Abstract
Many scarab beetles produce rigid projections from the body called horns. The exaggerated sizes of these structures and the staggering diversity of their forms have impressed biologists for centuries. Recent comparative studies using DNA sequence-based phylogenies have begun to reconstruct the historical patterns of beetle horn evolution. At the same time, developmental genetic experiments have begun to elucidate how beetle horns grow and how horn growth is modulated in response to environmental variables, such as nutrition. We bring together these two perspectives to show that they converge on very similar conclusions regarding beetle evolution. Horns do not appear to be difficult structures to gain or lose, and they can diverge both dramatically and rapidly in form. Although much of this work is still preliminary, we use available information to propose a conceptual developmental model for the major trajectories of beetle horn evolution. We illustrate putative mechanisms underlying the evolutionary origin of horns and the evolution of horn location, shape, allometry, and dimorphism.
Keywords: allometry, development, phenotypic plasticity, sexual selection, weapons
The origin and subsequent evolutionary diversification of complex morphological structures have long puzzled biologists (1–3). These traits may arise suddenly, and their size and complexity, as well as their lack of visible homology with existing structures (novelty), were thought for many years to be incompatible with traditional gradualistic views of genetic variation, selection, and evolution (4). Modern analytical techniques, including phylogenetic analyses and molecular genetics, have greatly improved our understanding of how complex structures arise, revealing in several instances how subtle perturbations to existing developmental mechanisms can generate substantial and unprecedented changes in animal form (4, 5). These studies demonstrate unequivocally that both novelty and complexity can arise from simple changes to development, and they illuminate how an understanding of development can inform studies of character evolution. We illustrate this approach using the example of beetle horns, skeletal outgrowths that function as weapons in intraspecific combat.
Beetles with horns include some of the most magnificent and bizarre organisms alive today. The sizes of these horns relative to the sizes of the beetles that bear them can dwarf even the most extreme antlers of ungulates, and the diversity of horn forms is breathtaking. Darwin used beetle horns when he first described sexual selection (6) and Teissier and Huxley used beetle horns when they first described the concept of relative growth and allometry (7, 8).
How did the first beetle horns arise? And once present, how were these structures modified so dramatically in form? In this article, we approach these questions from two vantages, comparative phylogenetic studies of horn evolution and developmental studies of the regulation of horn growth, and show that these disparate biological perspectives converge on the same basic conclusions regarding horn evolution: beetle horns do not appear to be difficult structures to gain or lose, and they appear capable of rapid and radical changes in form. We end this paper with a conceptual model for how beetle horns evolve. Specifically, we identify three developmental mechanisms that are now thought to underlie the principle trajectories of beetle horn evolution. This integration of perspectives comprises an important step in our attempts to elucidate the myriad ways in which these exaggerated structures have radiated in form. It also illustrates the more general theme of this colloquium: that “Darwinian” processes of selection, combined with subtle genetic variations in basic developmental processes, can account for the origin, and the subsequent diversification of even the most extreme animal structures.
A Natural History of Beetles with Horns
Beetle horns are weapons: they are used in combat between rival males over access to females (9–11). These contests tend to occur in physically restricted substrates, such as on branches or bamboo shoots or more commonly, inside the confines of tunnels. Tunnels can be the hollowed-out stems of plants, such as sugar cane, or burrows excavated into the soil. Regardless, long horns aid males in these battles over reproductive access to females [males with the longest horns win (12, 13)], and this can translate into higher fertilization success for these long-horned individuals (14). Thus, beetle horns are conspicuous morphological structures of known functional significance, and the more than a century of interest and observation of these animals, combined with the recent behavioral studies listed above, provide a rich ecological context for the historical and developmental studies we are about to describe.
Beetles with horns are primarily confined to the scarab superfamily (Coleoptera: Scarabaeoidea). Extant scarabs are diverse and successful, and include the bess beetles (Passalidae), stag beetles (Lucanidae), dung beetles (Scarabaeinae), flower beetles (Cetoniinae), May and June beetles (Melolonthinae), the chafers (Rutelinae), and the rhinoceros beetles (Dynastinae) (15). The earliest scarabs are thought to have been robust animals with bodies adapted to a lifestyle of burrowing (16–19), and they may have excavated tunnels into the soil beneath dinosaurs (17) or the stems of plants (20).
It is currently not known whether these ancestral scarabs had horns. Despite considerable effort in reconstructing the early history of the scarabs (18, 21–24), little attention has been given to the question of whether or not these animals had horns. The long-standing view has been that these ancestors probably did not have horns. Despite the literally thousands of scarab species with exaggerated horn morphologies, the majority of extant scarabs are hornless. In addition, the family and subfamilies most predominated by species with large horns (Geotrupidae, Scarabaeinae, Dynastinae) are widely separated within the scarabs as a whole [supporting information (SI) Fig. 5], leading to the view that scarab horns must have evolved independently many different times (25).
However, almost all of the extant subfamilies of scarabs possess rudimentary horns, and many of the predominantly “hornless” groups (e.g., Cetoniinae, Rutelinae) contain at least a few species with dramatic horns [e.g., Theodosia viridaurata (Cetoniinae), Ceroplophana modiglianii (Rutelinae)]. Where present, these horns generally occur in the same basic body regions as the horns of more “typical” horned scarabs (the anterior surface of the thoracic pronotum, which is a body region that was already universally enlarged in scarabs and thought to be an adaptation to a burrowing lifestyle, and the dorsal surface of the head). They also tend to have the same basic forms, and they nearly always exhibit the same patterns of horn “dimorphism” (females, small males lack the horns) characteristic of more typical horned scarabs. These observations led us recently to propose that the earliest scarabs may have had horns, as well as the developmental capacity to suppress horn growth facultatively (i.e., horn dimorphism; SI Fig. 5b) (26).
This alternative view of early horn evolution raises the exciting possibility that all descendant scarabs inherited the developmental capacity both to produce and to suppress horns. Extant species lacking horns, in this case, would be secondarily hornless. If true, it might take only relatively subtle genetic modifications to their development to reverse the suppression of horn growth, perhaps accounting for the “irregular” appearances of horned taxa nested within clades of otherwise hornless scarabs. It also could account for the surprising occurrence of mutant horned individuals, which sporadically appear within hornless taxa (27, 28).
Resolving the horn morphologies of the earliest scarabs may take some time. New scarab fossils are discovered each year (19), and these may reveal the shapes of these Jurassic beetles. Certainly, a great deal of information will come from the ongoing studies of horn development (described below). Comparing these mechanisms in different scarab subfamilies will provide important clues as to whether these weapons shared a common origin in their distant past. However, rooting the scarab tree is not necessary for drawing important conclusions about historical patterns of horn evolution. All phylogenies for the scarabs agree that their history was replete with a multitude of gains of horns. Regardless of whether these events represent a series of independent gains of new horns, or recurrent re-gains of ancestral horns, or both, the patterns of horn evolution that emerge from comparative studies of scarab morphology are indisputable. Two conclusions are especially clear.
Conclusion 1: Horns Are Easy to Gain and Lose.
Modern phylogenetic reconstructions of horn evolution reveal a history rich with gains and losses of these structures. One study of 48 species from the dung beetle genus Onthophagus (a mere 2% of this genus and <0.1% of the scarabs) concluded that there had been nine losses of one horn type and at least 15 gains of additional horn types, together contributing to over 25 changes in the physical location of horns (e.g., head versus thorax; see ref. 29). Another study of 45 genera sampled across the subfamily Scarabaeinae suggested that there had been at least three losses and eight gains of horns (30). Inferring ancestor states becomes problematic in these cases (31, 32), but all studies agree that beetle horns have arisen and been lost many, many times.
