Abstract
The hypothesis that dynamic actin filaments participate in specific aspects of synaptic plasticity was investigated at the Schaffer-collateral-CA1 pyramidal cell synapse of mouse hippocampus. Low concentrations (0.01–1 μM) of compounds that inhibit actin filament assembly were bath applied to hippocampal slices during extracellular recording of field excitatory postsynaptic potentials. Cytochalasin D, cytochalasin B, and latrunculin A all impaired the maintenance of LTP induced by brief high-frequency stimulation. This effect on LTP maintenance was specific, because none of the compounds affected basal synaptic transmission, paired-pulse facilitation, LTP induction, or post-tetanic potentiation. The effect of cytochalasin B was reversible. The results are consistent with a model in which dynamic actin filaments play an essential role in the molecular mechanisms underlying the early maintenance phase of LTP, such as growth of new synaptic connections or conversion of silent synapses.
Keywords: paired-pulse facilitation, cytochalasin , latrunculin, synapse
Actin filaments participate critically in many aspects of neuronal growth and development. Cellular actin exists in both monomeric (G-actin) and polymerized (F-actin) form. Equilibrium between these states is ATP dependent and regulated by a variety of actin-associated proteins. Actin filaments can be either stable, with their lengths precisely specified, or dynamic, undergoing rapid polymer elongation and shrinkage (1, 2). Stable actin filaments underlie specialized cellular structures, like muscle sarcomeres, brush border microvilli, and hair-cell stereocilia. Dynamic actin filaments mediate various types of cellular motility, including migration, membrane ruffling, and filopodial extension. Events mediated by dynamic actin filaments are arrested rapidly in the presence of actin assembly inhibitors (AAIs) like the cytochalasins and latrunculins (3, 4). These classes of compounds work by distinct mechanisms to induce net depolymerization of dynamic actin filaments, but they have comparatively little effect on more stable filaments that turn over slowly.
In neurons, the highest concentrations of actin filaments are found in growth cones of the immature nervous system and in dendritic spines of the mature nervous system (5, 6). Growth cones are the motile tips of developing neuronal processes that transduce extracellular guidance cues for steering the growing neurite. Cytochalasins inhibit growth cone motility rapidly and reversibly, causing the collapse of filopodia and lamellepodia at the leading edge (7). A role for dynamic actin filaments in growth cone motility is well established. In contrast, comparatively little is known about the function of actin in dendritic spines. Direct evidence now supports the view that actin filaments form the basis for structural integrity of dendritic spines. Jasplakinolide, a compound that specifically stabilizes F-actin, prevents spine collapse induced by exposure of cultured neurons to N-methyl-d-aspartate (NMDA) (8). Under normal physiological circumstances, much of the F-actin in spines is extremely stable, because spines persist for many hours in the presence of AAIs (9).
Recently, however, studies using high-resolution fluorescence microscopy have demonstrated that dendritic spines, like growth cones, exhibit rapid motility (10, 11). Although the overall size of a given spine remains fairly constant, the edges of the spine undergo dynamic rearrangements on the order of seconds to minutes, similar to the characteristic fluctuations seen in growth cone membranes. This rapid spine motility is arrested in the presence of AAIs, suggesting that spines contain highly dynamic actin filaments in addition to the stable filaments that mediate their long-term survival (12). Thus, dynamic actin filaments might play important roles in the normal function of dendritic spine synapses. The present study investigated the effect of disrupting dynamic actin filaments on synaptic function in region CA1 of the mature hippocampus, an area rich in glutamatergic synapses onto dendritic spines. The results indicate that the maintenance of LTP is blocked selectively when dynamic actin filaments are disrupted, but that normal synaptic transmission is unaffected.
Methods
Slice Preparation.
Methods were slightly modified from those described previously (13). Hippocampal slices were prepared from normal C57BL/6 male mice (32 ± 0.8 days old) from The Scripps Research Institute breeding colony. Animal care was in accordance with institutional and National Institutes of Health guidelines. Animals were anesthetized with 3% halothane, decapitated, and their hippocampal formations rapidly removed. Transverse slices 400 μm thick were cut on a McIlwain (TPI, O'Fallon, MO) tissue chopper and placed in ice-cold (1–3°C) artificial cerebrospinal fluid (ACSF), saturated with 95% O2/5% CO2, of the following composition (in mM): NaCl, 130; KCl, 3.5; NaH2PO4, 1.25; MgSO4, 1.5; CaCl2, 2; NaHCO3, 24; d-glucose, 10, pH 7.3 ± 0.1. Slices were transferred to a chamber and, after 25 min of incubation, completely submerged and continuously superfused with ACSF. Constant bath temperature was maintained during testing at 32 ± 0.2°C.
