Abstract
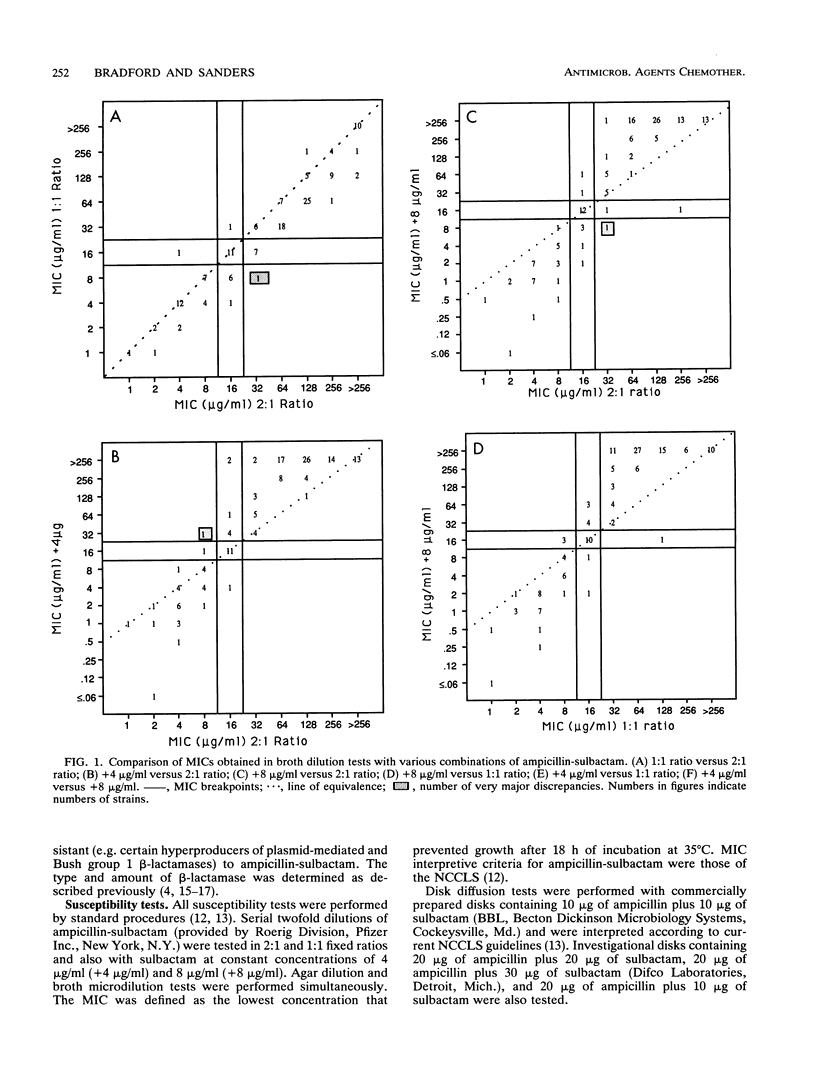
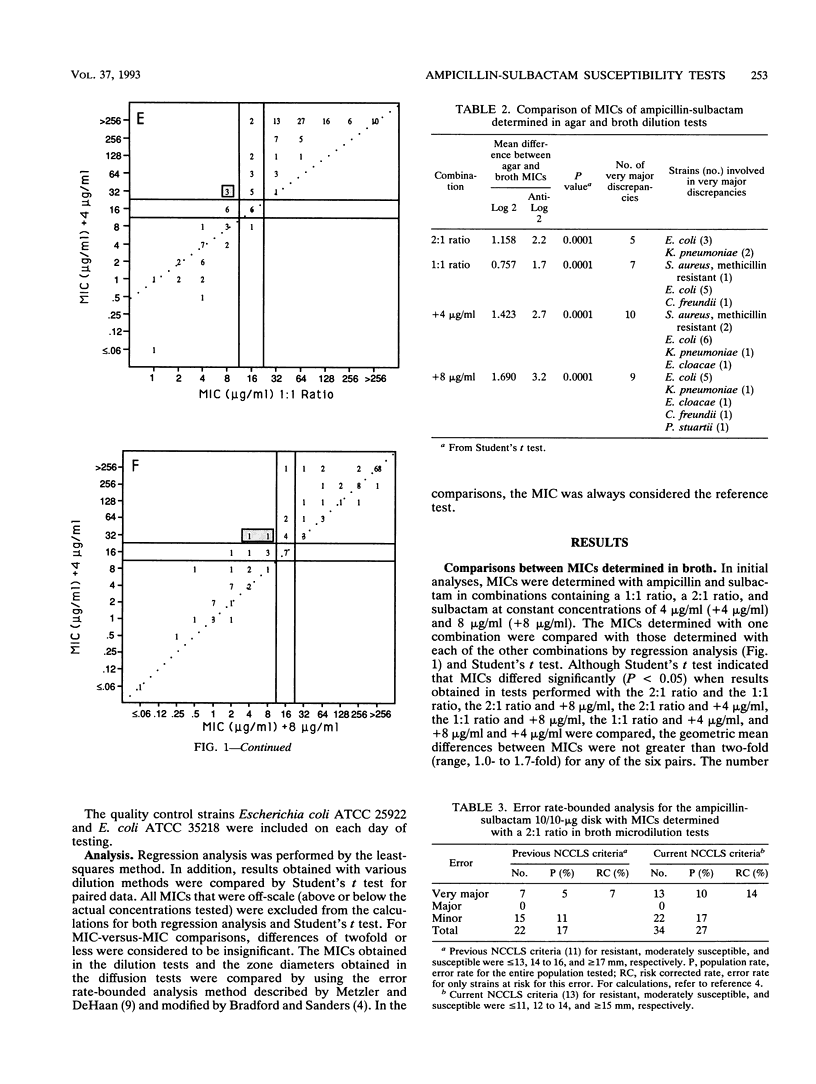
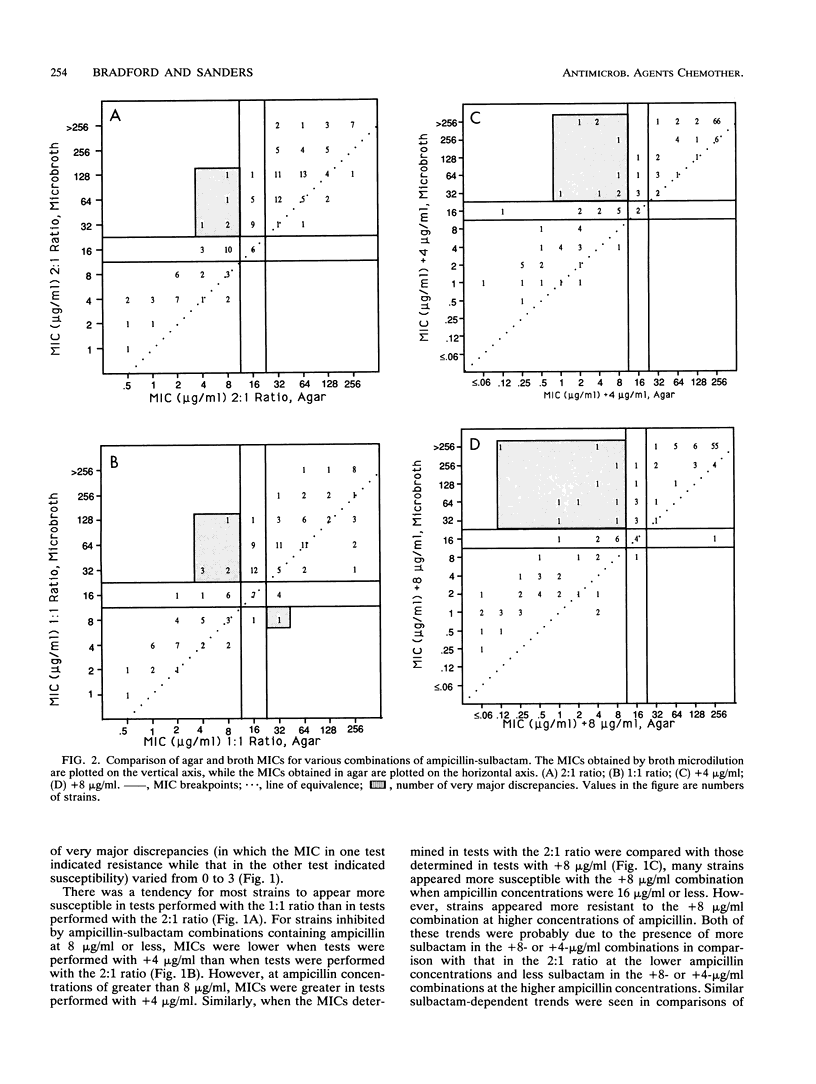
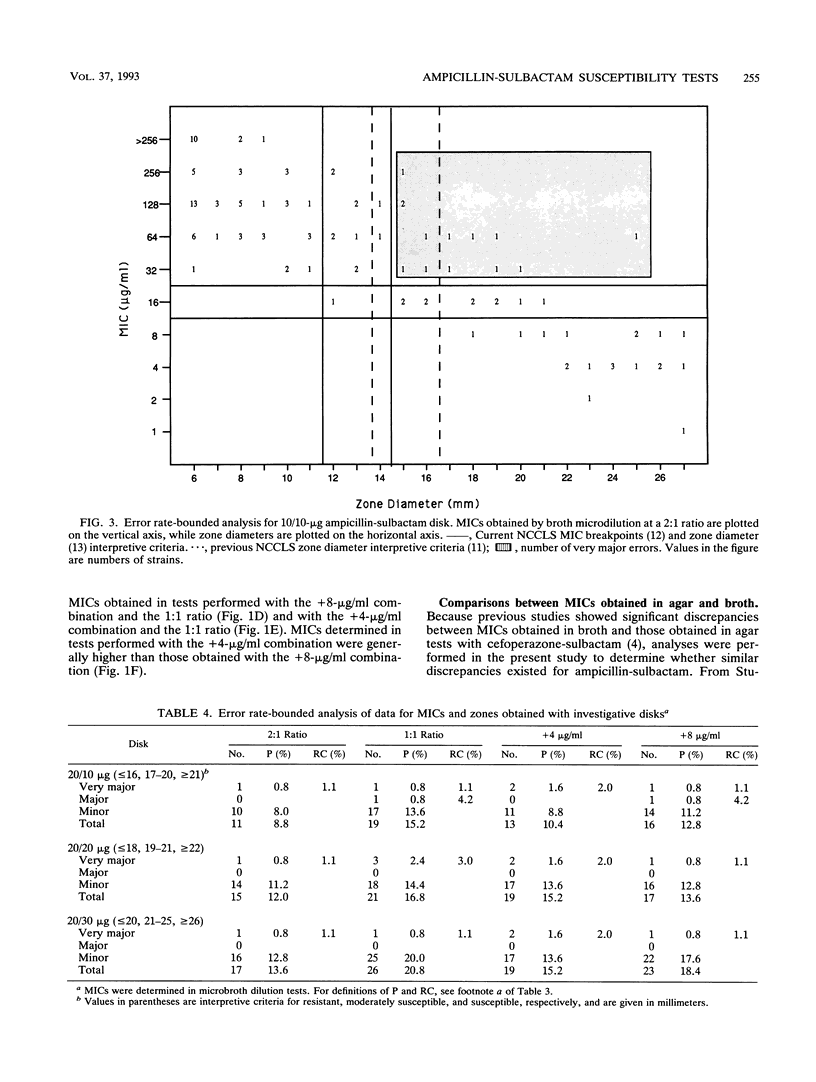
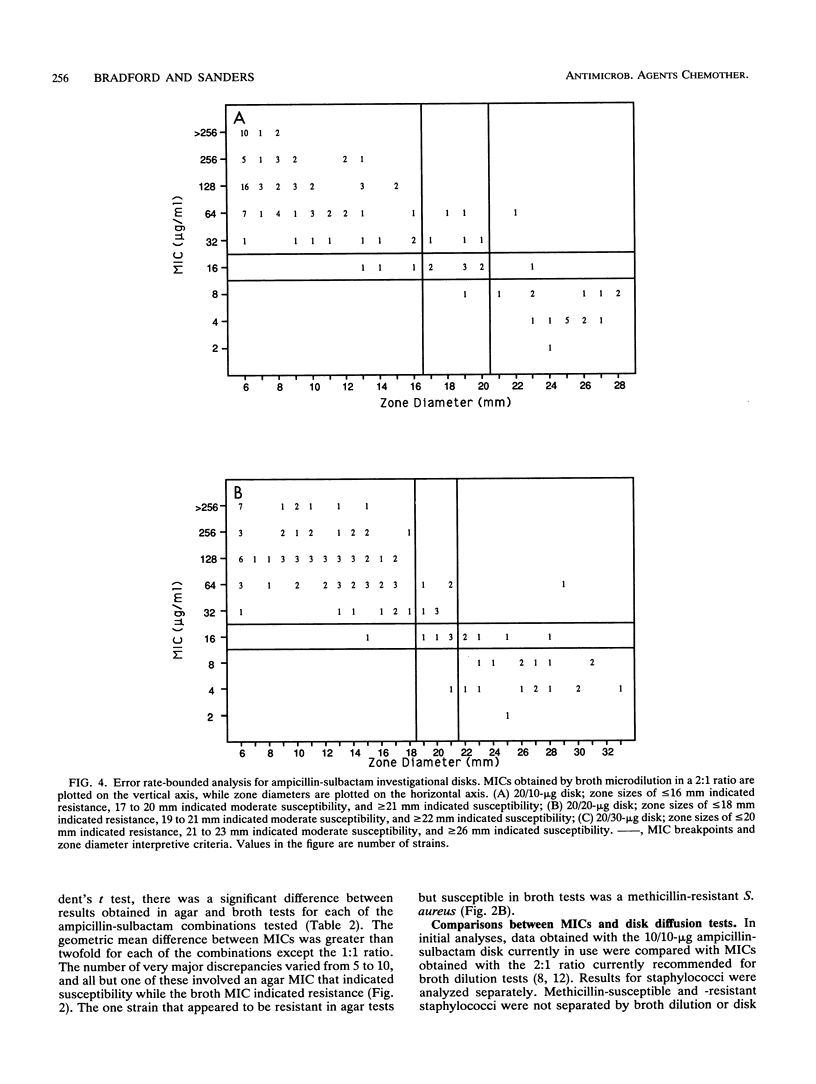
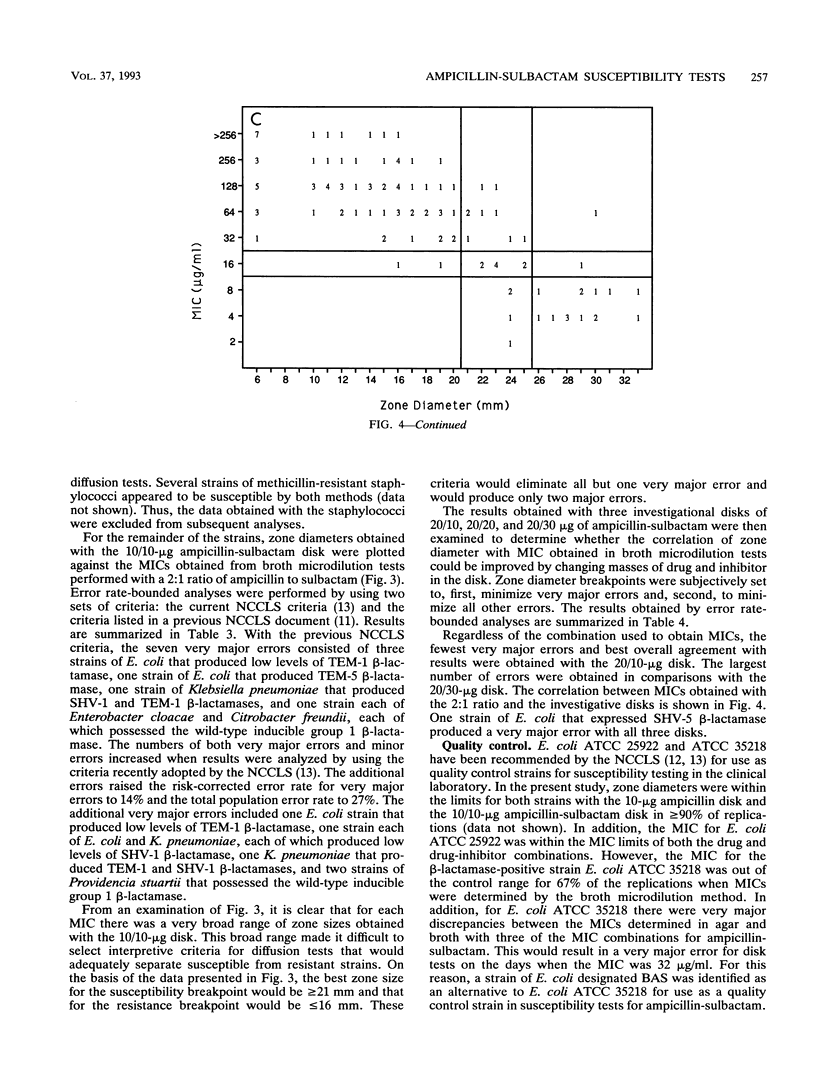
A predictor panel of clinical isolates that produce a variety of types and amounts of beta-lactamases was used to assess the accuracies of a variety of susceptibility tests for ampicillin-sulbactam. Combinations of ampicillin-sulbactam in ratios of 1:1 and 2:1 and with sulbactam held constant at concentrations of 4 and 8 micrograms/ml were examined in dilution tests performed in agar and broth. In addition, disks containing 10/10, 20/10, 20/20, and 20/30 micrograms of ampicillin-sulbactam were examined in diffusion tests. The results indicated that the MICs obtained in broth microdilution tests performed with each of the four combinations differed, on average, less than twofold. Of the disks