Abstract
Palladium (Pd) generally prefers low oxidation states. So far, no stable Pd compound with a +5 oxidation state is known. Here, we report two multinuclear Pd compounds containing Pd centers ligated by five silicon (Si) atoms. A thermal condensation reaction of [{1,2-C6H4(SiMe2)(SiH2)}PdII(Me2PCH2CH2PMe2)] (Me = methyl) afforded two stereoisomers of dinuclear PdII compounds and a trinuclear Pd compound as major products and a tetranuclear Pd compound as a minor product. The structures of the four Pd compounds were confirmed by single-crystal x-ray structure analysis. The dinuclear Pd compounds have a dimeric structure of [{1,2-C6H4(SiMe2)(SiH)}PdII(Me2PCH2CH2PMe2)] connected through a Si–Si single bond formed by dehydrogenation of two molecules of the starting compound. The trinuclear and tetranuclear Pd compounds proved to have Pd centers bonded to five Si atoms with normal Pd–Si single-bond distances. Theoretical calculations of the trinuclear and tetranuclear Pd compounds accurately reproduced their x-ray structures and suggested that all of the Pd–Si bonds of the central Pd atoms have a relatively high single-bond character.
Keywords: transition metal, hydrosilanes, oxidation states
Palladium (Pd) compounds provide useful catalysts for organic transformations to produce a wide range of organic compounds such as pharmaceuticals, agrochemicals, and organic materials for various applications (1, 2). Organopalladium compounds, typical intermediates in Pd-catalyzed organic transformations, generally prefer low formal oxidation states such as 0 and +2. Only relatively recently has the importance of organopalladium compounds with a +4 formal oxidation state been recognized (3, 4). The first stable organopalladium compounds with this +4 formal oxidation state, PdIV(C6F5)2Cl2(L–L) (L–L = bidentate neutral ligands), were isolated in 1975 (5); then, alkylpalladium(IV) species were also isolated and intensively studied (3, 6). However, an organopalladium compound with a formal oxidation state exceeding +4 has never been identified. On the other hand, highly electronegative fluorine ligands reportedly can produce +5 and +6 oxidation states in inorganic Pd compounds, although such species are unstable and have not been well characterized (7, 8). Electrochemical formation of PdO3 is also suggested (9). It is also known that more electropositive ligands such as hydride and silyl ligands can form stable transition-metal compounds with high formal oxidation states such as K2ReVIIH9, (Me5C5)IrVH4, and (Me5C5)MV(H)2(SiEt3)2 (M = Co, Rh, Ir) (10–14).
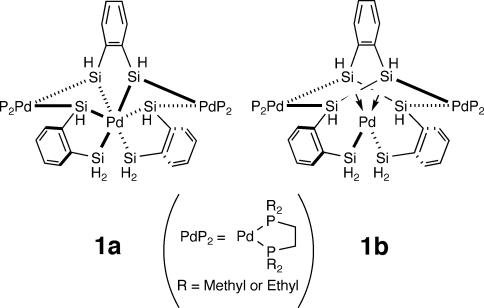
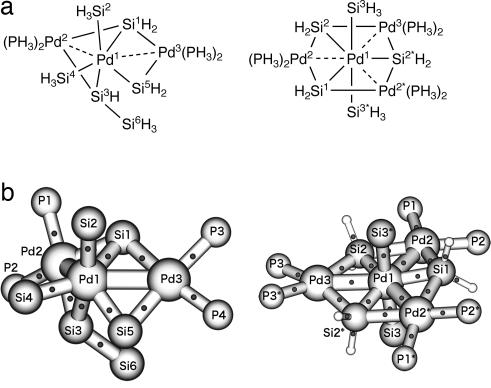
We have found that sterically less demanding chelating silyl ligands are useful in stabilizing silyl transition-metal compounds with high formal oxidation states (15) and reported silylpalladium(IV) (16) and silylnickel(IV) compounds (17). During our effort to synthesize silyl group 10 transition-metal compounds with formal oxidation states exceeding +4, we obtained a trinuclear Pd compound 1 (Fig. 1), which has a central Pd atom ligated by six Si atoms (18). X-ray structure analysis of compound 1 showed a possible Si–Si bonding interaction between two pairs of Si atoms and lead to two possible structural descriptions, 1a (six Pd–Si single bonds, formal oxidation state +6) and 1b (two Pd–Si single bonds and two Si–Si σ-bonds coordinating to the Pd atom, formal oxidation state +2) (18, 19). Theoretical calculations for compound 1 suggested that 1b more appropriately represented the bonding structure of this compound than did 1a (20, 21). In this article, we describe two multinuclear Pd compounds, each of which has a Pd center ligated by five Si atoms. X-ray structure analysis as well as theoretical calculations suggested that each Pd–Si bond of the central Pd atoms has a relatively high single-bond character.