Electrophysiology.
Orthodromic stimuli of 0.05 ms duration were delivered to the slices through an insulated bipolar tungsten electrode, after placing the electrode in stratum radiatum to activate the Schaffer-collateral pathway projecting to CA1. Glass microelectrodes with 1–4 MΩ resistance containing 3 M NaCl were positioned in stratum radiatum to record presynaptic fiber volleys followed by population excitatory postsynaptic potentials (pEPSPs). The polarity of the stimulus current was adjusted so that the stimulus artifact would not obscure the fiber volley potential. We isolated the NMDA–pEPSP by adding 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX, 10 μM), bicuculline (20 μM), and CGP55845A (1 μM) to ACSF with 0 mM Mg2+.
A typical experiment began with an input–output (I-O) curve that included stimulation intensities evoking threshold, 30–50% maximal, 50% maximal, and maximal pEPSP amplitudes. For baseline recordings, stimulus intensity was adjusted to evoke a pEPSP of approximately 30–50% of the maximal amplitude. Two successive pEPSP recordings at 0.5 Hz were averaged. Concomitant with baseline recordings (≥20 min), a paired-pulse protocol consisting of 20, 50, 100, and 200 ms intervals was delivered at stimulus intensities that evoked 30–50% of maximal pEPSP amplitudes. To assess drug effects on synaptic efficacy, we generated additional I-O curves at 20 and 40 min after the start of drug superfusion with the same stimulation intensities as those under control conditions. For additional paired-pulse facilitation (PPF) protocols, pEPSP amplitude was adjusted to the original control values.
After recording stable baseline pEPSPs for at least 30 min, LTP was elicited by applying two tetani of 100 Hz, 20 sec apart, at the same stimulus intensity as for the baseline values. Test responses were recorded for up to 180 min afterward. At least 40 min before or immediately after LTP induction, test compounds were applied to the superfusate for 5 to 180 min.
Two successive pEPSP recordings at 0.5 Hz were averaged for each data point. Population EPSPs were recorded with an Axoclamp-2A or -2B headstage interfaced with a PC and were acquired, digitized, stored, and analyzed using pclamp software (Axon Instruments, Foster City, CA).
Data Analysis.
We measured pEPSP and presynaptic volley amplitudes as the y axis difference between the highest peaks and the baseline value determined 10 ms preceding the stimulation and calculated the initial slopes (between the 10 and 60% points on the rising phase) of the pEPSP by using least-squares regression. We determined the pEPSP area between the time point after the presynaptic volley closest to baseline and the following trace/baseline intersection. In the figures, only slope measurements are shown, but parallel analysis of peak amplitudes gave the same results. All values are expressed as mean ± SEM. Statistically significant differences among control, treated, and/or untreated slices at each stimulation intensity and/or time point for I-O, LTP, paired-pulse intervals were probed by using the Mann–Whitney U test. P ≤ 0.05 was considered statistically significant.
Drugs.
Drugs were added through a continuous perfusion system to the bath from stock solutions (in 100% DMSO) at known concentrations. The final concentration of DMSO in the slice chamber never exceeded 0.1%, a concentration found in control experiments to have no detectable effects on the slices. CGP55845A was a gift from Novartis Pharma (Basel). CNQX and bicuculline were obtained from Tocris (Ballwin, MO), Latrunculin A from Molecular Probes, and all other chemicals, including cytochalasins, were from Sigma.
Results
For these studies, we used three well-characterized actin depolymerizing compounds: cytochalasin D, cytochalasin B, and latrunculin A, each of which has a unique chemical structure and distinct effects on actin filament dynamics in cells (3, 4). Latrunculins bind to G-actin in a 1:1 molar complex, thereby limiting the availability of actin monomers for polymerizing at either barbed or pointed ends of actin filaments (4). The cytochalasins have multiple complex effects on actin in vitro; however, at low concentrations, their primary effect in cells is to cap the barbed ends of actin filaments (3). These actin inhibitors selectively disrupt filaments undergoing rapid length excursions, leaving intact the more stable and slowly-turning-over actin filaments.
Actin Assembly Inhibitors Do Not Affect Normal Synaptic Transmission in CA1.
