Abstract
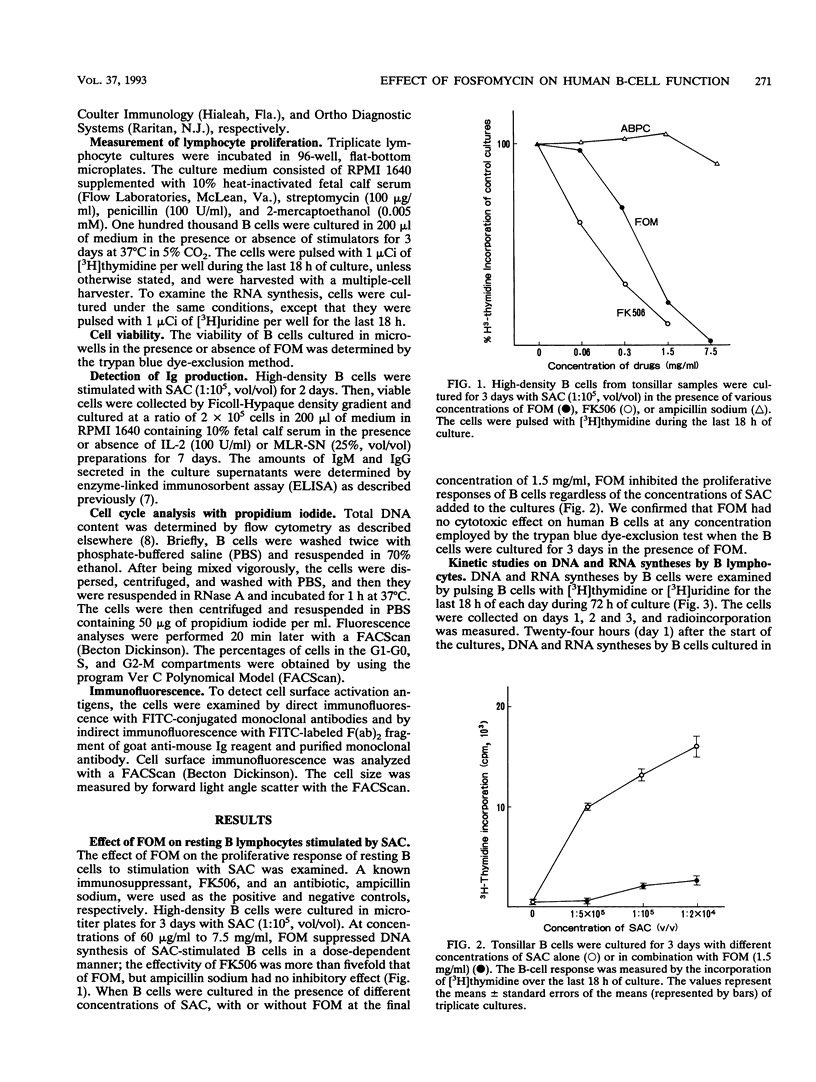
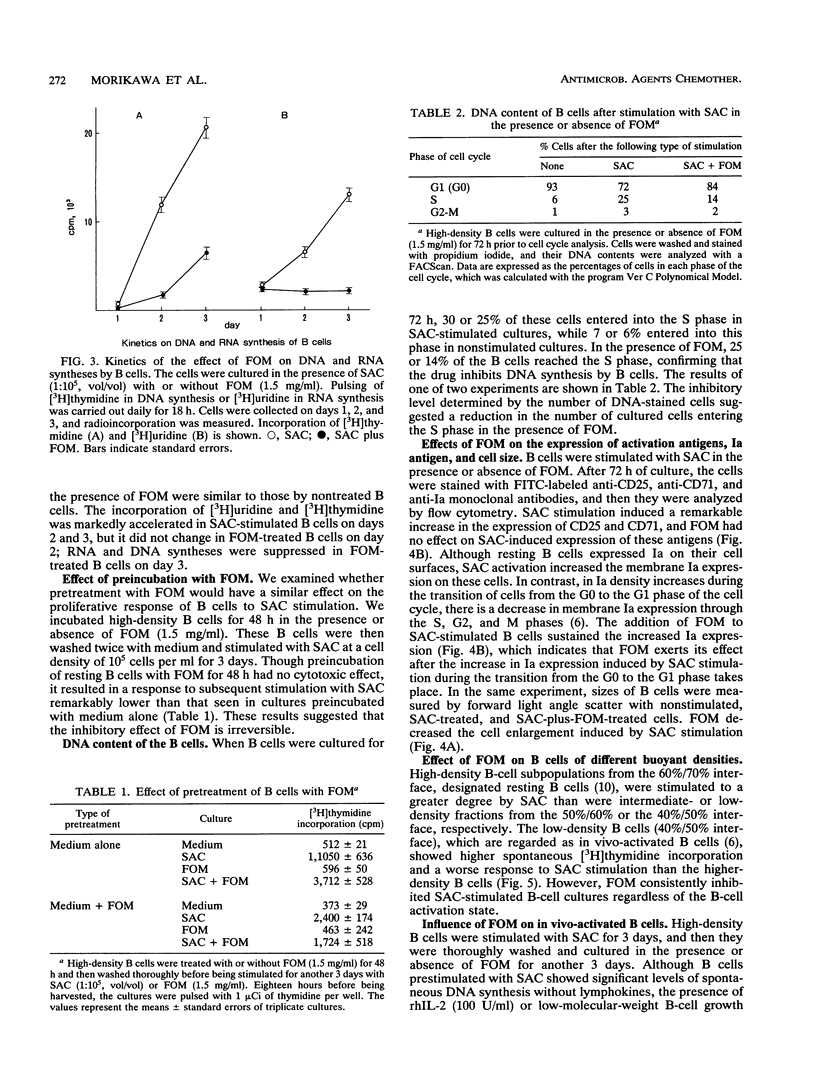
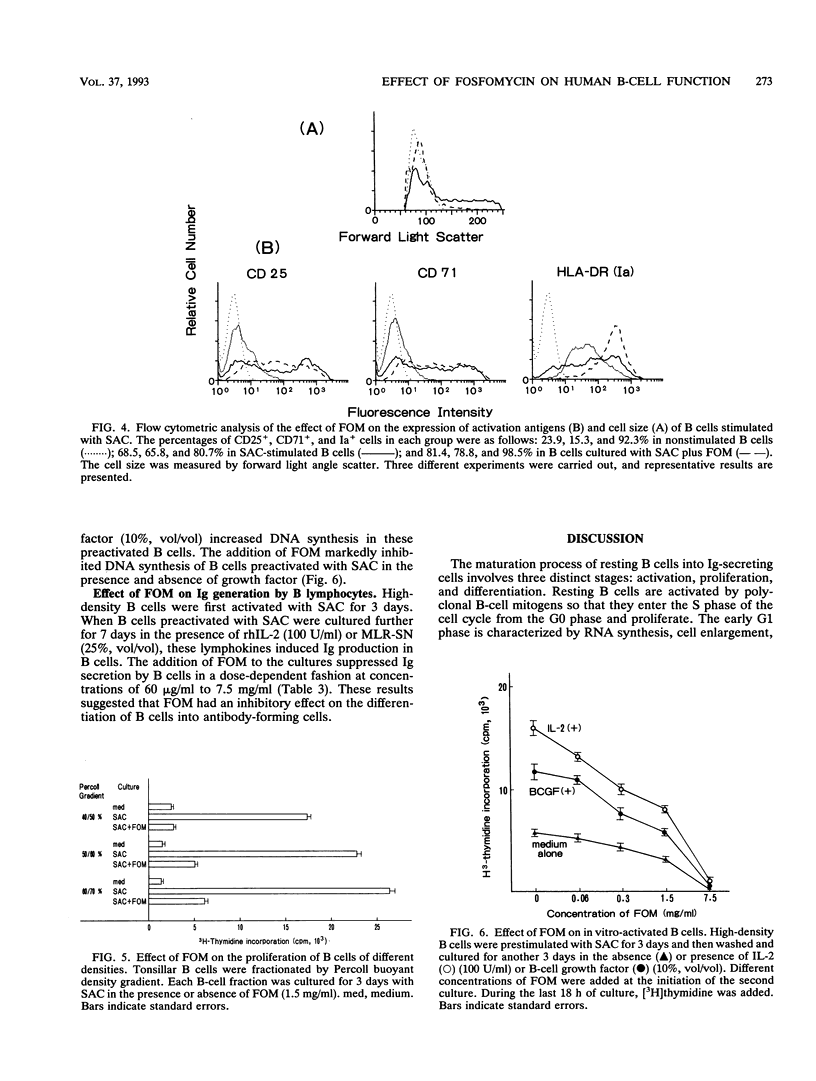
Fosfomycin (FOM) is an unique antibiotic which is chemically unrelated to any other known antimicrobial agent. Recent investigations have demonstrated that FOM inhibits histamine release from basophils. In this study, we examined the effect of FOM on human B-cell functions. FOM inhibited the proliferative response of resting B cells induced by Staphylococcus aureus Cowan 1 in a dose-dependent manner. FOM interfered with the transition from the G0 to the G1 phase of the cell cycle, leading to cell arrest. The proliferative response of in vivo-activated B cells and lymphokine-induced B-cell proliferation were also affected by FOM. In addition, FOM suppressed immunoglobulin secretion by antibody-producing B cells. Interestingly, FOM did not affect the expression of activation antigens such as the CD25 (interleukin-2 receptor) and CD71 (transferrin receptor) antigens. Moreover, FOM sustained the increased Ia expression on B-cell membranes induced by S. aureus Cowan 1 stimulation, which suggests that FOM may not block the role of B cells in antigen presentation in T-cell-B-cell interaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chesnut R. W., Grey H. M. Antigen presentation by B cells and its significance in T-B interactions. Adv Immunol. 1986;39:51–94. doi: 10.1016/s0065-2776(08)60348-x. [DOI] [PubMed] [Google Scholar]