Fig. 1.
Two structural description of compound 1.
Results and Discussion
The trinuclear Pd compound 1 was synthesized by the thermal condensation reaction of three molecules of Pd(II) compound [{1,2-C6H4(SiH2)2}PdII(R2PCH2CH2PR2)] 2. We simply modified the structure of the starting Pd compound 2 by putting two methyl groups on one of the two Si atoms. Because the Si–C bonds are much less reactive toward transition metals than are Si–H bonds, the modified compound [{1,2-C6H4(SiMe2)(SiH2)}PdII(dmpe)] 3 [dmpe = 1,2-bis(dimethylphosphino)ethane] was expected not to produce a compound similar to 1.
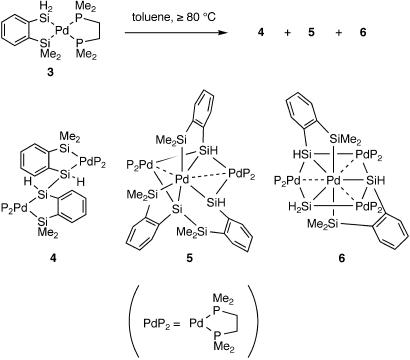
A thermal condensation reaction of compound 3, which was prepared by the reaction of 1,2-C6H4(SiMe2H)(SiH3) (22) with [Pd(PEt3)2(dmpe)], took place at 80°C or higher (Fig. 2). At 80°C in toluene, dinuclear compound 4 with a new Si–Si single bond was initially formed as a mixture of two stereoisomers as judged by 1H and 31P NMR spectroscopy. The structures of the two stereoisomers were confirmed to be meso- and dl-isomers by x-ray structure analysis (vide infra). With continued heating at 80°C for 2 days, trinuclear compound 5 started to emerge, and 31P NMR spectroscopy identified four signals with equal intensities for this compound. A separate experiment in an NMR tube heated at 100°C for 6 days afforded compounds 4 (meso- and dl-isomers in a 7:3 ratio) and 5 as the main products. The reaction slowed gradually, and the conversion of 3 reached ≈70% after 6 days. The yields of 4 and 5 estimated by 1H NMR integration were 55% and 39%, respectively, based on the converted 3. Compounds 4 and 5 were isolated by fractional solvent extraction/crystallization procedures. During the attempts to isolate and crystallize compound 5, a tiny amount of minor product, tetranuclear compound 6, was unexpectedly obtained as single crystals. Although compound 6 was formed in a low yield, the existence of 6 in the reaction mixture was easily confirmed by 1H NMR spectroscopy in tetrahydrofuran (THF)-d8, in which some of the signals of 6 were well separated from those of other compounds. The estimated yields of compound 6 in the reaction mixture varied (1–5%) depending on the reaction conditions. The product distribution largely depended on the concentration and reaction temperature. Higher concentration and higher reaction temperature favor the formation of 5 and 6.
Fig. 2.
Synthesis of compounds 4–6.
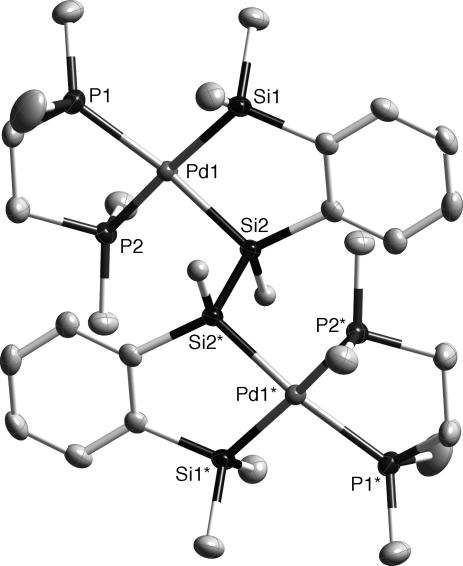
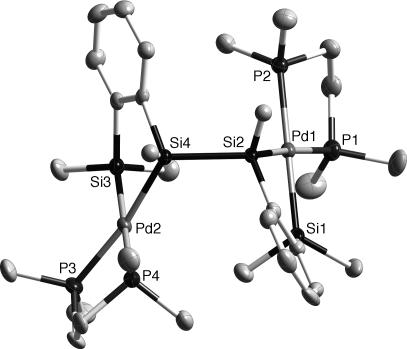
The structures of the two isomers of 4, 5, and 6, respectively, were unambiguously determined by the x-ray structure analysis. Figs. 3 and 4, respectively, show the molecular structures of meso- and dl-4. The Pd–Si, Pd–P, and Si–Si bond distances in meso- and dl-4 (Table 1) were all in their typical ranges.
Fig. 3.