To test whether AAIs affect normal synaptic transmission, cytochalasin D, latrunculin A, or cytochalasin B was superfused onto mouse hippocampal slices, and pEPSPs, or pharmacologically isolated NMDA–pEPSPs, were recorded in the dendritic region of CA1. By performing complete I-O curves at a series of increasing stimulation intensities, we established the intensity that evoked 30–50% of the maximal pEPSP amplitude for control recordings. After recording a stable baseline for at least 20 min, AAIs were continuously superfused into the slice medium. Neither concentration of cytochalasin D (1 μM and 0.1 μM) induced any detectable change in pEPSP slope or amplitude (Figs. 1 and 2) for over 90 min. Because there were no evident differences, data for the two concentrations were pooled for analysis. After 40 and 80 min of cytochalasin D superfusion, additional I-O curves performed at the same stimulation intensities as under control conditions also showed no detectable changes (Fig. 2A). Moreover, parallel analysis of the amplitude of the presynaptic volley, which is proportional to the number of presynaptic neurons recruited by stimulation, revealed no apparent changes during the entire recording time (Fig. 1B).
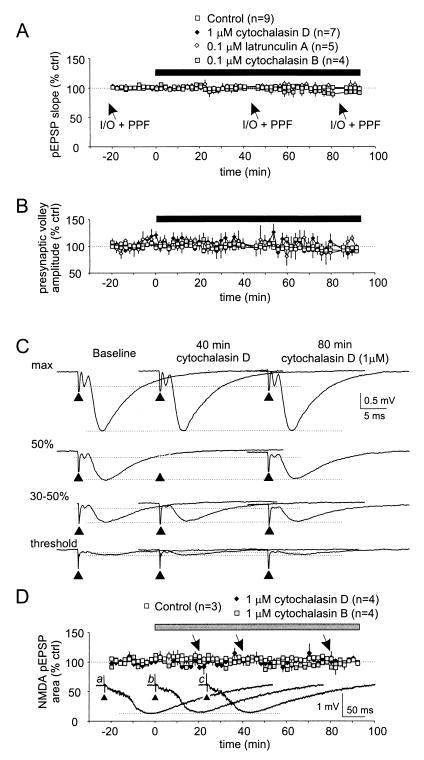
Figure 1.
Actin assembly inhibitors do not affect basal synaptic transmission. (A–C) Bath-applied cytochalasin D, cytochalasin B, and latrunculin A affect neither the pEPSP slope nor the presynaptic fiber volley amplitude. After establishing baseline recordings, cytochalasin D (1 μM, n = 4; 0.1 μM, n = 3; data pooled), cytochalasin B, or latrunculin A was continuously superfused (solid bar). Stimulation intensities were set to evoke 30–50% of maximal pEPSP amplitude. Data gaps at 40 and 80 min are because of additional I-O protocols. (A) Although the average pEPSP slope declined slightly at 80 min after cytochalasin B, none of the tested AAIs induced significant changes as measured after 20, 40, 60, and 80 min. (B) Parallel analysis of the corresponding fiber volley amplitudes showed no detectable changes. (C) Traces (averages of two) from a series of I-O curves taken under baseline, 40, and 80 min after the start of cytochalasin D superfusion. (D) NMDA–pEPSPs were isolated as described in Methods and cytochalasin D or cytochalasin B at 1 μM continuously superfused (gray bar). Neither the NMDA–pEPSP nor the corresponding presynaptic volley was significantly affected. Even after 80 min of cytochalasin treatment, the area of the NMDA–pEPSP did not vary significantly from control. Inset shows representative raw traces taken at 10 min before (a), 40 (b), and 80 (c) min (arrows) after the start of superfusing cyotchalasins. Arrowheads mark the stimulation artifacts (truncated).
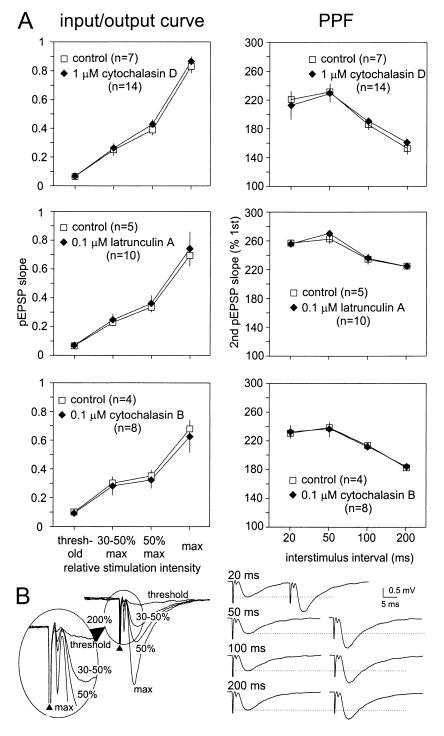
Figure 2.