Conclusion 2: Beetle Horns Change Rapidly and Dramatically in Form.
One recent attempt to characterize the vast diversity of scarab horn morphologies revealed the following four principal trajectories of horn evolution (26).
Horns vary in their physical location (SI Fig. 6).
There are five major regions of the body from which the horns can extend: three dorsal segments of the head (vertex, frons, clypeus) and the center or sides of the thoracic pronotum. Horns appear to be gained or lost independently at each of these body regions, and species can have all possible combinations of these horn types (29). Within any of these regions, horn location also may change; for example, when a single central head horn splits into a lateral pair of horns (SI Fig. 6 Lower).
Horns vary in shape (SI Fig. 7).
Even closely related species often differ extensively in horn shape, and phylogenetic studies suggest that there have been multiple and repeated transformations in horn shape (29). Common changes in shape appear to include the splitting of a single horn into two or even three horns accompanied by changes in horn location (see above), the addition of forks or branches to horns, and the transition from straight to curved horns.
Horns vary in their allometry (the scaling of horn lengths with among-individual variation in body size).
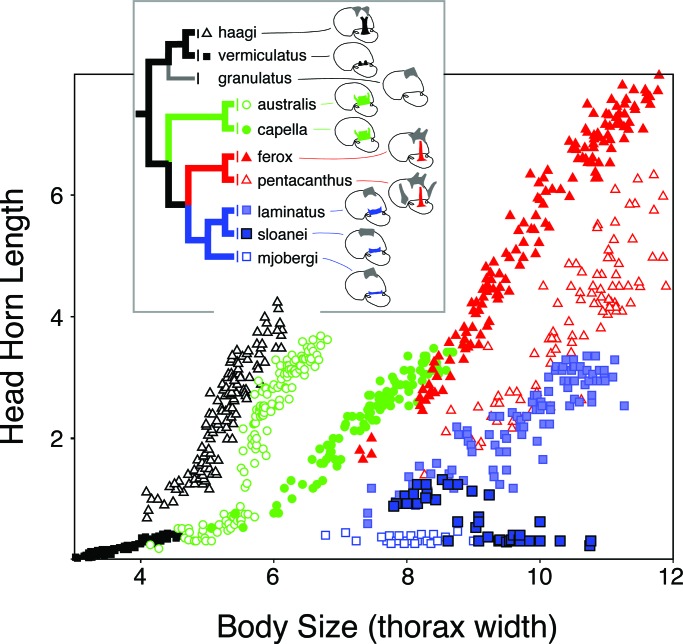
Allometric relationships reflect both the relative size of the horn and the developmental coupling of horn growth with among-individual variation in body size (see below). Horn allometries can diverge rapidly in the wild (33–35) and under selection in the laboratory (36), and the slopes, intercepts, and even the shapes of these allometries differ markedly among extant taxa (Fig. 1).
Fig. 1.
Evolution of horn allometry. Horn length–body size scaling relationships shown for the head horns of nine Australian species of Onthophagus, representing a well supported monophyletic clade within the phylogeny of Emlen et al. (29).
Horns vary in their dimorphism.
Scarab species differ in the presence/absence of horn dimorphism and even in the nature of their dimorphism (31, 37, 38) (SI Fig. 8). Two forms of dimorphism are widespread in these beetles, male dimorphism and sexual dimorphism. In most species with male dimorphism, males smaller than a critical, or threshold, body size dispense with horn production, resulting in horn lengths that scale according to a very different relationship than in large males. Females also often dispense with horn production, sometimes entirely, as in species where females never produce horns. In other cases females do produce the horn but the relative sizes of female horns differs from that of the males. Horn dimorphism also appears to have been gained and lost repeatedly in the history of the scarabs. One study of 31 species of the genus Onthophagus revealed at least 20 reversals in the presence/absence of horn dimorphism (31).
Combined, these four trajectories account for most of the extant diversity in horn forms. But identifying these trajectories does a great deal more than describe taxonomic patterns; it also provides an essential first step toward elucidating the underlying mechanisms responsible for generating this diversity of animal forms. Only by identifying biologically meaningful trajectories of morphological change is it possible to begin to consider how these changes are generated and which developmental and physiological mechanisms are involved. In the following sections, we briefly describe three mechanisms now thought to be involved in the development of a beetle horn, and we illustrate how each of these mechanisms could contribute to the above trajectories of horn evolution.
Three Steps to Building a Beetle Horn
Step 1: Making an Axis of Outgrowth.
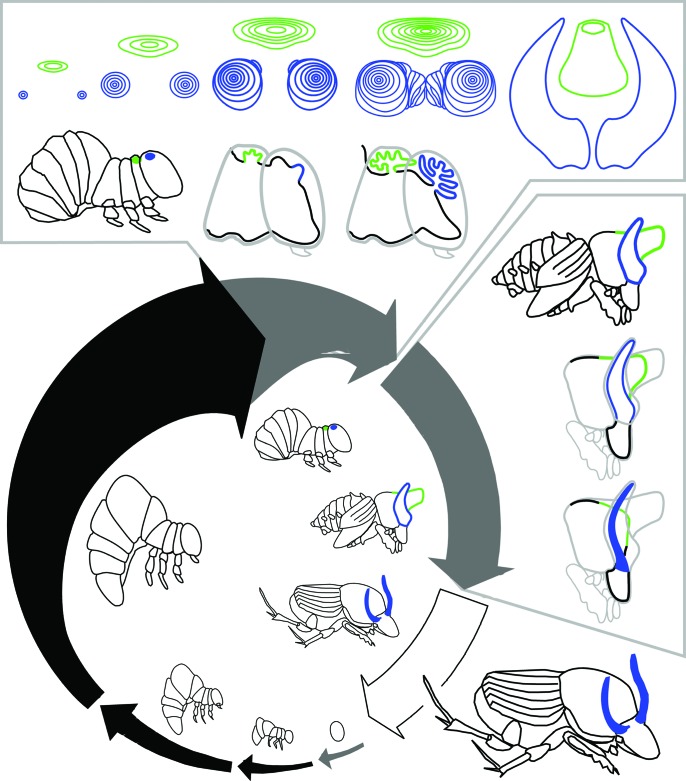
Beetle horns form as long tubes, localized regions of epidermal tissue that undergo a burst of proliferation at the end of the larval period, just before pupation (Fig. 2) (39). As these horn cells proliferate, they fold in on themselves to produce a compact disc of epidermal tissue that unfurls to its full length during the pupal molt. In these respects, beetle horns develop in a way very similar to the traditional appendages in beetles and other insects (e.g., wings or legs), and it now appears that similar mechanisms may be involved.
Fig. 2.
Development of beetle horns. Life cycle shown for the dung beetle Onthophagus taurus. After hatching, beetles pass through three larval instars before molting first into a pupa and then into an adult. Black arrows indicate feeding periods; gray arrows indicate nonfeeding periods. Arrow thickness approximates overall animal body size, and gaps between arrows indicate molting events. The final (third) larval instar can be divided into a feeding period and a nonfeeding prepupal period. Drawings inside the arrows illustrate egg, first through third larval instars, prepupa, pupa, and adult. Horn development can be divided into two stages: a period of horn growth when horn cell proliferation occurs, and a period of horn remodeling. The top box shows horn growth. Front view of thoracic (green) and head (blue) horn discs are shown, along with two profile views of the prepupal head and thorax during this stage. The side box shows horn remodeling. The drawings illustrate the profile of a large male just after pupation and the head and thorax of the same male at two later stages during the pupal period. The head horns are remodeled slightly, to form a pair of curved and slender adult horns. The pupal thoracic horn is removed completely, and is not present in adults. Close-up profiles of prepupa and pupa are adapted from figure 2 of ref. 48.