Thermal ellipsoid plots (50% probability level for Pd, P, Si, and C atoms) of meso-4 determined by single-crystal x-ray diffraction. Hydrogen atoms on the carbon atoms are omitted for clarity.
Fig. 4.
Thermal ellipsoid plots (50% probability level for Pd, P, Si, and C atoms) of dl-4 determined by single-crystal x-ray diffraction. Hydrogen atoms on the carbon atoms are omitted for clarity.
Table 1.
Selected bond distances (Å) for compounds meso- and dl-4
| Bond | meso-4 | dl-4 |
|---|---|---|
| Pd1–Si1 | 2.3634(10) | 2.3713(16) |
| Pd1–Si2 | 2.3405(9) | 2.3626(14) |
| Pd2–Si3 | 2.3632(17) | |
| Pd2–Si4 | 2.3613(15) | |
| Pd1–P1 | 2.3307(10) | 2.3259(16) |
| Pd1–P2 | 2.3391(9) | 2.3465(15) |
| Pd2–P3 | 2.3277(17) | |
| Pd2–P4 | 2.3402(15) | |
| Si–Si | 2.3720(13) | 2.355(2) |
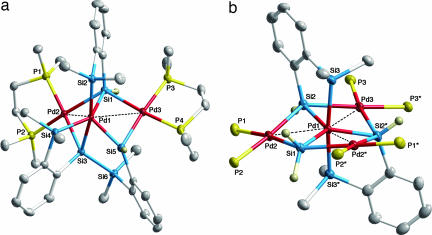
Fig. 5a shows the molecular structure of 5, and its structural features are as follows. The central Pd atom, Pd1, no longer retains the chelating phosphine ligand as is the case in 1 but instead is ligated by five Si atoms. The geometry of the Pd1(Si)5 moiety may be described as highly distorted, square-based pyramidal with Si5 at the apical position. The five Pd1–Si bond distances range from 2.2903(8) to 2.4015(8) Å (Table 2) and are comparable to typical Pd–Si single-bond distances [2.26–2.45 Å] (Cambridge Structural Database, Version 5.27, November 2005). These distances contrast with those in compound 1, in which four are long [2.437(3)–2.562(3) Å, average 2.483(3) Å for 1 bearing dmpe ligands]. One (Si6) of the six Si atoms in 5 is not bonded to Pd1 but forms a Si–Si single bond (Si3–Si6) with a common distance [2.3414(12) Å]. In compound 1, there are two short Si···Si contacts [2.488(4)-2.589(4) Å], whereas compound 5 has no such short Si···Si contact [the shortest Si···Si contact is 2.8919(13) Å, Si3···Si5]. The Pd···Pd distances [2.7677(3) and 2.7996(3) Å] suggest possible weak interatomic interactions. Another unique feature of compound 5 is that one (Si1) of the Si atoms is pentacoordinate. As mentioned above, the Pd1–Si1 distance is comparable to common Pd–Si single-bond distances, but the Pd2–Si1 [2.5273(10) Å] and Pd3–Si1 [2.5966(9) Å] bond distances are much longer than is common.
Fig. 5.
Thermal ellipsoid plots (50% probability level for Pd, P, Si, and C atoms) of compound 5 (a) and compound 6 (b) determined by single-crystal x-ray diffraction. Hydrogen atoms on the carbon atoms (for 5 and 6) and carbon atoms on the phosphorus atoms (for 6) are omitted for clarity.
Table 2.
