Serotonin [or 5-hydroxytryptamine (5-HT)] has a pervasive influence on brain activity, being released from axons originating largely in the raphe nucleus in the brainstem but spreading out to a greater or lesser extent into almost all brain areas. Unlike fast neurotransmitters at synapses, 5-HT is thought to act distantly from its site of release (1). One consequence of this diffuse mode of action is that the extracellular levels of 5-HT will be very sensitive to the rate at which it is removed. Like other monoamines, extracellular 5-HT is inactivated mainly by carriage across the cell membrane of presynaptic axons, the carrier being of just one type, called SERT (serotonin transporter). SERT has long been of interest, not least because it is the target of drugs used successfully for the treatment of depression and other psychiatric conditions. The paper by Chanrion et al. in this issue of PNAS (2) reveals unforeseen connections between SERT and another transmitter used even more widely in the brain, nitric oxide (NO).
NO is made by NO synthase enzymes, the dominant isoform in the brain being the neuronal type (or nNOS), which is activated to synthesize NO from l-arginine by the binding of Ca2+/calmodulin. NO formation has classically been linked to activation of the NMDA class of glutamate receptor in synapses (3), a relationship that is explained by the high permeability of NMDA receptor channels to Ca2+ and the physical association of nNOS with NMDA receptors through their mutual binding to the abundant postsynaptic density-95 protein (4). This binding occurs through specialized PDZ domains, which are found in a large number of proteins (estimated to be near 1,000 in humans) and are responsible for assembling multiprotein complexes in subregions of cells, such as synapses. SERT was already known to associate with a number of proteins through either one of its intracellular ends. Knowing that one such interacting protein, PICK1, contains a PDZ domain, Chanrion et al. (2) speculated that the carboxyl terminus of the SERT molecule may behave like a PDZ domain, in which case there may be other partners with PDZ domains waiting to be discovered. To examine this possibility, they fused a carboxyl-terminal SERT peptide to Sepharose beads and used proteomics to find out whether any proteins in homogenates of mouse brain bound selectively. The most abundant protein to stick turned out to be nNOS. When the SERT peptide terminal valine, which is indispensable for many PDZ domain interactions, was replaced by glutamate, the interaction was lost.
Hints of a relationship between NO and 5-HT in the brain have existed for some time. The majority of serotonergic neurons in the raphe nucleus also contain nNOS (5). In addition, there have been several reports of NO affecting extracellular 5-HT levels in various brain regions, although no consistent direction of change was found and the underlying mechanisms remain unclear (6). A more defined interaction between NO and 5-HT was already to be found at the level of SERT. As with conventional transmitters, the physiological effects of NO are exerted through specialized receptors. In this case, the receptors contain a guanylyl cyclase enzymatic domain whose catalytic activity is enhanced up to 1,000-fold or more when NO attaches to the ligand-binding site. The result is the generation of large quantities of cGMP from GTP and a subsequent engagement of one or more downstream pathways, including cGMP-dependent protein kinases. SERT has a number of putative phosphorylation sites for this and other kinases, and studies in cell lines had shown that NO, through cGMP, causes an increase in the maximum activity of SERT by ≈30% (7). This effect was blocked by inhibitors of cGMP-dependent protein kinases and was attributed to an increase in the amount of transporter present in the plasma membrane. Very recently, these results were extended to the CNS by the demonstration that cGMP stimulates 5-HT transport in midbrain synaptosomes and that one particular threonine residue is the likely phosphorylation site (8). Unlike in the cell lines, increased surface expression of SERT could not be detected, suggesting that cGMP acts mainly to enhance the intrinsic activity of the transporter. Such an action may explain the cGMP-dependent decrease in extracellular 5-HT observed in the hypothalamus in vivo in response to infusion of low NO concentrations and the opposite effect taking place when endogenous NO is reduced (9).
