed as effect size d and as heritability h2)
| Trait | d | Percent nonoverlap of observations | h2 | Refs. |
|---|---|---|---|---|
| Resting ventilation, liters/min | Male, 1.0Female, 1.1 | ≈55% (n = 320 Tibetan, 542 Andean) | Tibetan, 0.32 Andean, not significant | 20 |
| Tidal volume, ml | Male, 1.1Female, 0.8 | ≈55% (n = 320 Tibetan, 542 Andean) | No data | 20 |
| Respiration rate, breaths per minute | Male, −0.2 Female, −0.2 | ≈15% (n = 320 Tibetan, 542 Andean) | No data | 20 |
| HVR, Δliters/min per saturation, % | Male, 0.8Female, 0.8 | ≈47% (n = 320 Tibetan, 542 Andean) | Tibetan, 0.35 Andean, 0.22 | 20 |
| Oxygen saturation of hemoglobin, % | Male, −0.9 Female, −0.5 | ≈47%, ≈33% (n = 354 Tibetan, 381 Andean) | Tibetan, 0.35 Andean, NS | 26, 27 |
| Hemoglobin concentration, g/dl | Male, −2.2 and −0.7 Female, −2.4 | >82%; ≈43% (n = 136 Tibetan, 174 Andean) | Tibetan, 0.64 Andean, 0.89 | 25, 28 |
| 2,3-Bisphosphoglycerate mutase concentration | Male, −0.7 | ≈43% (n = 30 Tibetan, 30 Andean) | No data | 25, 41 |
| Erythropoietin concentration, milliunits/ml | Male, −0.2 | ≈15% (n = 30 Tibetan, 29 Andean) | No data | 25 |
| Exhaled NO, nmHg | Male, 1.2Female, 1.1 | ≈55% (n = 105 Tibetan, 144 Andean) | No data | 34 |
| Partial pressure of O2 in arterial blood, mmHg | Male, −0.8 | ≈47% (n = 10 Tibetan, 20 Andean) | No data | 24, 25 |
| Mean pulmonary artery pressure, mmHg | Male, −1.3 | ≈65% (n = 5 Tibetan, 11 Andean) | No data | 29 |
| Pulmonary artery systolic pressure, mmHg | Male and female, −1.3 | ≈65% (n = 57 Tibetan, 14 Andean) | No data | 30, 31 |
| Calf muscle capillary density, no/mm2 | Male, 1.3 | ≈65% (n = 5 Tibetan, 10 Andean) | No data | 64–66 |
| Mitochondrial volume density, % | Male, −0.3 | ≈21% (n = 5 Tibetan, 10 Andean) | No data | 64–66 |
Negative effect sizes reflect lower Tibetan mean values.
Are These Functional Adaptations Heritable?
To evaluate the hypothesis that natural selection accounts for the functional physiological characteristics of Tibetan highlanders relative to Andean highlanders or of highlanders relative to lowlanders, a primary consideration is the presence of heritable variation in the traits under consideration. However, the genetic underpinnings of these quantitative traits are mostly unknown (with the exception of nitric oxide). These traits are also influenced by individual characteristics, including age and sex.
Quantitative genetic techniques can be used to estimate the heritability (h2), the proportion of total variance in a trait attributable to the genetic relationships among individuals in the population. Theoretical values for h2 can range from 0 (no genetic variance) to 1 (all of the variance is genetic), with higher h2 values implying a greater potential for natural selection. It is calculated by using samples containing biological relatives and comparing the observed similarities in trait values of relatives with expectations based on the proportions of their shared genes. An absence of significant h2 does not mean there is no genetic influence on these traits; it simply means that no genetic variance is expressed, and therefore there is no potential for natural selection at the time of measurement. Genetic homogeneity could reflect past natural selection. Table 1 shows that Tibetan samples generally have higher h2 and thus greater potential for natural selection on many of the oxygen delivery traits described above. The presence among Tibetan, but not Andean, high-altitude natives of significant h2 for resting ventilation and oxygen saturation is indirect evidence of population genetic differences, because it reflects the presence of at least two alleles in the Tibetan sample (but not the Andean sample). Interestingly, a sample of people with one high-altitude native Tibetan parent and one low-altitude native Chinese parent was found to have high resting ventilation similar to Tibetans but low HVR, similar to Chinese residents at high altitude (46).
