Abstract
The complex cooperative behavior exhibited by wild chimpanzees generates considerable theoretical and empirical interest, yet we know very little about the mechanisms responsible for its evolution. Here, we investigate the influence of kinship on the cooperative behavior of male chimpanzees living in an unusually large community at Ngogo in Kibale National Park, Uganda. Using long-term field observations and molecular genetic techniques to identify kin relations between individuals, we show that male chimpanzees clearly prefer to affiliate and cooperate with their maternal brothers in several behavioral contexts. Despite these results, additional analyses reveal that the impact of kinship is limited; paternal brothers do not selectively affiliate and cooperate, probably because they cannot be reliably recognized, and the majority of highly affiliative and cooperative dyads are actually unrelated or distantly related. These findings add to a growing body of research that indicates that animals cooperate with each other to obtain both direct and indirect fitness benefits and that complex cooperation can occur between kin and nonkin alike.
Keywords: Pan troglodytes, genotyping, kin recognition, microsatellites, relatedness
The evolution of cooperation, among both human and nonhuman animals, remains one of the most important, unresolved questions in science (1–3). Male chimpanzee cooperation, which includes behaviors such as coalitionary aggression, hunting, sharing meat, and defending territories against males of other groups, has few parallels in the animal kingdom (4, 5). Because males typically compete for females, a resource that cannot be readily divided and shared, the complex and varied forms of male chimpanzee cooperation create a challenging puzzle that is not easily explained (5).
Cooperation in primates and other animals has frequently been attributed to kin selection, a process whereby individuals cooperate with relatives and gain indirect fitness benefits through the reproduction of kin (6–9). For example, in many Old World cercopithecine monkey species, females remain in their natal groups their entire lives and behave nepotistically toward their sisters and other maternal relatives, whereas males disperse among groups and cooperate less with each other (10). In contrast, male chimpanzees are philopatric and are much more affiliative and cooperative than female chimpanzees (11). Given these observations, it is not surprising that kin selection has historically been assumed to play a central role in the evolution of male chimpanzee cooperation (12, 13). Nevertheless, studies to date do not provide any evidence that male chimpanzees bias their social behavior toward maternal brothers (14–16), who should be readily recognizable by virtue of the life-long bonds they form with shared mothers (13).
Recent research shows that, in addition to the possibility of gaining indirect fitness benefits by cooperating with relatives, chimpanzees (16–18) and other animals may also gain substantial direct fitness benefits, increasing their own personal reproduction, by cooperating with unrelated individuals (1–3, 19). There are several ways an individual can gain direct fitness benefits through cooperation (2, 19). Two well known mechanisms are mutualism, which occurs when individuals derive immediate net fitness benefits by cooperating, and reciprocity, which takes place when one individual incurs a short-term cost from a cooperative act, but is repaid by the beneficiary at a later date (2). These processes may explain why male chimpanzees that are similar in age and dominance rank show high levels of affiliation and cooperation (16). Such individuals are likely to be familiar with one another, share similar social interests throughout their lives, and have similar access to resources and abilities to exchange them (16). Further observations indicate that, in captivity, unrelated chimpanzees engage in reciprocal bouts of grooming and trade grooming for food (18). Thus, direct rather than indirect fitness benefits may be the driving force behind chimpanzee cooperation.
To evaluate the direct and indirect fitness benefits derived by male chimpanzees who cooperate, we require information regarding the genetic relationships of these individuals. Such relationships, however, remain largely unresolved because previous studies have been limited to genetic data from mtDNA (14–16). These data are likely to furnish imprecise estimates of relatedness, because dyads that share mtDNA haplotypes can either be distant maternal relatives, e.g., first cousins, or more closely related, e.g., brothers. Furthermore, the finding that male chimpanzees bias their behavior toward age mates raises the possibility that they may be favoring paternal brothers. This could result if one or only a few males reproduce at any given time, so that age mates frequently have the same father (20). Such a scenario has been invoked to explain recent findings in rhesus macaques and baboons, where females affiliate more with their paternal sisters than they do with unrelated individuals (21–24). In sum, assessing the role of direct and indirect benefits in male chimpanzee cooperation requires information on both maternal and paternal sibling relationships.