The work by Chanrion et al. (2) forges new connections between 5-HT and NO synthase that have interesting reciprocal functional repercussions. When nNOS and SERT were coexpressed in a cell line, they bound to each other, and the uptake of 5-HT into the cells was simultaneously depressed as a result of a reduction in the maximum transport rate. This effect appeared to be independent of NOS enzymatic activity but was instead related to the binding of the two proteins together, because 5-HT uptake was unchanged if a mutant SERT lacking the carboxyl-terminal valine of the putative PDZ domain, which cannot bind nNOS, was used. By measuring the amount of SERT present at the cell surface relative to the total, the mechanism of reduced 5-HT uptake by nNOS was attributed to loss of ≈45% of the transporter located in the plasma membrane, not because of increased internalization but because its export to the membrane was inhibited. Next, Chanrion et al. explored the physiological relevance of the interaction. First, they showed that the maximal rate of 5-HT uptake and also the cell surface SERT expression in whole brain synaptosomes was increased in mice genetically deficient in nNOS. They then used a SERT carboxyl-terminal peptide sequence to inhibit SERT-nNOS interaction competitively and fused it with a HIV-1 Tat peptide to allow entry into cells. They showed the strategy to work in a cell line, and, when the method was applied to primary cultures of midbrain neurons, there was an ≈30% increase in 5-HT uptake. Finally, after i.v. injection into mice in vivo, the fusion protein entered the brain and caused a similar degree of enhancement of 5-HT uptake when measured ex vivo in synaptosomes. The control peptide (lacking the critical terminal valine residue) was inactive in both sets of experiments.
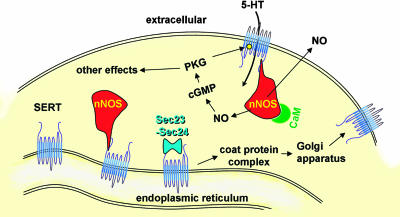
These findings indicate that nNOS retards the delivery of SERT to the plasma membrane by binding to its carboxyl-terminal PDZ-like sequence. Clues as to how this could be achieved came from the finding of Chanrion et al. (2) that, in addition to nNOS, the carboxyl terminus of SERT also bound two key components of the coat protein complex, which exports newly made proteins from the endoplasmic reticulum to the Golgi apparatus before incorporation into the plasma membrane (or elsewhere). The two proteins in question, called Sec23 and Sec24, are found linked together as heterodimers in the cytoplasm and function primarily to recognize (bind to) proteins in the endoplasmic reticulum destined for export (10). Thus, nNOS may compete with Sec23–Sec24 for binding to the SERT carboxyl terminus, resulting in reduced export of SERT to the cell membrane (Fig. 1).
Fig. 1.
SERT–nNOS interactions. Binding of nNOS to the SERT carboxyl terminus decreases SERT trafficking to the plasma membrane and thereby inhibits 5-HT uptake. Reciprocally, 5-HT uptake induces NO production from nNOS physically linked to SERT, through a calmodulin (CaM)-dependent mechanism. NO formed in association with 5-HT transport might, through cGMP and cGMP-dependent protein kinase (PKG), phosphorylate SERT, increasing its activity.
The counterpart to the negative influence of nNOS on the amount of SERT available at the cell surface was the finding by Chanrion et al. (2) that exposure of a cell line expressing both SERT and nNOS to 5-HT caused the formation of NO (detected by cGMP measurement). The obvious explanation would be that the cells possess one or more 5-HT receptor subtypes already known to couple to NO synthase activation. NO formation was undetectable in cells lacking SERT, however, and was blocked by inhibitors of 5-HT transport. The generation of NO was linked, therefore, to the operation of the 5-HT transporter. Chanrion et al. explored the possibility that 5-HT uptake may somehow raise intracellular Ca2+, but the results were negative. Nevertheless, studies with a Ca2+ chelator and with calmodulin inhibitors indicated that the nNOS activity was, as normal, dependent on Ca2+/calmodulin but, unusually for nNOS, did not require a rise in Ca2+. The maintenance of NO formation at resting cytosolic Ca2+ levels is reminiscent of the endothelial NO synthase isoform, on which phosphorylation of the enzyme confers this property (11). With nNOS, the activity depended on its physical interaction with SERT because it was undetectable when the SERT lacked the terminal valine residue needed for interaction with nNOS. Thus, although there have not yet been studies outside the model cell line, the results provide the first example of the operation of a transporter coupling to activation of a biochemical signaling pathway, a “metabotropic” function normally associated with receptors (Fig. 1).
