Glioblastoma multiforme (GBM) are the most common and aggressive adult primary brain tumors. Genetic alterations and their consequences in these malignant astrocytomas have been studied extensively and include (i) overexpression of growth factors and their corresponding receptors (fibroblast growth factor, epidermal growth factor, and platelet-derived growth factors), (ii) abnormalities of transduction signaling pathways (activation of PI3 kinase/AKT, RAS/MAP kinase, and protein kinase C), or (iii) disruption of cell cycle arrest (loss of p16INK4A and p14ARF, mutations in p53 protein, and PTEN) (1). Whether these modifications are causative or participate in tumor progression is a pivotal question that can best be answered by modeling glioma formation in mice. In this issue of PNAS, Kamnasaran et al. (2) combine genetically engineered murine (GEM) models of gliomas with a retroviral gene-trapping approach to identify new molecular alterations in human gliomas.
There has been substantial progress made recently in developing GEM models that recapitulate the genesis and progression of human malignancies. In such an effort, several publications from Guha (e.g., ref. 3) have described an astrocytoma mouse model using embryonic stem (ES) cell transgenesis to overexpress oncogenic Ras under the control of a GFAP promoter. These mice express various levels of oncogenic Ras where the highest producers develop astrocytomas with similarities to glioblastomas. Consequently, one strain of the model relies on the development of low-grade astrocytomas (LGA) that progress to anaplastic astrocytomas [high-grade astrocytomas (HGA)]. An alternative approach to inducing tumor formation in mice consists of somatic-cell gene transfer using tissue-specific, replication-competent avian leukosis virus-based retroviral vectors. In such a model, the combined transfer of genes encoding activated Ras and Akt to nestin-expressing CNS progenitors leads to the formation of gliomas (4). However, contrary to the mouse model of Guha, neither Ras nor Akt alone is sufficient to generate glioblastomas. The requirement for combined Ras and Akt signaling implies that these pathways may coordinately regulate some critical process that leads to glioma formation. In this line of view, in the GFAP-Ras model, astrocytoma cell lines established from transgenic newborn mice (B8-P0) showed moderate expression of the RAS transgene and were nontumorigenic, as opposed to those coming from 3-month-old mice (B8-3mth) that carry multiples genetic abnormalities. These findings directed the authors to hypothesize that Ras is not sufficient to transform a given astrocyte but provides a lower threshold for transformation.
The laboratory of Guha used the unbiased approach of gene trapping to discover novel molecular alterations in a murine Ras cancer model. The study led to the identification of GATA6 as a new tumor suppressor for gliomas. More importantly, it permitted the discovery of novel genetic alterations in corresponding human cancers. Basically, a retroviral gene trap cassette was used to infect B8-P0 astrocytes, triggering the transformation of a small subset of astrocytes that grew in anchorage-independent assays in soft agarose. Analyses of the gene-trapped astrocytes identified GATA6 in the majority of infected clones. Interestingly, in the more malignant astrocytomas, i.e., coming from B8-3mth, GATA6 expression was totally absent. GATA6 has been identified as a member of the GATA family of zinc-finger transcription factors. In previous reports, Koutsourakis et al. (5) had shown that GATA6 is involved in embryonic development. Moreover, GATA6 knockout mice arrest during the early gastrula stage and show defects in endoderm differentiation. All data converge to an essential role for GATA6 in lineage determination during development and maintenance of cell differentiation in adult tissues. At the brain level, GATA6 is normally expressed in the adult mouse and human brain, where nuclear expression was detected in neurons, astrocytes, and endothelial cells (6).