Bath-applied AAIs neither shift I-O curves nor alter PPF. (A) I-O curves were generated at stimulation intensities that evoked threshold, 30–50%, 50%, and maximal pEPSP amplitudes. PPF was tested at 20, 50, 100, and 200 ms intervals with intensities evoking 30–50% of maximal pEPSP amplitude. I-O curves and PPF protocols were performed during control recordings and compared with protocols taken 40 and 80 min after the start of AAI superfusion. No significant changes in I-Os or PPFs were detectable. (B Left) Traces of a typical I-O curve. In the enlarged section, note the increasing amplitude of the presynaptic volley after the stimulation artifact (arrowhead). (Right) Single traces from a PPF protocol. Although the presynaptic volley did not change in size, the amplitude of the second pEPSP was greatly potentiated at all intervals tested.
Cytochalasin B and latrunculin A were also tested, both at 0.1 μM. As with cytochalasin D, neither of these compounds induced significant alterations of the pEPSP slope (Fig. 1A) or amplitude for over 90 min. Although the average pEPSP slope declined slightly at 80 min (89.6 ± 6.4%) after cytochalasin B superfusion, these changes were not significantly different at any stimulation intensity tested within the corresponding I-O curve (Figs. 1A and 2A; P > 0.29, Mann–Whitney U test). The late phase of the pEPSP, usually referred as to the NMDA receptor-mediated component (14, 15), did not seem to be altered by AAI superfusion (Figs. 1C, 2B, and 3B). Nevertheless, we isolated pharmacologically the NMDA–pEPSP and superfused cytochalasin D and cytochalasin B at the highest concentration tested (1 μM) to confirm whether the NMDA response remained unaffected. Fig. 1D illustrates that neither the area nor the amplitude of NMDA receptor-mediated potentials (see Inset) was significantly altered by the continuous superfusion of cytochalasins for over 90 min. In addition, the presynaptic volley also remained unchanged (data not shown).
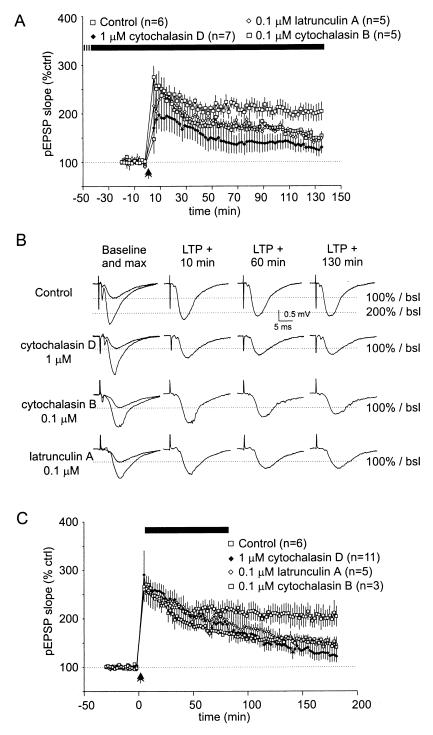
Figure 3.
AAIs selectively reduce LTP maintenance. (A and B) After superfusion of AAIs for at least 40 min, LTP was induced and responses recorded for 135 min. (A) Although cytochalasin D (1 μM, n = 4; 0.1 μM, n = 3; data pooled) seemed to reduce PTP compared with untreated (control) slices, these changes were not significant for the first 20 min after LTP induction. After a short potentiation to about 200% of baseline after 10 min, the pEPSP slope declined steadily to reach a plateau of about 140% after 60 min of LTP induction. Cytochalasin B also reduced PTP, although not significantly; responses were potentiated after 10 min to values similar to those in untreated slices but declined steadily afterward and reached 150% of baseline after 130 min of LTP induction. Like cytochalasin B, latrunculin A reduced PTP slightly; responses were potentiated for 10 min and declined to about 150% of baseline after 130 min of LTP induction. (B) Representative traces taken during baseline recording (maximal and 30–50% of maximal amplitude), 10, 60, and 130 min after LTP induction. (C) Maintenance of LTP is blocked selectively by AAIs. After recording control responses for at least 30 min, LTP was induced. Immediately afterward, AAIs were superfused for 60–80 min while recording continued. Cytochalasin D (1 μM, n = 7; 0.1 μM, n = 4; data pooled), cytochalasin B, and latrunculin A did not alter PTP but reduced LTP magnitude significantly, as measured at 120 and 180 min after LTP induction (pEPSP slope measurements). The dotted lines mark baseline (100%) amplitude.
To determine whether AAIs might affect the presynaptic locus at the tested concentrations, we studied paired-pulse function at 20, 50, 100, and 200 ms interstimulus intervals (Fig. 2). In area CA1 of the hippocampus, PPF is a presynaptic facilitation revealed by the second of a pair of stimulation pulses delivered at short intervals. It is widely believed that PPF is an efficient test to detect changes within presynaptic terminals (16, 17). The magnitude of PPF was estimated before and after AAI superfusion by plotting the slope of the pEPSP of the second of a pair of evoked potentials as percent of the slope of the first pEPSP vs. the interstimulus intervals (in milliseconds) of the two stimuli. Stimulation intensities were set to evoke 30–50% of maximal pEPSP amplitude. PPF protocols were performed subsequent to I-O curves at 40 and 80 min after the start of AAI superfusion. Neither cytochalasin D, cytochalasin B, nor latrunculin A led to any detectable changes in PPF, suggesting that AAIs do not have presynaptic effects under these experimental conditions.