We addressed this topic through a long-term study of wild chimpanzees at Ngogo, Kibale National Park, Uganda. We ascertained kin relationships among chimpanzees in the Ngogo community by genotyping 44 autosomal, 13 X-linked, and 13 Y-linked microsatellite loci and sequencing the first hypervariable region of the mtDNA of 142 individuals. Despite recent research showing that it is difficult to determine sibships in natural populations by using only autosomal microsatellite genotypes (25), we were able to establish maternal and paternal sibships with confidence because of the large number of loci used and the inclusion of three other types of markers with unique sex-specific inheritance patterns [see supporting information (SI) for details].
We followed individually identified adolescent and adult male chimpanzees to determine which dyads selectively affiliated and cooperated in the context of six social behaviors: associations, grooming, proximity maintenance, coalition formation, meat sharing, and joint participation in territorial boundary patrols. The unusually large size of the Ngogo chimpanzee community, with ≈150 individuals, including 12 maternal and 25 paternal sibling dyads among the 36–41 adolescent and adult males alive during the study period, permitted us to conduct robust tests of the effects of maternal and paternal sibship on male social behavior.
Results and Discussion
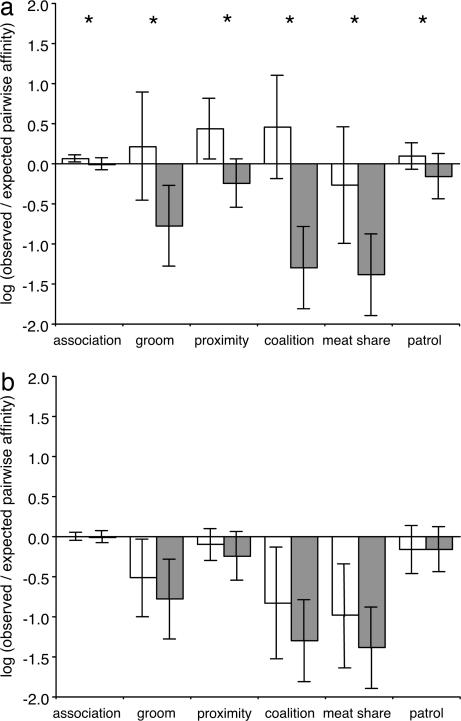
We found that male chimpanzees preferentially affiliated and cooperated with their maternal brothers (Fig. 1a). Resampling tests indicated that maternal brothers performed each of the six social behaviors significantly more often than did unrelated and distantly related dyads. These results held even when controlling for similarity in age and rank (restricted randomization two-factor ANOVAs (26); all 12 P values were less than sequential Bonferroni criteria). Thus, although male chimpanzees presumably achieve direct fitness benefits by forming mutualistic and reciprocal relationships with others (16–18), our results show that they are also likely to obtain indirect fitness benefits by selectively affiliating and cooperating with their maternal brothers.
Fig. 1.
Mean observed/expected pairwise affinity indices of maternal siblings (white bars) vs. unrelated dyads (gray bars) (a) and paternal siblings (white bars) vs. unrelated dyads (gray bars) (b). Positive values indicate that dyads performed the behavior more often than expected by chance, whereas negative values indicate that dyads engaged in the behavior less frequently than chance expectation. Error bars represent 1 SD. ∗, P < sequential Bonferroni α level assessed through a resampling test.
We also found that mtDNA haplotype sharing is a poor indicator of maternal sibship. Only 12 of 73 (16%) dyads that shared mtDNA haplotypes were confirmed as maternal brothers by using our approach combining multiple genetic systems. These data explain why prior research found that mtDNA haplotype sharing is a weak predictor of male chimpanzee social behavior (14–16).
In contrast to maternal brothers, paternal brothers did not engage in any of the six social behaviors more frequently than did unrelated and distantly related dyads (Fig. 1b). Although maternal brothers showed positive values, indicating clear biases in their behavior, both paternal brothers and unrelated and distantly related dyads consistently displayed negative mean observed/expected ratios for each of the six behaviors.