Another approach to detecting the genetic bases of these traits, so far applied only in the Andean population, is the use of admixture analysis (47–49). This approach takes advantage of the history of Spanish and African migration and intermarriage with the indigenous population in the Andean region and uses a panel of ancestry-informative genetic markers to quantify the proportion of Native American, European, and West African ancestry of each study participant. The contribution of the “proportion of Native American ancestry,” called genetic admixture, to variation in HVR has been estimated for samples of low-altitude natives from the Andean region who were acutely exposed to high altitude. A higher proportion of Native American ancestry was associated with lower ventilation during exercise and a lower HVR. However, there was no association of admixture with oxygen saturation upon acute exposure. These results support the hypothesis of distinctive genetic bases to the relatively low ventilation and HVR exhibited by Andean highlanders and support the finding of no heritable variance in oxygen saturation. Like the quantitative genetic approach, genetic admixture studies do not identify any specific genetic locus.
There is evidence for a major gene (an inferred locus whose alleles have a large quantitative effect) on oxygen saturation among Tibetans. Individuals with the inferred autosomal dominant allele average 6–10% higher oxygen saturation than their homozygous counterparts at the same altitude (50). Although the specific locus and alleles remain unknown, an individual's genotypic probability can be estimated. In a sample of nearly 700 women residing at ≈4,000 m, those estimated with high probability to be homozygous or heterozygous for the high oxygen saturation allele had more surviving children (3.7 as compared with 1.6 for women estimated to be homozygous for the low saturation allele), primarily because of lower infant mortality. Using these observations and assigning a Darwinian fitness coefficient of 1.0 to the women with the high-saturation genotype, the relative Darwinian fitness of women with the low-saturation genotype was only 0.44. For comparison, in the classic case of an environment with endemic falciparum malaria, a Darwinian fitness coefficient of 0.66 applies to homozygotes for normal hemoglobin A (51). High-altitude hypoxia may be an even stronger agent of natural selection than falciparum malaria. These findings suggest that the frequency of the high saturation allele may be increasing rapidly in the Tibetan population.
With respect to identifying specific genetic loci contributing to high-altitude functional adaptation, efforts so far have not been successful. They have mainly used the strategy of identifying plausible candidate genes and examining them for distinctive alleles or allele frequencies. Unusual genetic variants or allele frequencies have not been detected in the mitochondrial genome of Tibetans (52). A plausible candidate is the gene for myoglobin, a protein that contributes to oxygen storage and diffusion in skeletal and cardiac muscle. A screen of the myoglobin gene exon 2 for novel variants or deviations from Hardy–Weinberg equilibrium in a sample of Tibetans found little that was distinctive, apart perhaps from a higher frequency of one variant as compared with a U.S. sea-level population (53). Myoglobin gene expression was reported to be very high among Tibetans, regardless of altitude of residence (43).
Candidate genes for pulmonary vasodilators have been examined, based on the reasoning that alleles for high levels could improve blood flow and oxygen diffusion in the lung (54). One study reported a high frequency of a “wild-type” endothelial NO synthase (one of three enzymes catalyzing the synthesis of NO) haplotype in a Tibetan (Sherpa) sample as compared with a low-altitude sample (55). That study reported a lower level of circulating NO metabolites in the serum of the high-altitude sample. That finding is contrary to expectation based on the finding of high exhaled NO among Tibetans.
Another candidate gene is the transcription factor hypoxia-inducible factor 1 (HIF1) often called the “master regulator” of oxygen homeostasis, because it induces >70 genes that respond to hypoxia (56–58). An investigation of polymorphisms in the HIF1A gene of Tibetans (Sherpas) found a dinucleotide repeat in 20 Tibetans that was not found in 30 Japanese lowlander controls (59). However, no phenotypic data were reported. On the one hand, it seems unlikely that a transcription factor regulating the induction of dozens of genes accounts for the Tibetan–Andean differences, because a change of this sort would have many downstream effects. Perhaps more likely is genetic variation in one or more of the ≥70 genes induced by HIF1 or in the biochemical pathways in which they participate. On the other hand, considering that the Tibetan–Andean differences involve many traits and apparently have accumulated in a relatively short time of ≤25,000 years, perhaps a change in a regulatory mechanism is the underlying mechanism.
In summary, measures of oxygen transport reveal that Andean and Tibetan populations have large quantitative differences in numerous physiological and molecular traits involved in oxygen delivery. The hypothesis is that evolutionary processes have tinkered differently in the two founding populations and their descendents, with the result that the two populations followed different routes to the same functional outcome of successful oxygen delivery. That conclusion will remain tentative, however, until the responsible genes are identified.