The major adult structures in metamorphic insects form from isolated “pockets” of cells called imaginal discs, analogous in many ways to the limb buds of vertebrates (40). In Drosophila, these discs have been especially thoroughly studied (41, 42), but discs occur in all metamorphic insects, and arguably, disc-like patterns of growth occur in nonmetamorphic insects and other arthropods as well (40). These clusters of cells behave as remarkably autonomous units, and even when removed (in vitro) or transplanted, these discs are able to complete most of their growth and development, the result of a complex series of molecular and genetic interactions that unfolds within the disc (“patterning”; see refs. 41–45).
The full process of appendage patterning can be functionally subdivided into at least four hierarchical and relatively dissociable modules, each entailing the deployment of a specific and largely self-contained network of genetic interactions [specification of appendage identity (leg, antenna, wing, etc.), formation of an axis of outgrowth (proximal–distal), subdivision of the appendage into segments, and localization and growth of sensory structures, bristles, and hairs (45, 46)]. The portion of this patterning process that is most relevant to beetle horn development is the formation of an axis of outgrowth. Beetle horns do not have segments or joints, but they do have an axis of outgrowth. It now appears that horns form by deploying the outgrowth portion of the patterning cascade (26, 47–49). We do not describe the details of this pathway here (for reviews, see refs. 41–44). Instead, we highlight a few properties of this pathway that are especially relevant for understanding how beetle horns develop.
In an insect appendage, such as a Drosophila leg, the expression of patterning genes is confined to specific domains within the imaginal disc. These expression domains overlap partially, but not completely, with the domains of expression of other genes in the network, and the result is a spatially explicit mosaic of molecular signals defined by the boundaries of expression of the patterning genes. Cells physically located at the intersection of two of these boundaries, because of their position, come into contact with high concentrations of several different signals, including proteins of the patterning genes hedgehog (hh), wingless (wg), and decapentaplegic (dpp), and this critical combination of molecular signals causes these cells to become active organizers of the rest of the disc. These focal cells will give rise to the eventual distal/outermost tip of the new appendage.
Once their fate has been established, these focal cells begin expressing a new suite of patterning genes. The proteins of many of these genes diffuse outwards into the surrounding cells of the disc, activating additional tiers of patterning gene expression. This process both stimulates and coordinates cell proliferation within the disc such that there is a burst of localized growth concentrated around the focal cells. The result is a folded tube of epidermis that will subsequently unfurl to form the appendage.
Localized activation of this portion of the patterning pathway stimulates and coordinates the formation of a new body outgrowth. These molecular interactions define the precise location of a structure (which cells will form the distal tip of the structure) and the signals released from these focal cells direct the subsequent behavior of neighboring cells.
Like the patterning process as a whole, the outgrowth portion of the pathway is a cascade of molecular interactions that once started, unfolds to completion relatively autonomously. This means that exposing cells to the appropriate combination of signals can activate the entire module of the patterning cascade, and result in the formation of a complete (and new) body outgrowth. For example, juxtaposition of wg and dpp signals in an inappropriate region of a developing Drosophila wing disc initiates formation of a second axis of outgrowth: a new distal tip that subsequently generates a new wing (50, 51). This results in the formation of a bifurcated double wing blade, one wing blade that is the default outgrowth, and a second wing blade that is an aberrant outgrowth generated by activating this pathway in a second region of the disc. Similar juxtaposition of these same two signals in a Drosophila leg disc can generate a second fully formed leg attached to the original leg, again resulting in a bifurcated final structure (52, 53). Although these outgrowths are generated artificially in the laboratory, they beautifully illustrate the autonomous property of this pathway, and the potential for this pathway to underlie the evolution of novel morphological structures.
Although the molecular details of this process have been especially well studied in Drosophila leg discs, the basic elements of this outgrowth portion of the patterning pathway appear to be highly conserved across different imaginal discs within a species and across taxa; indeed, all arthropod body outgrowths that have been studied to date appear to use some form of this process in their development (e.g., refs. 54 and 55). Thus, the outgrowth portion of the patterning pathway is an evolutionarily conserved developmental module that leads to the formation of body outgrowths in diverse taxa, including horns in beetles.
All evidence to date suggests that beetle horns form their axis of outgrowth using this same basic patterning pathway. Eight patterning genes are already known to be expressed in horn discs during the period of disc cell proliferation, and most (but interestingly, not all) of these have domains of expression consistent with their putative role in the formation of the axis of outgrowth [dung beetle (Onthophagus) horns: wingless, decapentaplegic, distal-less, daschshund, aristaless, epidermal growth factor receptor, homothorax, extradenticle (refs. 47–49 and L.C.L. and D.J.E., unpublished data); rhinoceros beetle (Dynastinae) horns: wingless, decapentaplegic (L.C.L. and D.J.E. unpublished data)]. Ongoing research involves experiments that test for functional roles for these genes (e.g., by knocking down transcript abundance, using RNA interference methods), and more comprehensive examinations of the patterns of expression of these genes in individuals that differ in horn size and in species that differ in horn form. Future studies are likely to resolve this process in greater detail, but it is probably safe to conclude that the first stage of building a beetle horn involves the deployment of the outgrowth module of the appendage patterning process.
Step 2: Modulation of Horn Growth in Response to Nutrition.
The patterning of insect imaginal discs is not the whole story. Anyone who has reared insects in captivity knows that trait sizes are almost always phenotypically plastic. In particular, they are sensitive to nutrition. Somehow, the basic patterning and growth of structures must be modified in response to the conditions animals encounter as they develop, including and especially the larval nutritional environment.
Beetle horn growth depends critically on larval access to nutrition (56–60). Both horn size and body size are sensitive to variation in nutrition, with the consequence that there is a coupling of the amount of horn growth with overall body size. Iterated across a number of different individuals developing under a range of nutritive conditions and environments, the result of this phenotypic plasticity is allometry, the scaling of body parts with body size (Fig. 3). This highlights an important, but slightly counterintuitive point: nutrition-dependent phenotypic plasticity and allometry are related. In insects at least, they both result from physiological mechanisms that modulate the amount of trait growth in response to nutrition (61–63).
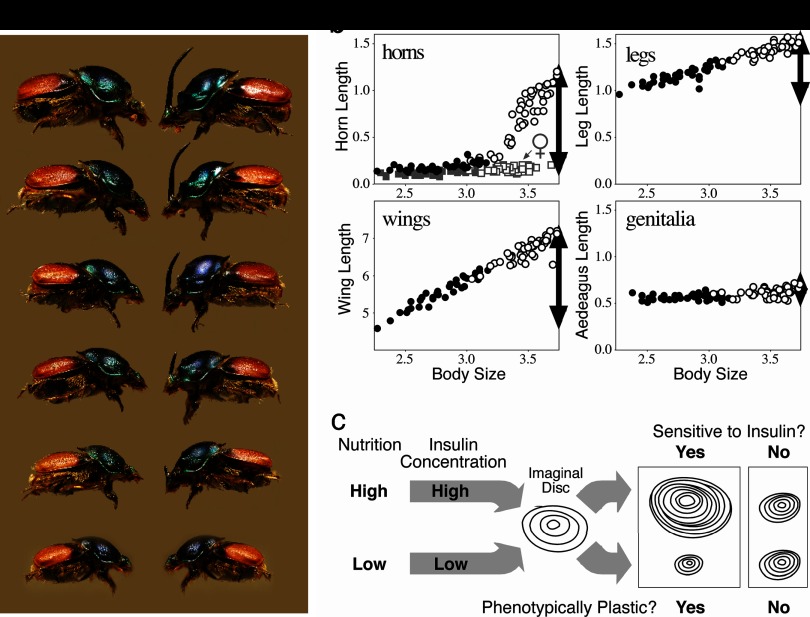
Fig. 3.