tested, the 20/10-micrograms ampicillin-sulbactam disk provided the best separation between susceptible and resistant strains when interpretive criteria for resistance was a zone size of < or = 16 mm and that for susceptibility was a zone size of > or = 21 mm. This disk also gave the highest overall agreement with MICs, regardless of the combination used in the broth microdilution test. Discrepancies between agar and broth microdilution MICs were greater than twofold, on average, and this necessitated recommendation of separate criteria for the two methods. Thus, a predictor panel was very useful in identifying the parameters of susceptibility tests that were most accurate in identifying strains that were susceptible and resistant to ampicillin-sulbactam.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N. In vitro activities of ampicillin-sulbactam and cefoperazone-sulbactam against oxacillin-susceptible and oxacillin-resistant staphylococci. Antimicrob Agents Chemother. 1990 Sep;34(9):1830–1832. doi: 10.1128/aac.34.9.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N. Proposed changes in interpretive criteria and potency of ampicillin and ampicillin-sulbactam disks for susceptibility tests. J Clin Microbiol. 1988 Apr;26(4):750–754. doi: 10.1128/jcm.26.4.750-754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C. Interpretive standards and quality control limits for susceptibility tests with ampicillin-sulbactam combination disks. J Clin Microbiol. 1984 Feb;19(2):134–139. doi: 10.1128/jcm.19.2.134-139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford P. A., Sanders C. C. Use of a predictor panel for development of a new disk for diffusion tests with cefoperazone-sulbactam. Antimicrob Agents Chemother. 1992 Feb;36(2):394–400. doi: 10.1128/aac.36.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoli-Richards D. M., Brogden R. N. Sulbactam/ampicillin. A review of its antibacterial activity, pharmacokinetic properties, and therapeutic use. Drugs. 