Acknowledgments
We thank the thousands of high-altitude natives on two continents who participated in this research and welcomed us into their communities. The research reported here was supported in part by grants from the National Science Foundation (Grant 215747), the National Institutes of Health (Grants M01 RR-00080, HL-60917, and M01 RR-018390), the Luce Foundation, and the National Geographic Committee on Research and Exploration. Amy Rezac and Jaleesa Avak prepared the figures. This research was conducted in collaboration with numerous scientists in the United States, including G. M. Brittenham, S. C. Erzurum, B. D. Hoit, K. P. Strohl, and members of their laboratories, and in collaboration with the Tibet Academy of Social Sciences, Lhasa, Tibet Autonomous Region, and the Instituto Boliviano de Biologia de Altura, La Paz, Bolivia.
Abbreviation
- HVR
hypoxic ventilatory response.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution I: Adaptation and Complex Design,” held December 1–2, 2006, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program is available on the NAS web site at www.nasonline.org/adaptation_and_complex_design.
The author declares no conflict of interest.
References
- 1.Trinkaus E. Annu Rev Anthropol. 2005;34:207–240. [Google Scholar]
- 2.Aldenderfer MS. Am Sci. 2003;91:542–549. [Google Scholar]
- 3.Ward MP, Milledge JS, West JB. High Altitude Medicine and Physiology. London: Oxford Univ Press; 2000. [Google Scholar]
- 4.Raymond J, Segre D. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- 5.Hochachka P, Rupert J. BioEssays. 2003;25:515–519. doi: 10.1002/bies.10261. [DOI] [PubMed] [Google Scholar]
- 6.Bekker A, Holland HD, Wang PL, Rumble D, III, Stein HJ, Hannah JL, Coetzee LL, Beukes NJ. Nature. 2004;427:117–120. doi: 10.1038/nature02260. [DOI] [PubMed] [Google Scholar]
- 7.Huey RB, Ward PD. Science. 2005;308:398–401. doi: 10.1126/science.1108019. [DOI] [PubMed] [Google Scholar]
- 8.Massabuau JC. Mech Ageing Dev. 2003;124:857–863. doi: 10.1016/s0047-6374(03)00147-7. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield GE, Gates J, Fleming S, Brooks GA, Sutton JR, Reeves JT. J App Physiol. 1992;72:1741–1748. doi: 10.1152/jappl.1992.72.5.1741. [DOI] [PubMed] [Google Scholar]
- 10.Buskirk ER. In: Man in the Andes: A Multidisciplinary Study of High Altitude Quechua. Baker PT, Little MA, editors. Stroudsburg, PA: Dowden, Hutchinson, and Ross; 1976. pp. 283–299. [Google Scholar]
- 11.Baker PT. In: Man in the Andes: A Multidisciplinary Study of High-Altitude Quechua. Baker PT, Little MA, editors. Stroudsburg, PA: Dowden, Hutchinson, and Ross; 1976. pp. 300–314. [Google Scholar]
- 12.Marconi C, Marzorati M, Cerretelli P. High Alt Med Biol. 2006;7:105–115. doi: 10.1089/ham.2006.7.105. [DOI] [PubMed] [Google Scholar]
- 13.Wu T, Kayser B. High Alt Med Biol. 2006;7:193–208. doi: 10.1089/ham.2006.7.193. [DOI] [PubMed] [Google Scholar]
- 14.Mazess RB, Picon-Reategui E, Thomas RB, Little MA. Aerosp Med. 1969;40:6–9. [PubMed] [Google Scholar]
- 15.Beall CM, Henry J, Worthman C, Goldstein MC. Am J Hum Biol. 1996;8:361–370. doi: 10.1002/(SICI)1520-6300(1996)8:3<361::AID-AJHB7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Picon-Reategui E. J Appl Physiol. 1961;16:431–434. doi: 10.1152/jappl.1961.16.3.431. [DOI] [PubMed] [Google Scholar]
- 17.Beall CM. In: Mountain Biodiversity; A Global Assessment. Korner C, Spehn EM, editors. New York: The Parthenon Publishing Group; 2002. pp. 199–210. [Google Scholar]
- 18.Moore LG. Respir Physiol. 2000;121:257–276. doi: 10.1016/s0034-5687(00)00133-x. [DOI] [PubMed] [Google Scholar]
- 19.Beall CM. Annu Rev Anthropol. 2001;30:423–446. [Google Scholar]