More broadly, the results of Chanrion et al. (2) raise a number of important questions about the role of nNOS in the regulation of 5-HT transmission. 5-HT neurons appear to fire mainly in a regular slow manner in vivo (12). This firing pattern should lead to a tonic level of extracellular 5-HT within the brain regions innervated by the axons, the concentration being set by the transporter activity (13). By diminishing the amount of SERT in the cell membrane, binding of nNOS should adjust extracellular 5-HT upward. In effect, nNOS would be acting like an endogenous antidepressant. Mice deficient in nNOS display abnormally impulsive and aggressive behaviors that are associated with a deficiency in 5-HT neurotransmission (14). It would be interesting to test whether disrupting the nNOS–SERT binding in vivo, shown to be feasible by Chanrion et al., replicates this behavior. In addition, if up-regulation of nNOS were to occur in 5-HT neurons, as it can in other neurons in response to various conditions (e.g., injury, neurotransmitters, neurotrophins; see ref. 3), the result should be lower SERT expression in the membrane, leading to an augmentation of 5-HT neurotransmission.
Finally, what could be the purpose of 5-HT transport generating an NO signal? If tonic 5-HT concentrations exist extracellularly in vivo then there will be tonic SERT activity and, should the findings of Chanrion et al. (2) apply, tonic NO synthesis. Tonic NO levels have only recently been recognized to be important in the CNS, including in regulating the membrane potential of central axons and in synaptic plasticity (15, 16). In these cases, NO comes from endothelial NO synthase located in the microcirculation. Perhaps, tonic NO from SERT-associated nNOS, by engaging the cGMP-dependent pathway that enhances intrinsic SERT activity (8), allows for a continual homeostatic fine-tuning of 5-HT uptake according to the ambient extracellular 5-HT concentration: the higher it is, the more rapidly it will be removed.
Footnotes
The author declares no conflict of interest.
See companion article on page 8119.
References
- 1.Bunin MA, Wightman RM. Trends Neurosci. 1999;22:377–382. doi: 10.1016/s0166-2236(99)01410-1. [DOI] [PubMed] [Google Scholar]
- 2.Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Proc Natl Acad Sci USA. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garthwaite J, Boulton CL. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- 4.Christopherson KS, Bredt DS. J Clin Invest. 1997;100:2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson KL, Waterhouse BD, Lin RC. J Comp Neurol. 2003;466:495–512. doi: 10.1002/cne.10912. [DOI] [PubMed] [Google Scholar]
- 6.Prast H, Philippu A. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- 8.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. J Biol Chem. 2007 Feb 19; doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- 9.Kaehler ST, Singewald N, Sinner C, Philippu A. Brain Res. 1999;835:346–349. doi: 10.1016/s0006-8993(99)01599-1. [DOI] [PubMed] [Google Scholar]
- 10.Gurkan C, Stagg SM, Lapointe P, Balch WE. Nat Rev Mol Cell Biol. 2006;7:727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- 11.Fulton D, Gratton JP, Sessa WC. J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 12.Hajos M, Allers KA, Jennings K, Sharp T, Charette G, Sik A, Kocsis B. Eur J Neurosci. 2007;25:119–126. doi: 10.1111/j.1460-9568.2006.05276.x. [DOI] [PubMed] [Google Scholar]
- 13.Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. J Neurochem. 2003;87:1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- 14.Nelson RJ, Trainor BC, Chiavegatto S, Demas GE. Neurosci Biobehav Rev. 2006;30:346–355. doi: 10.1016/j.neubiorev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Garthwaite G, Bartus K, Malcolm D, Goodwin DA, Kollb-Sielecka M, Dooldeniya C, Garthwaite J. J Neurosci. 2006;26:7730–7740. doi: 10.1523/JNEUROSCI.1528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopper RA, Garthwaite J. J Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]