Pretreatment with Actin Assembly Inhibitors Impairs LTP.
After superfusion of cytochalasin D, cytochalasin B, or latrunculin A for at least 40 min, we induced LTP using two trains of 100 pulses delivered at 100 Hz, recorded LTP for 135 min (Fig. 3 A and B), and plotted averaged pEPSP slopes as percentage of baseline responses (duration 20 min; stimulation intensities at 30–50% of maximal amplitude). Control (untreated) slices exhibited stable LTP of 214 ± 17% of baseline at 100 min and 211 ± 20% at 130 min after tetanus. Although post-tetanic potentiation (PTP) during the first 20 min after tetanus appeared to be reduced in the presence of AAIs, none of the values were significantly different from untreated control slices. However, all LTP magnitudes at 100 and 130 min were significantly reduced relative to control (P < 0.012). Specifically, slices incubated with cytochalasin D (1 μM, n = 4; 0.1 μM, n = 3; data pooled) showed reduced PTP but were subsequently potentiated to about 200% of baseline after 10 min, then declined steadily to reach a plateau of about 140% of baseline after 60 min of LTP induction. Slices incubated with cytochalasin B (0.1 μM, n = 5) also showed reduced PTP, although this was not significant. Responses were potentiated after 10 min to values similar to those in control slices but then declined steadily afterward and reached about 148% of baseline 130 min after tetanus. As with cytochalasin B, slices incubated with latrunculin A (0.1 μM, n = 5) showed nonsignificantly reduced PTP, were potentiated for 10 min, and declined to about 139% of baseline 130 min after tetanus.
LTP Maintenance Is Selectively Blocked by Actin Assembly Inhibitors.
To determine whether AAIs selectively affect LTP maintenance, as opposed to induction, we superfused drugs for 60 to 80 min starting shortly after LTP induction (Fig. 3C). Baseline values were recorded for at least 30 min at stimulation intensities that evoked 30–50% of maximal amplitude. In contrast to slices pretreated with AAIs, where PTP was noticeably affected and could have influenced LTP expression, PTP remained unchanged when AAIs were superfused shortly after LTP induction. In particular, after cytochalasin D superfusion (1 μM, n = 7; 0.1 μM, n = 4; data pooled), the mean pEPSP slope and amplitude continually decreased. At 100 min after LTP induction, LTP magnitude was 165 ± 17% of baseline (significantly different from untreated slices, P < 0.015) and further declined to 121 ± 12% of baseline after 180 min. Like the cytochalasin D effect, after 100 min of LTP induction, cytochalasin B (0.1 μM, n = 3) and latrunculin A (0.1 μM, n = 5) reduced the average pEPSP slope to 182 ± 12% and 165 ± 3% of baseline, respectively. After 180 min, both drugs reduced LTP magnitude significantly (P < 0.02) to 146 ± 15% and 140 ± 12% of baseline, respectively. To control for possible presynaptic effects of AAIs, we analyzed in parallel to the pEPSPs the presynaptic volley. The presynaptic volley amplitude did not change during the entire recording time, suggesting that there were no modifications of the fiber input (data not shown).
Effects of Low Concentrations of Cytochalasin B Are Reversible.