Experimental (x-ray diffraction) and calculated bond distances and Mayer bond orders for compounds 5 and 6
| Bond | Distance, Å |
Bond order | |
|---|---|---|---|
| Experimental | Calculated | ||
| Compound 5 | |||
| Pd1–Si1 | 2.3526(11) | 2.401 | 0.672 |
| Pd1–Si2 | 2.4015(8) | 2.457 | 0.747 |
| Pd1–Si3 | 2.3385(8) | 2.384 | 0.709 |
| Pd1–Si4 | 2.3464(11) | 2.405 | 0.729 |
| Pd1–Si5 | 2.2903(8) | 2.326 | 0.769 |
| Pd2–Si1 | 2.5273(10) | 2.564 | 0.542 |
| Pd2–Si3 | 2.3832(9) | 2.443 | 0.628 |
| Pd3–Si1 | 2.5966(9) | 2.652 | 0.455 |
| Pd3–Si5 | 2.4033(11) | 2.439 | 0.668 |
| Pd1–Pd2 | 2.7996(3) | 2.939 | 0.200 |
| Pd1–Pd3 | 2.7677(3) | 2.899 | 0.211 |
| Si3···Si5 | 2.8919(13) | 2.928 | 0.280 |
| Compound 6 | |||
| Pd1–Si1 | 2.3265(13) | 2.349 | 0.673 |
| Pd1–Si2 or Pd1–Si2* | 2.2944(9) | 2.32 | 0.747 |
| Pd1–Si3 or Pd1–Si3* | 2.4357(10) | 2.508 | 0.713 |
| Pd2–Si1 or Pd2*–Si1 | 2.5124(5) | 2.572 | 0.519 |
| Pd2–Si2 or Pd2*–Si2* | 2.5781(10) | 2.621 | 0.460 |
| Pd3–Si2 or Pd3–Si2* | 2.6093(9) | 2.649 | 0.449 |
| Pd1–Pd2 or Pd1–Pd2* | 2.7332(3) | 2.815 | 0.204 |
| Pd1–Pd3 | 2.7013(4) | 2.804 | 0.218 |
The molecular structure of compound 6 is shown in Fig. 5b. Compound 6 is crystallographically C2-symmetric. The geometry of the Pd1(Si)5 moiety can be described as distorted trigonal bipyramidal [or hexagonal bipyramidal for the Pd1(Si)5(Pd)3 moiety]. Five atoms (Pd1, Pd2, Pd2*, Pd3, and Si1) locate on the same plane, whereas two Si atoms (Si2 and Si2*) deviate from the plane by 0.59 Å. The Pd1–Si1, Pd1–Si2(Si2*), and Pd1–Si3(Si3*) bond distances of 2.3265(13), 2.2944(9), and 2.4357(10) Å, respectively, are similar to those of common Pd–Si single bonds. The axial Pd1–Si3(Si3*) bond distances are relatively long; this is partly because the two axial Si atoms [Si3 and Si3*] locate in trans to each other, and the strong trans-influence (or structural trans-effect) of Si atoms lengthens the Pd–Si bond distances (23). The three equatorial Si atoms (Si1, Si2, and Si2*) are pentacoordinate, like Si1 in compound 5. The Pd–Si distances for the outer Pd atoms [2.5124(5)–2.6093(9) Å] are much longer than those of common Pd–Si single bonds.
Although it is difficult and less meaningful to assign a formal oxidation state for each atom in cluster-type compounds (19, 20), an unusual +5 formal oxidation state may be assigned to the central Pd atoms (Pd1) in 5 and 6, if each Pd1–Si bond is simply counted to increase the formal oxidation state of Pd1 by +1. Therefore, we carried out density functional theory calculations for 5 and 6 to obtain further information about the structures of compounds 5 and 6. We also further evaluated the bonding of 5 and 6, using Mayer's bond order analysis (24) and Bader's atoms in molecule (AIM) analysis (25); the former was used for the bonding analysis of compound 1 (20), and the latter searches for bond critical points and indicates the existence of a chemical bond between two atoms sharing the bond critical point. Both calculated structures of 5 and 6 accurately reproduced the x-ray structures of 5 and 6. Table 2 summarizes selected bond distances obtained from x-ray analysis and density functional theory calculations and Mayer bond orders for 5 and 6 [see supporting information (SI) Tables 4 and 5 for Cartesian coordinates for the calculated structures of 5 and 6, and see SI Tables 6 and 7 for further lists of Mayer bond orders for 5 and 6]. For compound 5, the bond orders of the five Pd1–Si bonds (0.67–0.77) are higher than the highest value (0.63) calculated for the Pd–Si bonds of the central Pd atom in compound 1 (20). The total bond orders of the five Pd1–Si bonds (3.63) is also higher than that of the six central Pd–Si bonds (3.32) in 1 (20). The calculated bond orders and the bond distances determined by x-ray analysis suggest that the five Pd1–Si bonds in 5 have a relatively high single-bond character. The bonds between the pentacoordinate Si1 and terminal Pd atoms (Pd2 and Pd3) have lower bond orders (average 0.50), as expected from the long bond distances. The total (3.59) and individual bond orders (0.67–0.75) of the five Pd1–Si bonds in 6 are at a level similar to those of 5, suggesting that the five Pd1–Si bonds in 6 also have a relatively high single-bond character. The six outer Pd–Si bonds in 6 have lower bond orders (average 0.48), as expected from the long bond distances.
Because attempted AIM analysis on compounds 5 and 6 failed as a result of its inability to find bond critical points for the C–H bonds of the benzene rings of 5 and 6, we carried out AIM analysis on model systems M5 and M6 (Fig. 6a; see also SI Figs. 7 and 8), which were constructed by removing carbon atoms from 5 and 6, respectively. The Cartesian coordinates of core regions were fixed, while the positions of the substituted hydrogen atoms were optimized. Thus, the nature of the chemical bonds of the core regions in the model compounds M5 and M6 should be the same as those in the real systems. AIM analysis on M5 and M6 was successful and indicated the existence of bond critical points in all Pd1–Si bonds of M5 and M6 (Fig. 6b). This result also supports the bonding nature of Pd1–Si bonds of compounds 5 and 6. Mayer bond order analysis (Table 2) suggested a possible weak bonding interaction between Pd atoms in compounds 5 and 6 (bond order = 0.20–0.22) and also between Si3 and Si5 atoms (bond order = 0.28) in compound 5, although the Si3···Si5 distance is long. AIM analyses on the model compounds showed the existence of bond critical points only between Pd atoms in M6 but not between Pd atoms or Si3 and Si5 atoms in M5.