1987 Jun;33(6):577–609. doi: 10.2165/00003495-198733060-00003. [DOI] [PubMed] [Google Scholar]
- Itokazu G. S., Danziger L. H. Ampicillin-sulbactam and ticarcillin-clavulanic acid: a comparison of their in vitro activity and review of their clinical efficacy. Pharmacotherapy. 1991;11(5):382–414. [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Optimal dilution susceptibility testing conditions, recommendations for MIC interpretation, and quality control guidelines for the ampicillin-sulbactam combination. J Clin Microbiol. 1987 Oct;25(10):1920–1925. doi: 10.1128/jcm.25.10.1920-1925.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler C. M., DeHaan R. M. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J Infect Dis. 1974 Dec;130(6):588–594. doi: 10.1093/infdis/130.6.588. [DOI] [PubMed] [Google Scholar]
- Meyers B. R., Wilkinson P., Mendelson M. H., Walsh S., Bournazos C., Hirschman S. Z. Pharmacokinetics of ampicillin-sulbactam in healthy elderly and young volunteers. Antimicrob Agents Chemother. 1991 Oct;35(10):2098–2101. doi: 10.1128/aac.35.10.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Iaconis J. P., Bodey G. P., Samonis G. Resistance to ticarcillin-potassium clavulanate among clinical isolates of the family Enterobacteriaceae: role of PSE-1 beta-lactamase and high levels of TEM-1 and SHV-1 and problems with false susceptibility in disk diffusion tests. Antimicrob Agents Chemother. 1988 Sep;32(9):1365–1369. doi: 10.1128/aac.32.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Valcke Y. J., Rosseel M. T., Pauwels R. A., Bogaert M. G., Van der Straeten M. E. Penetration of ampicillin and sulbactam in the lower airways during respiratory infections. Antimicrob Agents Chemother. 1990 Jun;34(6):958–962. doi: 10.1128/aac.34.6.958. [DOI] [PMC free article] [PubMed] [Google Scholar]