Nutrition-dependent phenotypic plasticity and allometry in insects. (a) Female (left) and male (right) Proagoderus (Onthophagus) lanista, showing among-individual variation in body size and, in males, horn size. (b) Scaling relationships (allometries) for four morphological traits in the beetle O. acuminatus. Individuals reared with access to large food amounts (high nutrition) (open symbols) emerged at larger adult body sizes than full-sibling individuals reared with smaller food amounts (low nutrition) (closed symbols). Traits differed in how sensitive (plastic) their growth was to this variation in nutrition. Male horns were the most sensitive, and horn lengths were >10-fold longer in the largest individuals than they were in the smallest individuals (females of this species do not produce enlarged horns, and the height of the corresponding head region is indicated by the gray squares). Leg and wing development was also sensitive to nutrition, but legs were less plastic than wings or horns. Male genitalia were almost entirely insensitive to nutrition, and the size of the aedeagus was largely body size invariant. Horns, legs, and genitalia are plotted on the same scale to illustrate the relative plasticity (horns, legs) or canalization (male genitalia, female horns) of their development. Wings were much larger and are shown on their own scale. In all cases, the degree of plasticity/canalization (black arrows) is reflected in the steepness of the trait size–body size allometries. (c) Model for one developmental mechanism of allometry in insects. Larval nutritional state is reflected in circulating levels of insulins (and growth factors; data not shown), which modulate the rate of growth of each of the trait imaginal discs. Traits whose disc cells are sensitive to these signals exhibit greater nutrition-dependent phenotypic plasticity and steeper allometry slopes than other traits whose disc cells are less sensitive to these signals.
We illustrate this relationship with data from a diet-manipulation experiment, in which individuals from a number of different maternal lines were divided among either a high (large food amount) or a low (reduced food amount) nutrition environment. Larvae given poor nutrition emerged into adults with small body sizes that had short horns and legs and tiny wings (Fig. 3, closed circles). From the same families, siblings given large food amounts matured into adults with much larger body sizes that had much longer horns, legs, and wings (open circles in Fig. 3). Each of these traits is phenotypically plastic, because trait size is sensitive to the larval nutritional environment. In all cases, the magnitude of the plastic developmental response is reflected in the resulting population-level trait size versus body size allometries. Traits that are exquisitely sensitive to nutrition, such as horns, have the steepest allometry slopes; traits that are less sensitive to nutrition, such as legs, have shallower allometry slopes. Genitalia in these beetles are essentially not plastic at all (their growth is insensitive to larval nutrition), which is reflected in their respective trait allometry (Fig. 3b). Thus, traits vary in their sensitivity to the nutrition environment (plasticity), which is manifest across individuals as trait differences in allometry.
Any developmental mechanism of allometry is likely to involve whole-animal circulating signals whose levels (i) are sensitive to larval nutrition and (ii) modulate the growth of the different imaginal discs in accordance with the actual nutritional environment experienced by a larva. Several physiological pathways meet these criteria (reviewed in refs. 62 and 64), and we briefly describe the best studied of these, the insulin receptor (InR) pathway.
Cell proliferation requires high levels of protein synthesis, and in both insects and vertebrates, this process is regulated by the InR pathway (44, 65). In insects, insulin-like peptides secreted primarily by the brain, and probably in cooperation with growth factors secreted by the fat bodies, act as whole-animal circulating signals. When these signals reach the imaginal discs, they bind to InRs and activate a signal transduction cascade that controls the rate of cell proliferation within that disc (65–67). Both insulin and growth factor signal levels are sensitive to larval nutrition, and concentrations of these signals affect the rate of cell proliferation in the imaginal discs (68–70). Thus, cell proliferation should occur at a faster rate in large well-fed individuals, increasing the sizes of their traits relative to those of smaller or poorly fed individuals. Recent evidence suggests that insulin and the InR pathway comprise at least one of the developmental mechanisms modulating the amount of trait growth in response to nutrition in insects (70–72).
Understanding how growing insects respond to variations in their nutritional environment has been a focus of considerable recent research, and several important patterns have emerged from these studies (reviewed in refs. 64 and 73). Insects store nutrients in dispersed organs collectively called fat bodies, which may act as nutrient sensors that signal to the brain and other tissues information pertaining to the nutritional state of the animal (74). Body size appears to be assessed from the relative growth of the prothoracic gland. This endocrine organ communicates size information in the form of the secreted steroid hormone ecdysone (75, 76). Interactions between circulating levels of ecdysone and juvenile hormone, which is also sensitive to larval nutrition (77), coordinate the timing of many developmental events, including molting and metamorphosis (78, 79), as well as both the onset and cessation of cell proliferation in the different imaginal discs (40, 62). All these signals influence the insulin-producing cells in the brain and coordinate circulating levels of insect insulins (71, 75, 77).
By the time that the cells in a horn disc (or any of the traditional imaginal discs) initiate the outgrowth portion of the patterning cascade and begin their burst of proliferative growth, they are bathed in a milieu of circulating whole-animal physiological signals whose levels depend critically on the nutritional state of the animal. Several of these signals have been shown to modulate the rate of cell proliferation in these growing tissues in a way that couples their growth with nutrition. The result of this process is a beetle horn of the appropriate length relative to the final body size attained by that individual.
Step 3: Remodeling of Horns During the Pupal Period.
Both of the above developmental processes (steps 1 and 2) combine to stimulate cell proliferation and growth within developing horn discs. Together, they specify the total amount of growth that occurs. By the time the animal sheds its larval cuticle and molts into a pupa, these growth processes appear to be largely completed and the densely folded discs of horn tissue unfurl to form the fully extended fluid-filled tubes visible in pupae (Figs. 2 and 4).
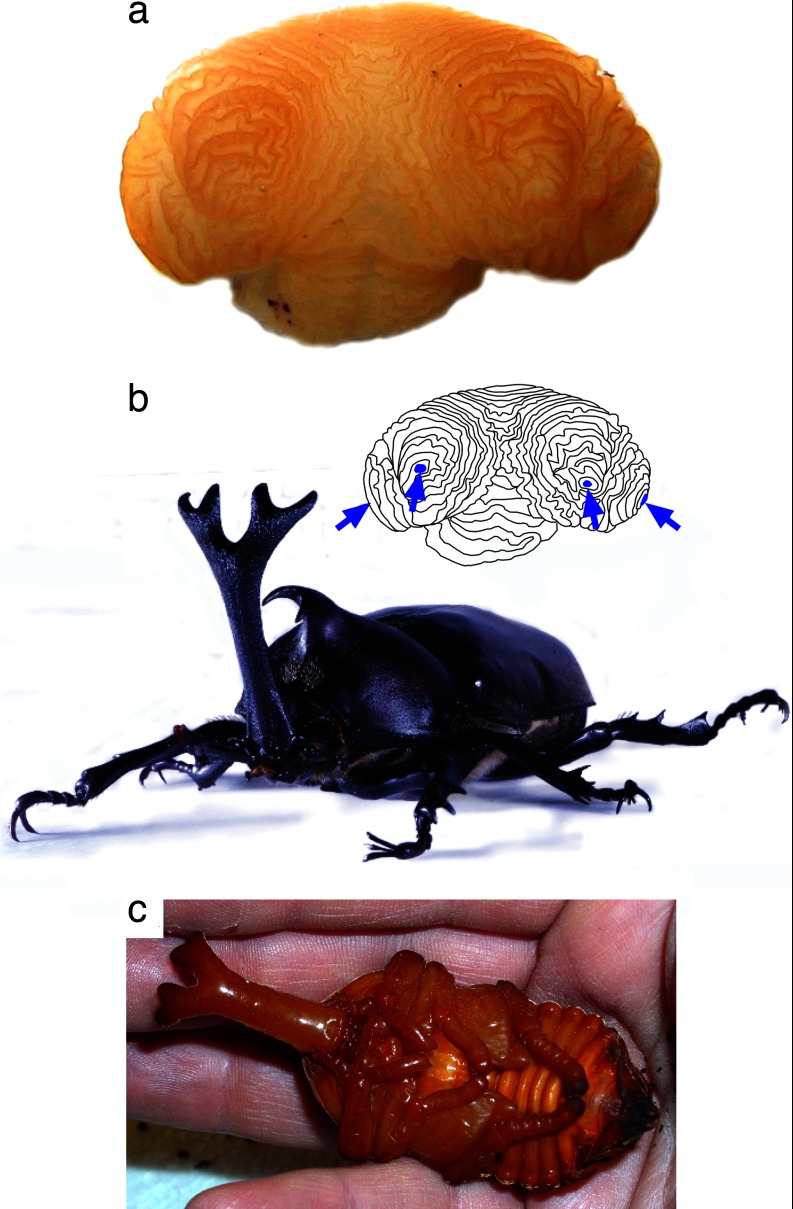
Fig. 4.