- 20.Beall CM, Brittenham GM, Strohl KP, Decker MJ, Goldstein MC, Blangero J, Williams-Blangero S, Almasy L, Worthman CM. Am J Phys Anthropol. 1997;104:427–447. doi: 10.1002/(SICI)1096-8644(199712)104:4<427::AID-AJPA1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Severinghaus JW, Bickler P. J Appl Physiol. 1994;77:313–316. doi: 10.1152/jappl.1994.77.1.313. [DOI] [PubMed] [Google Scholar]
- 22.Weil JV, Byrne-Quinn E, Sodal IE, Filley GF, Grover RF. J Clin Invest. 1971;50:186–195. doi: 10.1172/JCI106472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhuang J, Droma T, Sun S, Janes C, McCullough RE, McCullough RG, Cymerman A, Huang SY, Reeves JT, Moore LG. J Appl Physiol. 1993;74:303–311. doi: 10.1152/jappl.1993.74.1.303. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, McCullough RG, Sun S, Moore LG. Respir Physiol. 1996;103:75–82. doi: 10.1016/0034-5687(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 25.Winslow RM, Chapman KW, Gibson CC, Samaja M, Monge C, Goldwasser E, Sherpa M, Blume FD, Santolaya R. J Appl Physiol. 1989;66:1561–1569. doi: 10.1152/jappl.1989.66.4.1561. [DOI] [PubMed] [Google Scholar]
- 26.Beall CM, Strohl KP, Blangero J, Williams-Blangero J, Brittenham GM, Goldstein MC. Hum Biol. 1997;69:597–604. [PubMed] [Google Scholar]
- 27.Beall CM, Almasy LA, Blangero J, Williams-Blangero S, Brittenham GM, Strohl KP, Decker M, Vargas E, Villena M, Soria R, et al. Am J Phys Anthropol. 1999;108:41–51. doi: 10.1002/(SICI)1096-8644(199901)108:1<41::AID-AJPA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 28.Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, et al. Am J Phys Anthropol. 1998;106:385–400. doi: 10.1002/(SICI)1096-8644(199807)106:3<385::AID-AJPA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Groves BM, Droma T, Sutton JR, McCullough RG, Cullough REM, Zhuang J, Rapmun G, Sun S. J Appl Physiol. 1993;74:312–318. doi: 10.1152/jappl.1993.74.1.312. [DOI] [PubMed] [Google Scholar]
- 30.Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM. J Appl Physiol. 2006;99:1796–1801. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- 31.Antezana AM, Antezana G, Aparicio O, Noriega I, Velarde FL, Richalet JP. Eur Respir J. 1998;12:1181–1185. doi: 10.1183/09031936.98.12051181. [DOI] [PubMed] [Google Scholar]
- 32.Duplain H, Sartori C, Lepori M, Egli M, Allemann Y, Nicod P, Scherrer U. Am J Resp Crit Care Med. 2000;162:221–224. doi: 10.1164/ajrccm.162.1.9908039. [DOI] [PubMed] [Google Scholar]
- 33.Busch T, Bartsch P, Pappert D, Grunig E, Hildebrandt W, Elser H, Falke KJ, Swenson ER. Am J Respir Crit Care Med. 2001;163:368–373. doi: 10.1164/ajrccm.163.2.2001134. [DOI] [PubMed] [Google Scholar]
- 34.Beall CM, Laskowski D, Strohl KP, Soria R, Villena M, Vargas E, Alarcon AM, Gonzales C, Erzurum SD. Nature. 2001;414:411–412. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 35.Schneider A, Greene RE, Keyl C, Bandinelli G, Passino C, Spadacini G, Bonfichi M, Arcaini L, Malcovati L, Boiardi A, et al. J Hypertens. 2001;19:213–222. doi: 10.1097/00004872-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Huang SY, Sun S, Droma T, Zhuang J, Tao JX, McCullough RG, McCullough RE, Micco AJ, Reeves JT, Moore LG. J Appl Physiol. 1992;73:2638–2642. doi: 10.1152/jappl.1992.73.6.2638. [DOI] [PubMed] [Google Scholar]
- 37.Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Am J Phys Anthropol. 2001;114:42–53. doi: 10.1002/1096-8644(200101)114:1<42::AID-AJPA1004>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Moore LG, Brodeur P, Chumbe O, D'Brot J, Hofmeister S, Monge C. J Appl Physiol. 1986;60:1401–1406. doi: 10.1152/jappl.1986.60.4.1401. [DOI] [PubMed] [Google Scholar]
- 39.Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert TD, Parra E, Vargas E. Placenta. 2004;25:S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Hoppeler H, Vogt M, Weibel E, Fluck M. Exp Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- 41.Winslow RM, Monge CC, Statham NJ, Gibson CG, Charache S, Whittembury J, Moran O, Berger RL. J Appl Physiol. 1981;51:1411–1416. doi: 10.1152/jappl.1981.51.6.1411. [DOI] [PubMed] [Google Scholar]