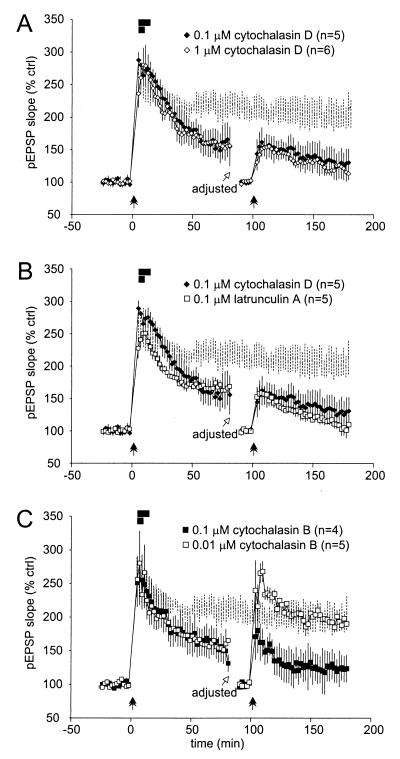
To test whether the reduction in LTP magnitude induced by AAIs was reversible, we superfused mouse hippocampal slices with low concentrations of AAIs for brief periods following LTP induction, then washed out the drugs after 5–10 min. After 80 min, an additional I-O curve was generated and stimulation intensity adjusted to 30–50% of the new maximal amplitude. We subsequently collected recordings for another 10 min at the new baseline settings before applying a second tetanus (Fig. 4). Cytochalasin D (1 μM, n = 6; 0.1 μM, n = 5) was superfused for 5 or 10 min. Both concentrations and application times significantly reduced LTP magnitude after 80 min, similar to when they were superfused continuously for 60–80 min (compare Fig. 4A to Fig. 3C). After the second LTP induction protocol, the pEPSP was potentiated for about 10 min but steadily declined to reach 113 ± 10% and 130 ± 21%, respectively, of control. Interestingly, these values are equivalent to the one obtained after only one LTP induction (Fig. 3C). It has been reported that cytochalasin D exhibits poor washout in intact preparations, but that effects of cytochalasin B are more readily reversible. Similarly, we observed that, in contrast to the effects of cytochalasin D and latrunculin A, the effects of cytochalasin B on LTP appeared to be at least partially reversible (Fig. 4C). Cytochalasin B superfused at 0.1 μM for 5 or 10 min (n = 3) reduced the maintenance of LTP induced by the first set of tetani. After washout, the second set of tetani induced a modest potentiation. Although this potentiation was less than in control slices, it represented a stable enhancement, because the pEPSP did not decline steadily toward baseline as with the other drugs (significantly different from baseline; P < 0.02). Indeed, a lower concentration of cytochalasin B (0.01 μM, n = 5) superfused for 5 min also reduced the magnitude of LTP in a manner indistinguishable from the effect of higher concentrations. However, we observed robust LTP after the second set of tetani. This enhancement declined only slightly and reached a plateau of about 200% of control after 30 min. Because this LTP magnitude resembled normal levels and was nondecremental, this finding suggests that cytochalasin B effects are reversible.
Figure 4.
Partial reversal of the impairment of LTP maintenance by AAIs. After recording at least 20 min, LTP was induced. Immediately afterward, AAIs were superfused for 5 and 10 min, respectively (solid bars). After an 80 min washout, the stimulation intensity was adjusted to the new 30–50% of maximal amplitude and responses recorded for an additional 10 min to reestablish control conditions. Tetani were delivered a second time and pEPSPs recorded for an additional 80 min. The ghostline indicates the level of LTP (mean ± SEM) from control slices in Fig. 3C. (A) Effects of cytochalasin D. Eighty minutes after LTP induction, both concentrations superfused for either 5 min or 10 min reduced LTP magnitude to about 150% of control. A second set of tetani delivered after washout could not induce stable LTP. (B) Effects of cytochalasin D compared with latrunculin A. As for cytochalasin D, latrunculin A superfused for 5 or 10 min irreversibly blocked LTP maintenance. (C) Effects of cytochalasin B. Although cytochalasin B superfused at low concentrations and for only 5 min reduced LTP magnitude as significantly as with higher concentrations and longer superfusion times, this effect was reversible, and the second set of tetani induced normal PTP and LTP magnitudes. LTP appeared stable.
Discussion
The purpose of this study was to determine whether manipulations of actin filament stability alter the basal or stimulus-dependent properties of synapses in area CA1 of mammalian hippocampus. The use of low but effective concentrations of actin inhibitors was designed to avoid nonspecific effects. The concentrations of latrunculin A and cytochalasins used here (0.01–1 μM) are similar to those that have been shown previously to alter actin filament organization in cultured cells, prevent lamellipodial ruffling in fibroblasts, inhibit motility and retrograde flow in neuronal growth cones, and arrest dendritic spine motility in hippocampal neurons (3, 4, 7, 10). Our results indicate that dynamic actin filaments are not involved critically in either pre- or postsynaptic aspects of basal synaptic transmission but are essential for the maintenance of stable LTP. Neither the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) nor NMDA receptor-mediated components of pEPSPs are affected by AAIs.
In addition, drug wash-out experiments show that the inhibition of LTP by AAIs is reversible. We therefore conclude that a resumption of actin filament turnover reestablishes conditions permissive for the maintenance of LTP. The fact that a transient (5–10 min) exposure of slices to AAIs immediately following the tetanus was sufficient to impair the maintenance of LTP, similar to that seen with prolonged exposures, suggests that dynamic actin filaments are required shortly after the initial induction phase of LTP. It will be of interest in future studies to determine the precise time window when dynamic actin filaments participate in mechanisms underlying LTP.