Development of a branched beetle horn. Horn disc from a late-stage prepupa of a male rhinoceros beetle (Trypoxylus [Allomyrina] dichotoma, Dynastinae) showing the folded tubes of epidermis (a) that, once unfolded, will comprise the branched adult horn (b). Four distinct axes of proximal–distal outgrowth are visible in this disc (blue arrows, inset) corresponding to each of the distal branch tips of the final horn. All branches are already formed by the time the animal pupates (a and c), suggesting that the evolution of a branched horn shape in this lineage resulted primarily from genetic modifications to the patterning processes that control cell proliferation (horn growth). However, the grooves between horn branches are more pronounced in the adult than in the pupa (b and c show the same individual), suggesting that some remodeling of horn shape also occurs during the pupal period.
One of the most exciting recent discoveries regarding beetle horn development was the observation by Moczek and colleagues (32, 37) that these pupal horns often undergo extensive remodeling during the pupal period. The regions of pupal horns that subsequently are removed by local apoptosis map to domains of expression of some of the same patterning genes that presumably were involved in the initial formation of the horn outgrowth (37, 49). Pupal remodeling of horns is only just beginning to be explored, but already it is clear that during this process, the final shape of the adult structure can be modified (e.g., by producing a narrower and/or more curved final horn; see Fig. 2), and it can also lead to the complete loss of the horn from the adult phenotype in some species. Many scarab species produce a thoracic pupal horn that is not retained in the adult, and in these species this horn is completely reabsorbed by all individuals during the pupal period (37). However, in other species with thoracic pupal horns, the horns are initially grown by all individuals but then selectively reabsorbed by only a subset (e.g., females, small males), resulting in either sexual dimorphism or male dimorphism in expression of the adult weapon (37).
In summary, the three steps to building a beetle horn involve separate and relatively dissociable developmental mechanisms. Deployment of a patterning gene network (mechanism 1), combined with permissive responses to nutrition-sensitive signals (mechanism 2), activates and coordinates a burst of cell proliferation that results in a densely folded disk of epidermal tissue (horn growth). As the animal pupates, this disk unfolds into an extended, fluid-filled tube that is visible in pupae. Subsequent remodeling by selective and domain-specific reabsorbtion of horn tissue during the pupal period (mechanism 3) specifies the final shape and size of the adult horns (horn remodeling). In the next section, we use these developmental mechanisms as a framework for beginning to predict how diversity in beetle horns may have been generated.
A Developmental Model for the Origin and Evolutionary Diversification of Beetle Horns
New Developmental Axes of Outgrowth: Origin of the First Beetle Horns?
Beetle horns arose as novel morphological structures. Although their development shares many similarities with the development of other “traditional” insect appendage imaginal discs, the horns do not themselves derive from these discs (at least so far as we know). Instead, they appear to have arisen as novel discs: new regions of epidermal tissue that at some point in the history of the scarabs began to behave like imaginal discs. Specifically, they began to form an axis of outgrowth. This strongly suggests that the evolution of the first beetle horns entailed the deployment of the outgrowth portion of the limb-patterning pathway in novel regions of the larval epidermis.
It is already clear that many (indeed most) of the genes involved with this portion of the patterning process are expressed in developing beetle horns, and this pathway is sufficiently autonomous that activation of the outgrowth module of the limb-patterning pathway may be sufficient to form an entire structure. We suspect that activation of this pathway alone could stimulate the growth of a full beetle horn.
The evolution of beetle horns most likely resulted from the localized cooption of the outgrowth module of an ancient and existing limb-patterning process (e.g., refs. 48 and 49). This would have generated new axes of outgrowth and new morphological structures that project outwards from the body surface. We suggest that this pathway was deployed independently at least twice, giving rise to the two most common horn locations: the center of the thoracic pronotum and the dorsal surface of the head. We also suspect that this occurred early in the history of the scarabs, possibly in the common ancestor to all of the scarabs. This Jurassic beetle is thought to have lived in burrows and could have used these early horns in an ecological context not unlike what we observe in extant taxa. Today, there are five recognizable body regions with horns, raising the possibility that the patterning pathway was independently deployed additional times as well. It is not yet clear how readily the horn foci migrate across body segment boundaries. For example, could an evolutionary shift in horn location from the back of the head (the vertex) to the center (frons) or front (clypeus) of the head result from a gradual migration of the position of the focal cells activating horn growth? Or must these involve new cooptions of the patterning process, combined with suppression of expression of an earlier horn? Species exist with all possible combinations of these five horn locations, which may indicate that each of these horn types arose de novo; but convincing answers to these questions will have to wait until additional studies of horn patterning have been conducted. Regardless of how many times this pathway was coopted, it is likely that once expression was initiated, this patterning process provided a viable mechanism for subsequent evolutionary modifications to horn form.
Changes in the Expression of Patterning Genes: Evolution of Horn Location and Shape?
If the patterning of beetle horns works in the same way that it does in other insect appendages such as Drosophila legs, then the patterning genes will have precise domains of expression that map to specific parts of the final structure. Critically, the process of patterning will be inextricably coupled with cell proliferation and disc growth. In Drosophila, many of the same signal interactions that specify the domains within a disc also stimulate and coordinate cell proliferation within those domains. This means that altered levels of expression of these patterning genes changes the final sizes of structures (43, 44, 80). Furthermore, because these genes control proliferation within specific subsets of the disc that map precisely to corresponding parts of the final appendage, altered expression of these genes is predicted to change the shape of the developing structure (e.g., the size of the tibia relative to the femur in an insect leg, or the size of the dorsal surface of the tibia relative to the ventral surface).
These two critical features of patterning (the explicit spatial map generated by these molecular signals and the local nature of their effects on cell proliferation) link this developmental process with morphological evolution. Even subtle genetic changes to the levels of expression of these patterning genes can have significant and predictable consequences for the shapes and sizes of adult insect appendages. For these reasons, the limb-patterning pathway has been a major focus for studies of the developmental basis for morphological evolution in arthropods. In the case of beetle horns, we think that subtle changes in the levels of expression of these patterning genes may underlie at least two types of changes in horn form, the evolution of horn location and horn shape (26).