- 42.Moore LG, Curran-Everett L, Droma TS, Groves BM, McCullough RE, McCullough RG, Sun SF, Sutton JR, Zamudio S, Zhuang JG. Int J Sports Med. 1992;13:S86–S88. doi: 10.1055/s-2007-1024605. [DOI] [PubMed] [Google Scholar]
- 43.Gelfi C, De Palma S, Ripamonti M, Eberini I, Wait R, Bajracharya A, Marconi C, Schneider A, Hoppeler H, Cerretelli P. FASEB J. 2004;18:612–614. doi: 10.1096/fj.03-1077fje. [DOI] [PubMed] [Google Scholar]
- 44.Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. J Appl Physiol. 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 46.Curran L, Zhuang J, Sun SF, Moore LG. J Appl Physiol. 1997;83:2098–2104. doi: 10.1152/jappl.1997.83.6.2098. [DOI] [PubMed] [Google Scholar]
- 47.Brutsaert TD, Spielvogel H, Soria R, Caceres E, Buzenet G, Haas JD. J Appl Physiol. 1999;110:435–455. doi: 10.1002/(SICI)1096-8644(199912)110:4<435::AID-AJPA5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Palacios JA, Rivera M, Rodriguez I, Leon-Velarde F. J Appl Physiol. 2003;95:519–528. doi: 10.1152/japplphysiol.01088.2002. [DOI] [PubMed] [Google Scholar]
- 49.Brutsaert TD, Parra E, Shriver M, Gamboa A, Palacios JA, Rivera M, Rodriguez I, Leon-Velarde F. Am J Phys Anthropol. 2004;123:390–398. doi: 10.1002/ajpa.10319. [DOI] [PubMed] [Google Scholar]
- 50.Beall CM, Song K, Elston RC, Goldstein MC. Proc Natl Acad Sci USA. 2004;101:14300–14304. doi: 10.1073/pnas.0405949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Firschein IL. Am J Hum Genet. 1961;13:233–254. [PMC free article] [PubMed] [Google Scholar]
- 52.Torroni A, Miller JA, Moore LG, Zamudio S, Zhuang J, Droma T, Wallace DC. Am J Phys Anthropol. 1994;93:189–199. doi: 10.1002/ajpa.1330930204. [DOI] [PubMed] [Google Scholar]
- 53.Moore LG, Zamudio S, Zhuang J, Droma T, Shohet RV. High Alt Med Biol. 2002;3:39–47. doi: 10.1089/152702902753639531. [DOI] [PubMed] [Google Scholar]
- 54.Wilkins MR, Aldashev A, Morrell NW. Lancet. 2002;359:1539–1540. doi: 10.1016/s0140-6736(02)08528-8. [DOI] [PubMed] [Google Scholar]
- 55.Droma Y, Hanaoka M, Basnyat B, Ariyal A, Neupane P, Pandit A, Sharma D, Miwa N, Ito M, Katsuyama Y, et al. High Alt Med Biol. 2006;7:209–220. doi: 10.1089/ham.2006.7.209. [DOI] [PubMed] [Google Scholar]
- 56.Semenza GL. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 57.Semenza GL. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 58.Semenza GL. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 59.Suzuki K, Kizaki T, Hitomi Y, Nukita M, Kimoto K, Miyazawa N, Kobayashi K, Ohnuki Y, Ohno H. Med Hypotheses. 2003;61:385–389. doi: 10.1016/s0306-9877(03)00178-6. [DOI] [PubMed] [Google Scholar]
- 60.Hurtado A. In: Handbook of Physiology Section 4: Adaptation to the Environment. Dill DB, editor. Washington, DC: Am Physiol Soc; 1964. pp. 843–859. [Google Scholar]
- 61.Beall CM, Strohl K, Blangero J, Williams-Blangero S, Brittenham GM, Goldstein MC. Hum Biol. 1997;69:597–604. [PubMed] [Google Scholar]
- 62.Beall CM. Integr Comp Biol. 2006;46:18–24. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]
- 63.Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P. Int J Sports Med. 1990;11(Suppl 1):S3–S9. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- 64.Desplanches D, Hoppeler H, Tushcer L, Mayet MH, Spielvogel H, Ferretti G, Kayser B, Leuenberger M, Grunenfelder A, Favier R. J Appl Physiol. 1996;81:1946–1951. doi: 10.1152/jappl.1996.81.5.1946. [DOI] [PubMed] [Google Scholar]
- 65.Kayser B, Hoppeler H, Claassen H, Cerretelli P. J Appl Physiol. 1991;70:1938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- 66.Desplanches D. Pflügers Arch. 1993;425:263–267. doi: 10.1007/BF00374176. [DOI] [PubMed] [Google Scholar]