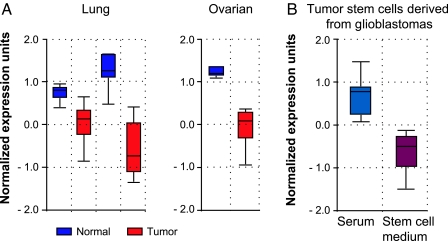
The present study of Kamnasaran et al. (2) brings a role for GATA6 acting as a tumor suppressor in gliomas: The trapped clones expressing a lower level of GATA6 showed increased proliferation and were able to grow intracranially as invasive malignant astrocytomas after injection into mice. A novel function arose after comparison of the level of GATA6 with tumor grade: GATA6 is abundantly present in LGA, whereas it is absent in HGA. This suggests an important role for the loss of GATA6 expression in tumor progression. One of the most promising findings in the study of Kamnasaran et al. comes from the use of the gene-trapping approach in GEM models that led to the identification of GATA6 alterations in the corresponding human GBM. As observed in the Ras astrocytomas model, GATA6 expression was absent in a panel of human GBM cell lines and human GBM operative specimens. Of particular interest, the authors elucidated one mechanism responsible for the loss of GATA6 expression in mouse and human gliomas. In the B8-3mth astrocytes, they identified a mutation in the DNA binding domain of the transcription factor. Similarly, in human GBM, mutations were detected within the DNA binding domain and the C terminus of the protein and were associated with LOH. In transactivation assay, mutations in human GATA6 resulted in decreased activity. This emphasizes the relevance of loss of GATA6 expression and function in human GBM and the further selection of the population during tumor progression. In the literature, great discrepancy arises from the level of GATA6 expression in different cancer types. Similar to glioblastomas, in ovarian cancer (7) and adrenocortical (8) tumors, GATA6 is absent or mislocalized, whereas normal tissues express abundant levels. A close-up at the cancer profiling database ONCOMINE (www.oncomine.org) revealed a highly significant down-regulation of GATA6 in lung and ovarian tumors compared with the normal tissues (Fig. 1A). On the other hand, GATA6 was found to be overexpressed in human colon cancer (9) and ubiquitously expressed in esophageal cancer (10). Overall, these data suggest that the regulation of GATA6 is tumor-type-specific.
Fig. 1.
GATA6 down-regulation in tumors and glioma stem cells. (A) Relative GATA6 expression of normal and human lung adenocarcinoma tissues (Left) from two different microarray analyses of the cancer profiling database ONCOMINE. GATA6 is found to be down-regulated (red) in tumor samples as compared with control lung (blue). Similar results were found in human ovarian cancer (Right). (B) Relative GATA6 expression in tumor stem cells derived from human glioblastomas showing higher levels when cells are exposed to serum (blue) versus stem cell medium supplemented with growth factors (red). In all cases, the ONCOMINE software, according to published cancer gene expression data, plotted the bar charts.
Looking further at the biological function of GATA6, the induction of differentiation during embryonic development raises the intriguing hypothesis for a role in ES cell behavior. In this regard, knockdown of Nanog in human ES cells resulted in the induction of differentiation markers such as GATA4 and GATA6 (11). In the last few years, stem cells have been detected in solid tumors that might represent the cells of origin of these solid tumors. So far, no study has looked at the expression of GATA6 in neural cancer stem cells: A loss of expression may provide an advantage for proliferation. However, gene expression profiles from Lee et al. (12), available at ONCOMINE, present very promising data for GATA6 expression in cancer stem cells. This microarray compares the expression profile of stem cells derived from GBM when treated with either serum or growth factors. The serum induced differentiation of the neurospheres, associated with a decrease in stem cell marker expression (12). In looking at GATA6 expression from this study by using ONCOMINE, this tumor suppressor is found to be highly expressed when cells are exposed to serum and down-regulated when they are cultured in stem cell medium (Fig. 1B). This may suggest a role for GATA6 in stem cell differentiation. It is unknown whether forced expression or knockdown of GATA6 in neurospheres can alter their ability to maintain cancer stem cell characteristics and tumorigenicity. Cancer stem cells were also shown to confer the chemoresistance phenotype due to their quiescent state, capacity for DNA repair, and accumulation of mutations. In the later case, mutations in the GATA6 gene might have an impact on cell survival and further regrowth of the tumor. Again, no information is available on the response to treatment of a GEM model in which the expression of GATA6 was modified.