Because cells exposed to high levels of hh, wg, and dpp signals become the distal tip of an appendage, their domains of expression determine the precise physical location of a horn. Genetic changes to any of these domains (e.g., an increase in the expression of wg) would shift the relative location of the domain boundaries, changing the respective point of intersection. Thus, a different cluster of cells would be induced to become the distal tip, and there would be a shift in the precise physical location of the outgrowth. Consequently, genetic modifications to the domains of expression of these patterning genes comprise a plausible mechanism for this trajectory of horn evolution, for example, the migration of a horn from the center to the sides of the head (SI Fig. 6 Lower).
In addition, because changes in the domains of expression of these same genes can duplicate or bifurcate appendages, this same process could give rise to a multitude of evolutionary changes in horn shape. One horn could be split into 2 or even 3, as in Onthophagus fuliginosus (SI Fig. 7). This mechanism could even account for the addition of forks or branches to horns (Fig. 4). Comparative studies of horn patterning are still in their infancy, but the behavior of this pathway in the appendages of other insects, combined with existing evidence for species differences in horn patterning (e.g., refs. 47 and 49) suggest that this mechanism underlies at least some of the evolutionary diversification of horn form.
Changes in the Sensitivity of Horn Cells to Insulin: Evolution of Horn Allometry?
Insulin signaling couples trait growth with nutrition, and for this reason, this pathway comprises another likely mechanism for horn evolution. The InR pathway is activated within each of the imaginal discs, and the sensitivity of each disc to these insulin signals will determine to a large extent how that particular structure will grow. Consequently, genetic changes in the expression or activity of elements in the InR pathway could cause specific traits to become more or less sensitive to insulin signals, with profound consequences for subsequent patterns of growth of that trait. Increased sensitivity of horn disc cells to insulin is predicted to increase the rate of growth of that trait overall and enhance the sensitivity of that trait to nutrition. Thus, it should lead to increased nutrition-dependent phenotypic plasticity in horn growth, a steeper population-level horn allometry slope, and, in the best-fed individuals, a disproportionately larger final horn size.
It is noteworthy that all of these properties (enhanced nutrition-sensitivity, steep allometry slopes, and disproportionately large final trait sizes) are characteristic of the most extreme and exaggerated morphological structures in insects (63) and of the enlarged ornaments and weapons of sexual selection in general (81, 82). It is tempting to speculate that the evolution of extreme sizes in these charismatic traits resulted from something as simple as genetic changes to the sensitivity of their cells to insulin or other nutrition-dependent physiological signals.
One way to begin to test these ideas involves a comparison of insulin sensitivity across the different traits within a species. We predict that traits that have steep and positive allometry slopes should be exquisitely sensitive to insulin signals (e.g., wings, horns). Other traits that are insensitive to nutrition and have shallow allometry slopes should be relatively insensitive to insulin signals (e.g., the genitalia). What we are suggesting is that the degree of phenotypic plasticity or canalization of trait expression could result from disc-specific differences in their sensitivity to circulating insulin signals (64).
In Drosophila trait differences in nutrition-dependent plasticity and allometry result at least in part from disc-specific differences in their responsiveness to insulin signals. Recent experiments by Shingleton et al. (72) showed that traits like wings were sensitive to both insulin and to perturbations to the InR, whereas the genitalia were not. Growth of the genitalia was almost entirely unaffected by perturbations to the InR. This important study confirmed that activity of the InR pathway does affect trait allometry. Results from several other studies where genetic perturbations to elements of this pathway were examined show this as well (e.g., refs. 70 and 76).
We have used quantitation of relative transcript abundances of the InR gene as our first measure of insulin pathway activity in beetle imaginal discs, and our preliminary results indicate that horn, leg, wing, and genital discs differ predictably in their relative activities of this pathway during the period of disk growth (L.C.L. and D.J.E., unpublished results). Reduced activity of this pathway also appears to be one of the mechanisms used by scarabs to truncate horn growth, in this case, in the horn discs of small males and females of the species Onthophagus nigriventris (ref. 26 and L.C.L. and D.J.E., unpublished data). Consequently, the insulin pathway now appears a likely candidate mechanism for the development and evolution of trait plasticity and trait allometry in insects generally and in beetle horns specifically. Although we initially illustrated this point by examining how the different traits within a species have diverged (e.g., horns versus wings versus genitalia), it is important to recognize that this same process can also account for population and species differences in the expression of a single trait (Fig. 1). This process could explain evolutionary shifts in both the relative size of a trait and the evolution of extreme or exaggerated trait sizes.
Changes in the Amount of Horn Resorption During the Pupal Period: Evolution of Horn Shape?
Programmed cell death is an integral part of animal development and can lead to significant remodeling of appendages. Cell death is responsible for generating interdigital spaces in tetrapod limb buds (83, 84) and for creating cavities in the developing inner ear (85). In insects, programmed cell death sculpts head morphology in flies (86) and remodels the outer margins of butterfy wings (87). Interestingly, programmed cell death has also been shown to underlie sexual dimorphism and caste differences in insect wing morphology (88–90), a situation analogous in many respects to what Moczek et al. (37, 49) have observed with beetle horns.
The preliminary findings of Moczek and colleagues (32, 37) suggest that variation in the spatial domains of expression of the patterning gene dll during the prepupal (horn growth) period map to subsequent variation observed in the amount of resorption of horn tissue. The involvement of patterning signals in this process of pupal horn remodeling would be exciting, because it would suggest parallels with the molecular mechanisms involved with tissue remodeling in other taxa (e.g., refs. 84, 91, and 92), and because it would illustrate yet another route to beetle horn evolution. Genetic changes to the spatial domains of expression of patterning genes could underlie evolutionary changes in horn shape through their effects on the relative locations and amounts of cell death in pupal horns, rather than (or in addition to) any effects that they may have on proliferation. Pupal remodeling appears to be widespread, at least within the genus Onthophagus (37), and this process could lead to the carving of spaces between horns (analogous to the spaces between vertebrate limb digits) and to fine-scale sculpting of horn barbs, branches, or curves.
The Many Routes to Horn Dimorphism.
In this article, we have focused on the developmental mechanisms underlying the evolutionary origin of horns and the subsequent diversification of horn forms. For space reasons, we have not elaborated on the mechanisms generating dimorphism in horn expression. However, it is already clear from the few species that have been studied to date that scarabs use a variety of means to shut off horn growth. Indeed, they appear to be remarkably good at it. Both the patterning and insulin pathways are required for horn growth, and a disruption or truncation in the activities of either pathway could halt the proliferation of horns and result in a hornless adult phenotype. Our studies measuring transcript abundances for the patterning gene wg and the InR gene in the species O. nigriventris suggest that both pathways may be involved. Both pathways showed reduced activities in the horn discs of small males and females (which grow only minimal horns) compared with same-stage horn discs from large males (which grow full horns). In addition, Moczek (37) showed that differential amounts of pupal remodeling also contribute to horn dimorphism in this same species: females and small males reabsorb greater amounts of horn tissue than large males. Thus, developmental studies from just this one species implicate three possible mechanistic routes to the suppression of horn growth and to the evolution of horn dimorphism. Other studies by Moczek and colleagues (37, 47–49) have begun to relate domains of expression of patterning genes and relative amounts of pupal remodeling with horn dimorphism in additional Onthophagus species. These studies also reveal a variety of mechanisms for shutting off horn growth.
Conclusions
Even this preliminary examination of the mechanisms of beetle horn development reveals a great deal about their capacity for evolution. The conclusions from these studies of development are remarkably similar to the ones we get by mapping horns onto a phylogeny: it may not be hard to gain a horn. The outgrowth portion of the limb-patterning pathway is sufficiently autonomous that initiating this cascade may be all that is needed to get a fully formed horn. This might be possible in a single step or within a single beetle generation, as suggested by the sporadic appearance of mutant individuals that emerge with fully formed horns from species that are otherwise entirely hornless. It certainly could account for the numerous irregular appearances of horned taxa securely nested within clades of otherwise hornless species.