Fig. 6.
AIM analysis. (a) Structure of model compounds M5 and M6. (b) AIM analysis results for model compounds M5 and M6. Some of hydrogen atoms are omitted for clarity. Dots between the atoms represent the bond critical points.
The formation of silylpalladium compounds with unusual Pd centers ligated by five Si atoms represents one of interesting features of Si ligands in transition metal chemistry. Although interest in the chemistry of transition metal compounds with Si ligands is rapidly growing (26), the field is still at the initial stage of development compared with the organometallic chemistry of transition metals. We believe Si ligands will provide further unique chemistry of transition metals and lead to the development of unique catalyses.
Materials and Methods
All manipulations of air-sensitive materials were carried out under a nitrogen atmosphere by using standard Schlenk tube techniques or in a glovebox filled with argon or nitrogen. Toluene-d8, THF-d8, and benzene-d6 were distilled from Na/benzophenone ketyl. All other anhydrous solvents were purchased from Kanto Chemicals or Aldrich and degassed before use. 1H (499.1 MHz), 29Si (99.1 MHz), and 31P (202.0 MHz) NMR spectra were recorded on a JEOL LA500 spectrometer. Chemical shifts are given in parts per million, using external references [tetramethylsilane (0 ppm) for 1H and 29Si, and 85% H3PO4 (0 ppm) for 31P] or residual solvent signal for the 1H NMR in THF-d8 (the residual solvent signal at lower field position was set to 3.60 ppm), and coupling constants are reported in hertz.
Synthesis of [{1,2-C6H4(SiMe2)(SiH2)}Pd(dmpe)], 3.
A toluene solution (5 ml) of Pd(PEt3)4 (300 mg, 0.52 mmol) and dmpe (78 mg, 0.52 mmol) was stirred at room temperature overnight. Volatiles were removed under vacuum to leave Pd(PEt3)2(dmpe) as a viscous solid. To the solid was added toluene (5 ml) and 1,2-C6H4(SiMe2H)(SiH3) (87 mg, 0.52 mmol) at 0°C. During the addition of 1,2-C6H4(SiMe2H)(SiH3), a small amount of gas evolution was observed. Then the mixture was stirred at room temperature for 12 h under a nitrogen atmosphere. Removal of the volatiles under vacuum and washing the residual solid with hexane gave 3 in 72% yield (157 mg) as a pale brown powder. The product is practically pure and used for the following reactions without further purification. 1H NMR (toluene-d8) δ 0.73 (t, 6H, JP-H = 2), 0.92–1.04 (m, 16H), 5.57 (t, 2H, JP-H = 10, JSi-H = 158), 7.32 (t, 1H, JH-H = 7), 7.36 (t, 1H, JH-H = 7), 7.82 (d, 1H, JH-H = 7), 8.07 (d, 1H, JH-H = 7). 31P NMR (toluene-d8) δ 13.2 (br). 29Si{1H} NMR (toluene-d8, dept) δ −17.7 (d, JP-Si = 137, (JH-Si = 158), SiH2). The measurement parameters were adjusted to the silicon atoms with directly bound hydrogen atoms. The JH-Si value was determined by 1H-coupled spectrum. 29Si{1H} NMR (toluene-d8, inept) δ 31.8 (d, JP-Si = 145, SiMe2). The measurement parameters were adjusted to the silicon atoms with two methyl groups. Anal. Calcd. for C14H28P2PdSi2: C, 39.95; H, 6.71. Found C, 40.14; H, 6.65.
Thermal Condensation Reaction of 3.
Isolation of compounds 4 and 5.