Investigation of the upstream and downstream events regulating GATA6 may allow advances in the treatment of glioblastomas. Kamnasaran et al. (2) propose a role for GATA6 in cell cycle regulation of astrocytoma cells: Its forced expression induced an arrest of the cells in the G1 phase. Corroborating this data, it has been shown recently that the tumor suppressor LKB1 forms a complex with GATA6 to induce p21(cip) through a p53-independent mechanism (14). Previous studies demonstrated that GATA6 is expressed in vascular smooth muscle cells but down-regulated when vascular smooth muscle cells are induced to proliferate (15). Transfection of GATA6 into these cells inhibited the entry into S phase by increasing p21 expression (16). This may be of particular interest at the brain level considering that GATA6 is expressed in endothelial cells of normal tissues. Very recently, Calabrese et al. (16) demonstrated that in glioblastomas, the stem cells reside at a perivascular niche. Loss of the tumor suppressor GATA6 may provide a more favorable environment for stem cell survival. Of particular interest, Kamnasaran et al. (2) provide a possible role for GATA6 in astrocytoma angiogenesis by showing an inhibition of VEGF expression after constitutive reexpression of GATA in human glioma cell lines. Surprisingly, in the B8-3mth Ras model, loss of p19ARF and mutations in p53 are detected, whereas none of these modifications are found in the trapped clones. Kamnasaran et al. concluded that additional molecular alterations, independent of GATA6 mutations, contribute to tumorigenesis. The article by Kamnasaran et al. clearly demonstrates that GEM models of glioblastomas represent effective tools for identifying genetic alterations that may play important roles in human glioblastomagenesis. This intriguing aspect of the study highlights the benefits of using mouse models to address human diseases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 8053.
References
- 1.Sanai N, Alvarez-Buylla A, Berger MS. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 2.Kamnasaran D, Qian B, Hawkins C, Stanford WL, Guha A. Proc Natl Acad Sci USA. 2007;104:8053–8058. doi: 10.1073/pnas.0611669104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A. Cancer Res. 2001;61:3826–3836. [PubMed] [Google Scholar]
- 4.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 5.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. Development (Cambridge, UK) 1999;126:723–732. [PubMed] [Google Scholar]
- 6.Kamnasaran D, Guha A. Dev Brain Res. 2005;160:90–95. doi: 10.1016/j.devbrainres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Capo-chichi CD, Roland IH, Vanderveer L, Bao R, Yamagata T, Hirai H, Cohen C, Hamilton TC, Godwin AK, Xu XX. Cancer Res. 2003;63:4967–4977. [PubMed] [Google Scholar]
- 8.Rahman NA, Kiiveri S, Siltanen S, Levallet J, Kero J, Lensu T, Wilson DB, Heikinheimo MT, Huhtaniemi IT. Reprod Biol. 2001;1:5–9. [PubMed] [Google Scholar]
- 9.Shureiqi I, Zuo X, Broaddus R, Wu Y, Guan B, Morris JS, Lippman SM. FASEB J. 2007;21:743–753. doi: 10.1096/fj.06-6830com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo M, House MG, Akiyama Y, Qi Y, Capagna D, Harmon J, Baylin SB, Brock MV, Herman JG. Int J Cancer. 2006;119:2078–2083. doi: 10.1002/ijc.22092. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda SY, Tsuneyoshi N, Sumi T, Hasegawa K, Tada T, Nakatsuji N, Suemori H. Genes Cells. 2006;11:1115–1123. doi: 10.1111/j.1365-2443.2006.01000.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Setogawa T, Shinozaki-Yabana S, Masuda T, Akiyama T, Matsuura K. Biochem Biophys Res Commun. 2006;343:1186–1190. doi: 10.1016/j.bbrc.2006.03.077. [DOI] [PubMed] [Google Scholar]
- 14.Lepore JJ, Cappola TP, Mericko PA, Morrisey EE, Parmacek MS. Arterioscler Thromb Vasc Biol. 2005;25:309–314. doi: 10.1161/01.ATV.0000152725.76020.3c. [DOI] [PubMed] [Google Scholar]
- 15.Perlman H, Suzuki E, Simonson M, Smith RC, Walsh K. J Biol Chem. 1998;273:13713–13718. doi: 10.1074/jbc.273.22.13713. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]