It also does not appear to be difficult to lose horns. To truncate horn growth, scarabs employ numerous mechanisms, any of which could lead to the sudden loss of horns from a lineage. Subsequent breakdowns in these suppressive mechanisms could just as easily lead to sudden regains of horns. There is no question that the history of beetle horns is a story of repeated gains, losses, and re-gains of these weapons. We suggest that comparative studies of horn evolution and developmental studies of horn growth both attest to the relative ease with which growth of these structures can be turned on or off.
Finally, it does not appear to be difficult to change horn morphology. All three of the mechanisms now thought to be involved with horn development are likely candidates for genetic changes in horn form. We now suspect that subtle genetic changes in just a few elements within these mechanisms might be sufficient to generate all four of the principal trajectories of horn evolution: changes in horn location, shape, allometry and dimorphism.
One hundred and thirty five years ago, Darwin noted that sexual selection appeared to have acted “especially effectively” in scarab beetles (ref. 6, p. 371), and 55 years ago, Gilbert Arrow, then curator of the British Museum, noted that these beetles appeared to have a “special tendency toward the acquisition of horns” (ref. 25, p. 94). Today, we are finally able to elucidate the mechanisms underlying these observations. We now understand a lot about how beetles make a complex structure like a horn, and we are beginning to visualize how these horns might change in form. In essence, we are starting to elucidate what that “special tendency” of the scarabs was, and these insights from development are transforming how we think about the patterns of horn evolution.
Acknowledgments
We thank Kerry Bright and two anonymous reviewers for providing helpful comments on the manuscript and Mary Liz Jameson, Brett Ratcliffe, and Andrew Smith for help with scarab history and diversity. This work was funded by National Science Foundation CAREER Award no. IBN-0092873 (to D.J.E.).
Abbreviation
- InR
insulin receptor.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution I: Adaptation and Complex Design,” held December 1–2, 2006, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program is available on the NAS web site at www.nasonline.org/adaptation_and_complex_design.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701209104/DC1.
References
- 1.Darwin C. On the Origin of Species by Means of Natural Selection. London: John Murray; 1859. [Google Scholar]
- 2.Goldschmidt R. The Material Basis of Evolution. New Haven: Yale Univ Press; 1940. [Google Scholar]
- 3.Mayr E. In: Evolution After Darwin. Tax S, editor. Chicago: Univ of Chicago Press; 1960. pp. 349–380. [Google Scholar]
- 4.Müller GB, Wagner GP. Ann Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- 5.Nitecki MH. Evolutionary Innovations. Chicago: Univ Chicago Press; 1990. [Google Scholar]
- 6.Darwin C. The Descent of Man and Selection in Relation to Sex. New York: Random House, Modern Library; 1871. [Google Scholar]
- 7.Teissier G. Bull Soc Zool Fr. 1935;60:292–307. [Google Scholar]
- 8.Huxley JS. Problems of Relative Growth. London: Methuen; 1932. [Google Scholar]
- 9.Eberhard WG. Psyche. 1978;83:292–298. [Google Scholar]
- 10.Hongo Y. Behaviour. 2003;140:501–517. [Google Scholar]
- 11.Iguchi Y. Entomol Rev Jpn. 2001;56:11–14. [Google Scholar]
- 12.Rasmussen JL. J Insect Behav. 1994;7:67–82. [Google Scholar]
- 13.Pomfret JC, Knell RJ. Anim Behav. 2006;71:567–576. [Google Scholar]
- 14.Hunt J, Simmons L. Proc Royal Soc London Ser B. 2001;268:2409–2414. doi: 10.1098/rspb.2001.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratcliffe BC, Jameson ML. 2006. www.museum.unl.edu/research/entomology/guide/guide-introduction/guide/intro.html.
- 16.Crowson RA. The Biology of Coleoptera. London: Academic; 1981. [Google Scholar]
- 17.Scholtz CH, Chown SL. In: Biology, Phylogeny, and Classification of the Coleoptera. Pakaluk AFJ, Slipinski SA, editors. Warsaw, Poland: Muzeum i Instytut Zoologii PAN; 1995. pp. 335–374. [Google Scholar]
- 18.Iablokoff-Khnzorian SM. Entomologische Abhandlungen der Statlichen Museum für Tierkunde in Dresden. 1977;41:135–199. [Google Scholar]
- 19.Krell F-T. Coleopterists Soc Monogr. 2006;5:120–143. [Google Scholar]
- 20.Lameere A. Bull Ac Belgique. 1904;1904:1327–1364. [Google Scholar]
- 21.Browne DJ, Scholtz CH. Syst Entomol. 1995;20:145–173. [Google Scholar]
- 22.Browne J, Scholtz CH. Systc Entomol. 1999;24:51–84. [Google Scholar]
- 23.Kohlmann B. Coleopterists Soc Monogr. 2006;5:19–34. [Google Scholar]
- 24.Smith ABT, Hawks DC, Heraty JM. Coleopterists Soc Monogr. 2006;5:35–46. [Google Scholar]
- 25.Arrow GJ. Horned Beetles. The Hague: Dr. W. Junk; 1951. [Google Scholar]
- 26.Emlen DJ, Szafran Q, Corley LS, Dworkin I. Heredity. 2006;97:179–191. doi: 10.1038/sj.hdy.6800868. [DOI] [PubMed] [Google Scholar]
- 27.Ballerio A. Folia Heyrovskiana. 1999;7:221–228. [Google Scholar]
- 28.Ziani S. Boll Assoc Romana Entomol. 1995;49:3–4. [Google Scholar]
- 29.Emlen DJ, Marangelo J, Ball B, Cunningham CW. Evolution (Lawrence, Kans) 2005;59:1060–1084. [PubMed] [Google Scholar]
- 30.Emlen DJ, Philips TK. Coleopterists Soc Monogr. 2006;5:47–56. [Google Scholar]
- 31.Emlen DJ, Hunt J, Simmons LW. Am Nat. 2005;166:S42–S68. doi: 10.1086/444599. [DOI] [PubMed] [Google Scholar]
- 32.Moczek AP, Cruickshank TE, Shelby JA. Evolution (Lawrence, Kans) 2006;60:2329–2341. [PubMed] [Google Scholar]
- 33.Moczek AP, Hunt J, Emlen DJ, Simmons LW. Evol Ecol Res. 2002;4:587–601. [Google Scholar]
- 34.Kawano K. Am Nat. 2002;159:255–271. doi: 10.1086/338512. [DOI] [PubMed] [Google Scholar]
- 35.Rowland JM. Aust J Zool. 2003;51:213–258. [Google Scholar]
- 36.Emlen DJ. Evolution (Lawrence, Kans) 1996;50:1219–1230. doi: 10.1111/j.1558-5646.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 37.Moczek AP. Am Nat. 2006;168:711–729. doi: 10.1086/509051. [DOI] [PubMed] [Google Scholar]
- 38.Eberhard WG, Gutierrez EE. Evolution (Lawrence, Kans) 1991;45:18–28. doi: 10.1111/j.1558-5646.1991.tb05262.x. [DOI] [PubMed] [Google Scholar]
- 39.Emlen DJ, Nijhout HF. J Insect Physiol. 1999;45:45–53. doi: 10.1016/s0022-1910(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 40.Truman JW, Riddiford LM. Ann Rev Entomol. 2002;47:467–500. doi: 10.1146/annurev.ento.47.091201.145230. [DOI] [PubMed] [Google Scholar]