Chimpanzee paternal brothers are as closely related to each other as are maternal siblings, and it is unclear why pairs of the former fail to affiliate and cooperate like the latter. One possibility is that maternal brothers are much more abundant than paternal brothers and that, because individuals are necessarily constrained in the total number of close social relationships they can form, a bond with a maternal brother leaves an individual unavailable for interactions with a paternal brother (23, 24). However, the number of individuals who had paternal siblings but did not have any maternal siblings was higher among the Ngogo males (11 of 21 = 52%) than among females in the primate populations where paternal sibling nepotism has been documented (Cayo Santiago rhesus macaques: 8 of 51 = 15.7% A. Widdig, personal communication; Amboseli baboons: 17 of 41 = 41.5% S. C. Alberts and J. Altmann, personal communication). Furthermore, at Ngogo, dyads of paternal brothers where one or both of the individuals lacked a maternal brother did not affiliate or cooperate more frequently than unrelated and distantly related dyads (Resampling test: P > sequential Bonferroni criteria for associations and patrolling, the only two social behaviors for which sufficient data were available for all four paternal sibling dyads where one or both individuals of the dyad lacked a maternal sibling). Thus, demographic factors cannot account for the lack of nepotism among paternal brothers at Ngogo.
A second potential explanation is that male chimpanzees do not preferentially cooperate with their paternal brothers because they cannot reliably recognize them. Phenotype matching and familiarity because of age proximity are two mechanisms that primates can use to recognize their paternal siblings (27). In phenotype matching, individuals compare the phenotypic cues (such as odor) of others with the phenotypes of themselves or their known relatives to determine their kin relations. Studies conducted in captivity and in the wild have produced contradictory, but mainly negative, results regarding the ability of primates to use phenotype matching to identify paternal relatives (10, 21–24, 28–31). One study of rhesus monkeys has implicated phenotype matching by showing among members of the same age cohort, paternal sisters interacted slightly, but statistically significantly, more than did unrelated individuals in two of five social behaviors (22). Females in the same population, however, preferred to interact with their maternal sisters at rates well above chance in all five behaviors (22). Further research will be necessary to determine whether these results represent type I error, as has recently been shown to be the case in baboons. Although one study of baboons reported a weak phenotype matching effect (23), it has not been replicated in subsequent research using a much larger sample of females (24).
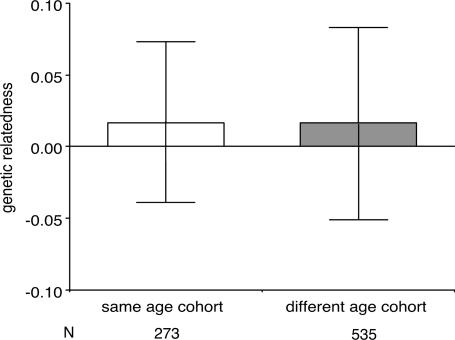
Similarly, whether age proximity can be used to identify paternal siblings remains unclear (Table 1). In the three primate populations where paternal sibling nepotism has been studied (rhesus macaques at Cayo Santiago, baboons at Amboseli, and chimpanzees at Ngogo), the majority of similarly aged dyads do not consist of paternal siblings (Table 1, second row). Age proximity may not be a reliable cue for paternal sibship in these populations because male reproductive skew at any given time is not extreme, and males produce offspring throughout their entire adult life rather than only during a narrow time window (32–36). At Ngogo, patterns of male reproduction result in a situation where members of different age cohorts are as closely related to each other as individuals of the same age cohort (Fig. 2). Additional studies are necessary to evaluate whether the patterns shown by the Cayo Santiago rhesus macaques, Amboseli baboons, and Ngogo chimpanzees are typical of these and other primate species.
Table 1.