Dynamic Actin Filaments Are Not Essential for Basal Synaptic Transmission or Short-Term Plasticity.
We have shown that continuous superfusion of AAIs for over 90 min affects neither pEPSPs nor pharmacologically isolated NMDA–pEPSPs over a series of stimulation intensities. In addition, the presynaptic fiber volley and PPF remain unchanged. We therefore conclude that dynamic actin filaments do not influence postsynaptic cellular mechanisms required for synaptic transmission during basal synaptic activity. Furthermore, because disturbances in transmitter release or other presynaptic changes are expected to alter PPF, our results also suggest that dynamic actin filaments in the presynaptic compartment do not take part in basal synaptic transmission or in short-term plasticity. Populations of actin filaments in axons and nerve terminals might thus be stable and play exclusively structural roles.
Our findings stand somewhat in contrast to a study reporting that, even after as little as 10–15 min, latrunculin B and cytochalasin D significantly reduced basal pEPSPs and disrupted PPF, in addition to reducing LTP induction and magnitude (18). Although differences in species or developmental stage (these authors used rats 2–3 weeks of age) might account for the dissimilar findings, it seems likely that the more widespread effects observed by Kim and Lisman could be caused by the use of much higher drug concentrations (2–10 μM) that may have nonspecific effects or disrupt more stable actin filament populations. Indeed, Kim and Lisman observed gradual declines in baseline parameters even in the presence of vehicle alone or in the absence of any drug. This may indicate that their slice preparations were vulnerable to nonspecific actions of the drugs. We chose to use only very low concentrations of AAIs, because cytochalasins and latrunculins are extremely potent and can be toxic to hippocampal neurons at higher concentrations (9).
Potential Functions of Dynamic Actin Filaments in LTP.
In neurons, actin filaments are present in many subcellular compartments, including the submembranous cytoskeleton; however, they are most highly concentrated in growth cones and in dendritic spines. Both growth cones and spines contain dynamic actin filaments. In addition, developing hippocampal neurites express dynamic filopodia, which can play a role in synaptogenesis (19–21). Actin filaments mediate the dynamic behavior of filopodia (2). However, in the mature brain, filopodia and growth cones are relatively rare (21). For the present studies, we used mice 32 days of age, when dendritic spines are the primary locus enriched in actin filaments (5, 8, 22, 23). Spines appear to contain both stable and dynamic populations of actin filaments (12). The functional significance of dynamic actin filaments in spines is presently unknown. Actin participates in a wide variety of biological activities, including cell adhesion, membrane trafficking, and signal transduction. All of these functions conceivably contribute to mechanisms underlying LTP. In ultrastructural studies, actin filaments are observed in direct contact with the postsynaptic density, with the plasma membrane, and with vesicular structures and the spine apparatus (reviewed in refs. 5 and 12). Recently, the β isoform of CaMKII was shown to bind actin filaments and to target CaMKII to synapses (24). In addition, both NMDA and AMPA receptors interact directly or indirectly with actin (9, 25, 26). Presently it is unclear which of these linkages involves dynamic vs. stable F-actin.
The cellular and synaptic mechanisms in CA1 that contribute to LTP induction, expression, and maintenance are still a matter of debate (27, 28). It is widely accepted that initial changes in synaptic efficacy are primarily postsynaptic, and a growing body of evidence suggests that structural remodeling of synaptic connections is essential in the maintenance phase of LTP and other enduring forms of synaptic plasticity. Recent imaging studies provide direct evidence for the activity-dependent growth of new postsynaptic elements in hippocampus. New filopodia or spines are induced specifically in dendrites consequent to stimulation that elicits LTP (29, 30). It seems likely that dynamic F-actin participates in the rapid emergence of such protrusions, which in turn form new synapses. Insertion of new AMPA receptors at existing synapses has also been identified as an important step during early LTP maintenance (31). Depolymerization of dynamic actin might directly block this process (9).
Under the conditions used here, the application of actin perturbing agents is not expected to disrupt stable actin filaments or induce collapse of dendritic spines. Instead, we hypothesize that low concentrations of AAIs selectively disrupt the translocation of AMPA receptors, the production of new spiny protrusions, the rapid motility of existing spines, or a combination of these phenomena. Further experiments are required to identify the precise nature and location of actin filaments essential for LTP maintenance. Indeed, whereas dynamic filaments in dendrites and spines are logical candidates, our studies do not rule out a role for dynamic actin in other regions of the neuron or in glial cells.
Intrinsic Mechanisms Regulating Dynamic Actin Filaments and LTP.