A toluene solution (10 ml) of 3 (507 mg, 1.20 mmol) was heated in an oil bath at 90°C for 44 h, at 95°C for 51 h, and then at 100°C for 47 h. After cooling to room temperature, volatiles were removed under vacuum to give crude solid products. NMR measurement of the crude products showed that the conversion of 3 was ≈68% and the formation of 4 (≈39%, meso-4/dl-4 = 72/28) and 5 (≈23%) as major products. The crude products were dissolved in THF (≈20 ml) and filtered through a membrane filter (Sartorius Minisart SRP 15, no. 17573). The filtrate was evacuated to remove volatiles, and the remaining solid was extracted with hexane (3 × 4 ml) and hexane/toluene mixture (1/1, 3 × 3 ml), leaving a solid mixture mainly consisting of 4 and 5. The solid was dissolved in toluene (20 ml) by heating at 100°C for a few minutes. After cooling to room temperature, the supernatant was transferred to another Schlenk tube to remove a small amount of undissolved materials on the bottom. A few crystals of 4 were added to the solution to induce crystallization, and the mixture was kept at room temperature for 1 day to give almost colorless crystals of 4. The supernatant was removed by decantation, and the remaining crystals were washed with toluene (1 × 1 ml and 2 × 0.5 ml) and hexane (1 × 0.5 ml and 2 × 1 ml) and dried under vacuum to give 65 mg of 4 (meso-4:dl-4 = 78:22). The supernatant was evacuated to remove the solvent, and the resulting solid was dissolved in 16 ml of 1,2-dimethoxyethane (DME) by heating in an oil bath at 100°C for a few minutes. Keeping the solution at room temperature for 1 day gave orange-red crystals of 5 containing DME as a cocrystallization solvent. The supernatant was removed by decantation, and the remaining crystals were washed with DME and hexane to give 27 mg (6%) of 5. The separated supernatant was evacuated to remove the solvent, and the resulting solid was recrystallized from toluene to give additional 4 (58 mg). Total isolated yield of 4 was 24%. Compound 4: 1H NMR (benzene-d6, 4:1 mixture of meso- and dl-isomers) δ 0.43 (d, 6H of meso-isomer, JP-H = 3); 0.69–1.15 (m, 38H of meso-isomer and 44H of dl-isomer); 5.62 (ddd, 2H of dl-isomer, JP-H = 4, 8, 23, Si-H); 5.86 (ddd, 2H of meso-isomer, JP-H = 5, 8, 19, Si-H); 7.21–7.37 (m, 4H of meso-isomer and 4H of dl-isomer); 7.67 (d, 2H of meso-isomer, JH-H = 7); 7.81 (d, 4H of dl-isomer, JH-H = 8); 8.07 (d, 2H of meso-isomer, JH-H = 7). 31P NMR (benzene-d6, 4:1 mixture of meso- and dl-isomers) δ value 10.2 (d, meso-isomer, JP-P = 13); 10.6 (d, dl-isomer, JP-P = 15); 10.9 (d, meso-isomer, JP-P = 13); 13.3 (d, dl-isomer, JP-P = 15). Anal. calcd. for C28H54P4Pd2Si4: C, 40.04; H, 6.48. Found C, 40.43; H, 6.48. Compound 5. 1H NMR (THF-d8) δ −0.10 (s, 3H, SiCH3), −0.08 (s, 3H, SiCH3), −0.03 (s, 3H, SiCH3), 0.07 (d, 3H, JP-H = 8, PCH3), 0.13 (d, 3H, JP-H = 7, PCH3), 0.20 (d, 3H, JP-H = 9, PCH3), 0.43 (d, 3H, JP-H = 8, PCH3), 0.45 (s, 3H, SiCH3), 0.70 (s, 3H, SiCH3), 0.72 (s, 3H, SiCH3), 1.44 (d, 3H, JP-H = 7, PCH3), 1.54 (d, 3H, JP-H = 9, PCH3), 1.59 (d, 3H, JP-H = 7, PCH3), 1.61 (d, 3H, JP-H = 8, PCH3), 1.50 - 1.85 (m, 8H, PCH2CH2P), 6.86–6.90 (m, 2H), 7.00–7.11 (m, 4H), 7.15 (dt, 1H, JH-H = 1 and 7), 7.37–7.40 (m, 1H), 7.45–7.49 (m, 1H), 7.60–7.67 (m, 2H), 7.76–7.85 (m, 2H), 8.01 (d, 1H, JH-H = 7 Hz). 31P{1H} NMR (THF-d8) δ 15.7 (dd, JP-P = 10 and 31), 17.3 (dd, JP-P = 12 and 29), 18.9 (dd, JP-P = 10 and 29), 21.0 (dd, JP-P = 12 and 31). 29Si{1H} NMR (THF-d8, nne) δ −21.1 (d, JP-Si = 6, Si6), 25.0 (s, Si2 (or Si4)), 38.7 (d, JP-Si = 18, Si4 (or Si2)), 129.8–132.0 (m, Si1), 173.1 (d, JP-Si = 113, Si3), 210.7 (ddd, JP-Si = 12, 34, 122, Si5). The assignment is based on the coupling pattern of the signals as well as the prediction by the theoretical calculations (Table 3). IR (KBr pellet, cm−1) 1999 (Si–H), 1972 (Si–H). Anal. calcd. for C36H64P4Pd3Si6·C4H10O2 C, 40.08; H, 6.22. Found C, 40.27; H, 6.23.
Table 3.