- 41.Weihe U, Milán M, Cohen SM. Comp Mol Insect Sci. 2005;1:305–347. [Google Scholar]
- 42.Kojima T. Dev Growth Differ. 2004;46:115–129. doi: 10.1111/j.1440-169X.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 43.Serrano N, O'Farrell PH. Curr Biol. 1997;7:R186–R195. doi: 10.1016/s0960-9822(97)70085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston LA, Gallant P. BioEssays. 2002;24:54–64. doi: 10.1002/bies.10021. [DOI] [PubMed] [Google Scholar]
- 45.Held LI., Jr . Imaginal Discs: The Genetic and Cellular Logic of Pattern Formation. New York: Cambridge Univ Press; 2002. [Google Scholar]
- 46.Carroll SB, Grenier JK, Weatherbee SD. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design. Malden, MA: Blackwell; 2001. [Google Scholar]
- 47.Moczek AP, Nagy LM. Evol Dev. 2005;7:175–185. doi: 10.1111/j.1525-142X.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- 48.Moczek AP. Heredity. 2006;97:168–178. doi: 10.1038/sj.hdy.6800871. [DOI] [PubMed] [Google Scholar]
- 49.Moczek AP, Rose D, Sewell W, Kesselring BR. Dev Genes Evol. 2006;216:655–665. doi: 10.1007/s00427-006-0087-2. [DOI] [PubMed] [Google Scholar]
- 50.Campbell G, Weaver T, Tomlinson A. Cell. 1993;74:1113–1123. doi: 10.1016/0092-8674(93)90732-6. [DOI] [PubMed] [Google Scholar]
- 51.Zecca M, Basler K, Struhl G. Development (Cambridge, UK) 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 52.Diaz-Benjumea FJ, Cohen B, Cohen SM. Nature. 1994;372:175–179. doi: 10.1038/372175a0. [DOI] [PubMed] [Google Scholar]
- 53.Gibson MC, Schubiger G. Development (Cambridge, UK) 1999;126:1591–1599. doi: 10.1242/dev.126.8.1591. [DOI] [PubMed] [Google Scholar]
- 54.Panganiban G, Nagy L, Carroll SB. Curr Biol. 1994;4:671–675. doi: 10.1016/s0960-9822(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 55.Jockusch EL, Williams TA, Nagy LM. Dev Genes Evol. 2004;214:324–338. doi: 10.1007/s00427-004-0412-6. [DOI] [PubMed] [Google Scholar]
- 56.Emlen DJ. Proc R Soc London. 1994;256:131–136. [Google Scholar]
- 57.Hunt J, Simmons LW. Evolution (Lawrence, Kans) 2000;54:936–941. doi: 10.1111/j.0014-3820.2000.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 58.Moczek AP, Emlen DJ. J Evol Biol. 1999;12:27–37. [Google Scholar]
- 59.Iguchi Y. Ann Entomol Soc Am. 1998;91:845–847. [Google Scholar]
- 60.Karino K, Seki N, Chiba M. Ecol Res. 2004;19:663–668. [Google Scholar]
- 61.Stern DL, Emlen DJ. Development (Cambridge, UK) 1999;126:1091–1101. doi: 10.1242/dev.126.6.1091. [DOI] [PubMed] [Google Scholar]
- 62.Emlen DJ, Allen CE. Integr Comp Biol. 2004;43:617–634. doi: 10.1093/icb/43.5.617. [DOI] [PubMed] [Google Scholar]
- 63.Emlen DJ, Nijhout HF. Annu Rev Entomol. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. [DOI] [PubMed] [Google Scholar]
- 64.Shingleton A, Frankino A, Flatt T, Nijhout HF, Emlen DJ. BioEssays. 2007 doi: 10.1002/bies.20584. in press. [DOI] [PubMed] [Google Scholar]
- 65.Weinkove D, Leevers SJ. Curr Opin Genet Dev. 2000;10:75–80. doi: 10.1016/s0959-437x(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 66.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 67.Claeys I, Simonet G, Poels J, Van Loy T, Vercammen L, De Loof A, Vanden Broeck J. Peptides. 2002;23:807–816. doi: 10.1016/s0196-9781(01)00666-0. [DOI] [PubMed] [Google Scholar]
- 68.Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Curr Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 69.Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. Development (Cambridge, UK) 1999;126:211–219. doi: 10.1242/dev.126.2.211. [DOI] [PubMed] [Google Scholar]
- 70.Goberdhan DC, Wilson C. Dev Genes Evol. 2002;212:196–202. doi: 10.1007/s00427-002-0226-3. [DOI] [PubMed] [Google Scholar]
- 71.Nijhout HF. Dev Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 72.Shingleton AW, Das J, Vinicius L, Stern DL. PLoS Biol. 2005;3:1607. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgar BA. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- 74.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 75.Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemont C, Antoniewski C, Carre C, Noselli S, Leopold P. Science. 2005;310:667–670. doi: 10.1126/science.1119432. [DOI] [PubMed] [Google Scholar]
- 76.Mirth C, Truman JW, Riddiford LM. Curr Biol. 2005 Oct 25; doi: 10.1016/j.cub.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 77.Tu MP, Tatar M. Aging Cell. 2003;2:327–333. doi: 10.1046/j.1474-9728.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert LI, Rybczynski R, Tobe SS. In: Metamorphosis: Postembryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. Gilbert LI, Tata JR, Atkinson BG, editors. San Diego: Academic; 1996. pp. 60–108. [Google Scholar]
- 79.Nijhout HF. Insect Hormones. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 80.Campbell G. Nature. 2002;418:781–785. doi: 10.1038/nature00971. [DOI] [PubMed] [Google Scholar]
- 81.Cotton S, Fowler K, Pomiankowski A. Proc Biol Sci. 2004;271:771–783. doi: 10.1098/rspb.2004.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andersson M. Sexual Selection. Princeton: Princeton Univ Press; 1994. [Google Scholar]
- 83.Zuzarte-Luis V, Hurle JM. Int J Dev Biol. 2002;46:871–876. [PubMed] [Google Scholar]
- 84.Rodriguez-Leon J, Merino R, Macias D, Ganan Y, Santesteban E, Hurle JM. Nat Cell Biol. 1999;1:125–126. doi: 10.1038/10098. [DOI] [PubMed] [Google Scholar]
- 85.Fekete DM, Homburger SA, Waring MT, Riedl AE, Garcia LF. Development (Cambridge, UK) 1997;124:2451–2461. doi: 10.1242/dev.124.12.2451. [DOI] [PubMed] [Google Scholar]
- 86.Lohmann I, McGinnis N, Bodmer M, McGinnis W. Cell. 2002;110:457–466. doi: 10.1016/s0092-8674(02)00871-1. [DOI] [PubMed] [Google Scholar]
- 87.Kodama R, Yohida A, Mitsui T. Dev Genes Evol. 1995;204:418–426. doi: 10.1007/BF00360849. [DOI] [PubMed] [Google Scholar]
- 88.Nardi JB, Godfrey GL, Bergstrom RA. J Morphol. 2005;209:121–131. doi: 10.1002/jmor.1052090110. [DOI] [PubMed] [Google Scholar]
- 89.Lobia S, Niitsu S, Fujiwara H. J Insect Sci. 2003;3:11–17. doi: 10.1093/jis/3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sameshima S, Miura T, Matsumoto T. Evol Dev. 2004;6:336–341. doi: 10.1111/j.1525-142X.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- 91.Rusconi JC, Hays R, Cagan RL. Cell Death Differ. 2000;7:1063–1070. doi: 10.1038/sj.cdd.4400767. [DOI] [PubMed] [Google Scholar]
- 92.Adachi-Yamada T, O'Connor MB. Dev Biol. 2002;251:74–90. doi: 10.1006/dbio.2002.0821. [DOI] [PubMed] [Google Scholar]