Spines likely possess mechanisms for regulating actin filament dynamics; such mechanisms could modulate the stability of LTP and other forms of plasticity. Several actin-binding proteins and receptor–actin linkages are attractive candidates for mediating morphological plasticity in neurons (12, 32). One regulatory factor is the calcium/calmodulin-dependent protein phosphatase calcineurin. Calcineurin is enriched in dendritic spines and regulates actin filament stability in response to strong excitatory stimuli (8). Interestingly, transgenic mice overexpressing a constitutively active form of calcineurin were impaired in the maintenance of LTP and in the establishment of long-term memory (33, 34). It is reasonable to speculate that a mechanism underlying this effect of calcineurin overexpression might involve disruption of dynamic actin filaments in spines. The identification of additional actin regulatory molecules will advance our understanding of the relationship between physiological and morphological plasticity in the nervous system.
Acknowledgments
We thank D. Allison and K. Lindsley for technical support. This work was supported by National Institutes of Health (MH47680, G.R.S.; NS37311, S.H.) and Novartis (T.K., G.R.S.). This is manuscript no. 13202-CB from The Scripps Research Institute.
Abbreviations
- AAIs
actin assembly inhibitors
- I-O
input–output
- pEPSP
population excitatory postsynaptic potential
- PTP
post-tetanic potentiation
- PPF
paired-pulse facilitation
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100139797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100139797
References
- 1.Fowler V M. Curr Opin Cell Biol. 1996;8:86–96. doi: 10.1016/s0955-0674(96)80052-4. [DOI] [PubMed] [Google Scholar]
- 2.Mitchison T J, Cramer L P. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 3.Cooper J A. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector I, Shochet N R, Blasberger D, Kashman Y. Cell Motil Cytoskeleton. 1989;13:127–144. doi: 10.1002/cm.970130302. [DOI] [PubMed] [Google Scholar]
- 5.Fifkova E. Brain Res. 1985;356:187–215. [PubMed] [Google Scholar]
- 6.Matus A, Ackermann M, Pehling G, Byers H R, Fujiwara K. Proc Natl Acad Sci USA. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forscher P, Smith S J. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halpain S, Hipolito A, Saffer L. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allison D W, Gelfand V I, Spector I, Craig A M. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer M, Kaech S, Knutti D, Matus A. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 11.Dunaevsky A, Tashiro A, Majewska A, Mason C, Yuste R. Proc Natl Acad Sci USA. 1999;96:13438–13443. doi: 10.1073/pnas.96.23.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halpain S. Trends Neurosci. 2000;23:141–146. doi: 10.1016/s0166-2236(00)01576-9. [DOI] [PubMed] [Google Scholar]
- 13.Krucker T, Toggas S M, Mucke L, Siggins G R. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- 14.Wigstrom H, Gustafsson B. J Physiol. 1986;81:228–236. [PubMed] [Google Scholar]
- 15.Collingridge G L, Herron C E, Lester R A. J Physiol (London) 1988;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Creager R, Dunwiddie T, Lynch G. J Physiol (London) 1980;299:409–424. doi: 10.1113/jphysiol.1980.sp013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess G, Kuhnt U, Voronin L L. Neurosci Lett. 1987;77:187–192. doi: 10.1016/0304-3940(87)90584-2. [DOI] [PubMed] [Google Scholar]
- 18.Kim C H, Lisman J E. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dailey M E, Smith S J. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziv N E, Smith S J. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 21.Fiala J C, Feinberg M, Popov V, Harris K M. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drenckhahn D, Frotscher M, Kaiser H W. Brain Res. 1984;300:381–384. doi: 10.1016/0006-8993(84)90851-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaech S, Fischer M, Doll T, Matus A. J Neurosci. 1997;17:9565–9572. doi: 10.1523/JNEUROSCI.17-24-09565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen K, Teruel M N, Subramanian K, Meyer T. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 25.Rosenmund C, Westbrook G L. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- 26.Wyszynski M, Lin J, Rao A, Nigh E, Beggs A H, Craig A M, Sheng M. Nature (London) 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- 27.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 28.Malinow R, Mainen Z F. Science. 1996;271:1604–1605. doi: 10.1126/science.271.5255.1604. [DOI] [PubMed] [Google Scholar]
- 29.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 30.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 31.Shi S H, Hayashi Y, Petralia R S, Zaman S H, Wenthold R J, Svoboda K, Malinow R. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 32.Matus A. Curr Opin Neurobiol. 1999;9:561–565. doi: 10.1016/S0959-4388(99)00018-5. [DOI] [PubMed] [Google Scholar]
- 33.Mansuy I M, Winder D G, Moallem T M, Osman M, Mayford M, Hawkins R D, Kandel E R. Neuron. 1998;21:257–265. doi: 10.1016/s0896-6273(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 34.Winder D G, Mansuy I M, Osman M, Moallem T M, Kandel E R. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]