- Eisen H., Kahn S. Mimicry in Trypanosoma cruzi: fantasy and reality. Curr Opin Immunol. 1991 Aug;3(4):507–510. doi: 10.1016/0952-7915(91)90012-p. [DOI] [PubMed] [Google Scholar]
- Ida S., Shindoh Y., Takishima T. Effect of antibiotics on immediate hypersensitivity reactions in vitro: suppression of IgE-mediated histamine release from peripheral blood basophils by fosfomycin. Microbiol Immunol. 1987;31(10):975–984. doi: 10.1111/j.1348-0421.1987.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Differential expression of cell activation markers after stimulation of resting human B lymphocytes. J Immunol. 1984 Jun;132(6):2857–2861. [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. Level of mIa expression on mitogen-stimulated murine B lymphocytes is dependent on position in cell cycle. J Immunol. 1983 Feb;130(2):626–631. [PubMed] [Google Scholar]
- Morikawa K., Oseko F., Morikawa S. The role of CD45RA on human B-cell function: anti-CD45RA antibody (anti-2H4) inhibits the activation of resting B cells and antibody production of activated B cells independently in humans. Scand J Immunol. 1991 Sep;34(3):273–283. doi: 10.1111/j.1365-3083.1991.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Kehrl J. H., Butler J. L., Fauci A. S. Sequential requirements for cell cycle progression of resting human B cells after activation by anti-Ig. J Immunol. 1984 Jan;132(1):176–180. [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]