Calculated and observed 29Si NMR chemical shifts [δ (ppm)] of compounds 5 and 6
| Si | Compound 5 |
Compound 6 |
||
|---|---|---|---|---|
| Calculated | Observed | Calculated | Observed | |
| Si1 | 122.1 | 130.9 | 101.6 | 103.0 |
| Si2 (Si2*) | 25.8 | 25.0 | 147.0 (147.1) | 139.3 |
| Si3 (Si3*) | 194.8 | 173.1 | 24.6 (24.5) | 19.0 |
| Si4 | 43.5 | 38.7 | — | — |
| Si5 | 202.7 | 210.7 | — | — |
| Si6 | −32.1 | −21.1 | — | — |
The atomic numbering refers to that in Fig. 5.
Isolation of compound 6.
A toluene solution (100 ml) of 3 (910 mg, 2.16 mmol) was heated in an oil bath at 100°C for 6 days. The estimated molar ratio of 3/4 (two isomers)/5/6 by 1H NMR spectroscopy was 73/19/7.7/0.3 at this stage. The solution was evacuated to reduce the volume to ≈40 ml and then was heated in an oil bath at 100°C for 58 h. The estimated molar ratio of 3/4 (two isomers)/5/6 by 1H NMR spectroscopy was 48/34/17/1 at this stage. The mixture was evacuated to dryness and washed with hexane (2 × 7 ml and 3 × 5 ml) to leave solid materials. The solid was dried under vacuum, dissolved in toluene (7 ml), and heated in an oil bath at 100°C for 84 h. The estimated molar ratio of 3/4 (two isomers)/5/6 by 1H NMR spectroscopy was 29/38/30/3 at this stage. The mixture was evacuated to dryness and reacted with 1,2-C6H4(SiMe2H)(SiH3) (111 mg, 0.67 mmol) in THF (10 ml) at 40°C for 11 h [this hydrosilane selectively reacted with compound 4 to form an unidentified product(s), which has much higher solubility in hexane than does 4 and can be easily removed from the mixture by solvent extraction]. The mixture was evacuated to dryness and washed with hexane (4 × 5 ml), hexane/toluene (3/2) mixture (4 × 5 ml), and hexane/toluene (1/1) mixture (3 × 7 ml). The residue was dissolved in THF (20 ml), filtered through a membrane filter (Sartorius Minisart SRP 15, no. 17573), and evacuated to dryness to give yellow-brown solid (179 mg). 1H and 31P NMR spectroscopy showed that the solid consisted of 5 and 6 in a 92:8 molar ratio. Fortunately, compound 6 has higher solubility in DME but much lower solubility in diethyl ether than does 5. The solid was extracted with DME (6 ml) to give a mixture of 5 and 6 in a 3:4 molar ratio and to leave pure 5 (153 mg, 19% yield). Then, the mixture (after evacuation) was extracted with diethyl ether (1 × 3 ml, 1 × 2 ml, 1 × 1 ml) to leave compound 6 with >80% purity by 1H and 31P NMR spectroscopy (9 mg). Compound 6: 1H NMR (THF-d8) δ −0.48 (s, 6H, SiCH3), −0.37 (s, 6H, SiCH3), 0.03 (d, 6H, JP-H = 7, PCH3), 1.36–1.40 (m, 12H, PCH3), 1.48–1.51 (m, 6H, PCH3), 1.56–1.58 (m, 6H, PCH3), 1.68 (d, 6H, JP-H = 7, PCH3), 1.50–1.90 (m, 12H, PCH2CH2P), 6.06 (tt, 2H, JP-H = 7 and 20, SiH2), 6.62 (t, 2H, JH-H = 7, aromatic H), 6.91 (t, 2H, JH-H = 7, aromatic H), 6.94 (d, 2H, JH-H = 7, aromatic H), 7.76–7.93 (br m, 2H, SiH), 7.89 (d, 2H, JH-H = 7, aromatic H). The assignments of SiH2 and SiH signals are based on the coupling patterns of the signals and the prediction by the theoretical calculations. 31P{1H} NMR (THF-d8) δ 11.7–12.6 (m), 14.1 (s). 29Si{1H} NMR (THF-d8, dept) δ 102.0–103.9 (m, Si1), 138.1–140.5 (m, Si2 and Si2*). The measurement parameters were adjusted to the silicon atoms with directly bound hydrogen atoms. The assignments of the signals are based on the prediction by the theoretical calculations (Table 3). 29Si{1H} NMR (THF-d8, inept) δ 19.0 (s, Si3 and Si3*). The measurement parameters were adjusted to the silicon atoms with two methyl groups.
X-Ray Data Collection, Structure Determination, and Refinement.
For details on x-ray data collection, structure determination, and refinement for compounds meso-4, dl-4, 5, and 6, see SI Materials and Methods and SI Data Set.
Computational Details.
The calculations for geometry optimizations, vibrational frequency analysis, NMR chemical shifts, Mayer's bond-order analysis, and AIM analysis were carried out with the Gaussian 03 program package (27). For further details on computations, see SI Materials and Methods.
Acknowledgments
Y.-H.L. thanks the Japan Society for the Promotion of Science for a postdoctoral fellowship. This work was supported by Ministry of Education, Science, Sports, and Culture (Japan) Grants-in-Aid for Scientific Research 15350037 and 05F05666.
Abbreviations
- AIM
atoms in molecule
- DME
1,2-dimethoxyethane
- dmpe
1,2-bis(dimethylphosphino)ethane.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference nos. 626200–626203).
This article contains supporting information online at www.pnas.org/cgi/content/full/0700450104/DC1.
References
- 1.Tsuji J. Palladium Reagents and Catalysts. Chichester, UK: Wiley; 2004. [Google Scholar]
- 2.Malleron J-L, Fiaud J-C, Legros J-Y. Handbook of Palladium-Catalyzed Organic Reactions. San Diego: Academic; 1997. [Google Scholar]
- 3.Canty AJ. Acc Chem Res. 1992;25:83–90. [Google Scholar]
- 4.Elsevier CJ. Coord Chem Rev. 1999;185/186:809–822. [Google Scholar]
- 5.Uson R, Fornies J, Navarro R. J Organomet Chem. 1975;96:307–312. [Google Scholar]
- 6.Byers PK, Canty AJ, Skelton BW, White AH. J Chem Soc Chem Commun. 1986:1722–1724. [Google Scholar]
- 7.Timakov AA, Prusakov VN, Drobyshevskii YV. Russ J Inorg Chem. 1982;27:1704–1705. [Google Scholar]
- 8.Sokolov VB, Drobyshevskii YV, Prusakov VN, Ryzhkov AV, Khoroshev SS. Proc Acad Sci USSR. 1976;229:503–505. [Google Scholar]
- 9.Chausse V, Regull P, Victori L. J Electroanal Chem. 1987;238:115–128. [Google Scholar]
- 10.Abrahams SC, Ginsberg AP, Knox K. Inorg Chem. 1964;3:558–567. [Google Scholar]
- 11.Gilbert TM, Bergman RG. Organometallics. 1983;2:1458–1460. [Google Scholar]
- 12.Fernandez MJ, Maitlis PM. J Chem Soc Chem Commun. 1982:310–311. [Google Scholar]
- 13.Fernandez MJ, Maitlis PM. Organometallics. 1983;2:164–165. [Google Scholar]
- 14.Brookhart M, Grant BE, Lenges CP, Prosenc MH, White PS. Angew Chem Int Ed. 2000;39:1676–1679. doi: 10.1002/(sici)1521-3773(20000502)39:9<1676::aid-anie1676>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Shimada S, Tanaka M. Coord Chem Rev. 2006;250:991–1011. [Google Scholar]
- 16.Shimada S, Tanaka M, Shiro M. Angew Chem Int Ed Engl. 1996;35:1856–1858. [Google Scholar]
- 17.Shimada S, Rao MLN, Tanaka M. Organometallics. 1999;18:291–293. [Google Scholar]
- 18.Chen WZ, Shimada S, Tanaka M. Science. 2002;295:308–310. doi: 10.1126/science.1067027. [DOI] [PubMed] [Google Scholar]
- 19.Crabtree RH. Science. 2002;295:288–289. doi: 10.1126/science.1067921. [DOI] [PubMed] [Google Scholar]
- 20.Sherer EC, Kinsinger CR, Kormos BL, Thompson JD, Cramer CJ. Angew Chem Int Ed. 2002;41:1953–1956. [PubMed] [Google Scholar]
- 21.Aullón G, Lledós A, Alvarez S. Angew Chem Int Ed. 2002;41:1956–1959. [PubMed] [Google Scholar]
- 22.Shimada S, Rao MLN, Hayashi T, Tanaka M. Angew Chem Int Ed. 2001;40:213–216. doi: 10.1002/1521-3773(20010105)40:1<213::AID-ANIE213>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Coe BJ, Glenwright SJ. Coord Chem Rev. 2000;203:5–80. [Google Scholar]
- 24.Mayer I. Chem Phys Lett. 1983;97:270–274. [Google Scholar]
- 25.Bader RFW. Chem Rev. 1991;91:893–928. [Google Scholar]
- 26.Corey JY, Braddock-Wilking J. Chem Rev. 1999;99:175–292. doi: 10.1021/cr9701086. [DOI] [PubMed] [Google Scholar]
- 27.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr, Vreven T, Kudin KN, Burant JC, et al. Gaussian 03. Wallingford, CT: Gaussian, Inc; 2004. Revision C.02. [Google Scholar]