Distribution of paternal siblings within and across age cohorts in three primate populations
| Ngogo chimpanzees | Cayo rhesus | Amboseli baboons | |
|---|---|---|---|
| No. of paternal sib dyads | 25 | 26 | 57 |
| Dyads in same age cohort that are paternal sibs (%) | 14 of 276 (5.1) | 8 of 63 (12.7) | 27 of 72 (37.5) |
| Dyads in different age cohort that are paternal sibs (%) | 11 of 544 (2.0) | 18 of 461 (3.9) | 30 of 432 (6.9) |
| Times more likely that members of the same age cohort are paternal sibs than are members of different age cohorts: | 5.1%/2.0% = 2.5 | 12.7%/3.9% = 3.3 | 37.5%/6.9% = 5.4 |
Members of the same age cohort have an age difference of <5 years in chimpanzees, 6 months in rhesus, and 1 year in baboons. Baboon data are from S. Alberts and J. Altmann, personal communication; rhesus data are from A. Widdig, personal communication.
Fig. 2.
Mean genetic relatedness of dyads belonging to the same (white bars) and different (gray bars) age cohorts. Error bars represent 1 SD. A resampling test indicated that the means do not differ. Twelve maternal sibling dyads were excluded from this analysis.
Taken together, these results suggest that primates may affiliate and cooperate with members of the same age cohort to obtain direct, rather than indirect, fitness benefits. Although rhesus macaque and baboon females bias their behavior significantly more to their paternal sisters than to nonrelatives, such biases are not nearly as strong as those displayed toward maternal sisters (21–24). This point, our finding that male chimpanzees do not preferentially interact with their paternal brothers, and the lack of a reliable paternal sibling identification mechanism in primates suggest that paternal kin effects may arise as a byproduct of individuals maximizing their own fitness by cooperating with age mates, who only sometimes happen to be paternal siblings.
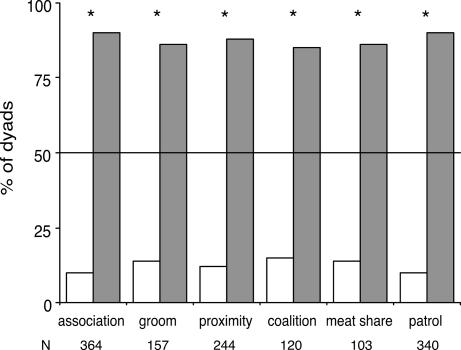
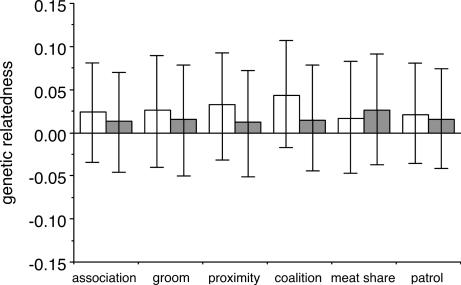
Although prior studies of nepotism in monkeys may have underestimated the direct fitness benefits obtained by animals, our data show that male chimpanzees cooperate to obtain direct, as well as indirect, benefits. We determined the number of male pairs that performed each of the six behaviors at levels above chance expectation. For each of the six behaviors, there were many more pairs of males that cooperated above chance expectation than there were closely related pairs. Because of this, individuals that affiliated and cooperated at levels exceeding chance were more likely to be unrelated or distantly related than they were to be closely related (Fig. 3). Furthermore, the mean genetic relatedness between individuals who engaged in behaviors more often than expected by chance did not exceed that of dyads who displayed the behaviors less often (Fig. 4). Collectively, these results show that most affiliative and cooperative behavior between males at Ngogo can be explained by individuals striving to maximize their direct rather than their indirect fitness.
Fig. 3.
Percentage of closely related dyads (white bars) and distantly related dyads (gray bars) that engaged in social behaviors more often than expected by chance. Percentages sum to 100 for each of the six social behaviors. ∗, P < sequential Bonferroni α level assessed through a resampling test.
Fig. 4.
Mean genetic relatedness of dyads that performed affiliative and cooperative behaviors more often than expected by chance (white bars) and dyads that engaged in the behaviors less often (gray bars). Error bars represent 1 SD. A resampling test indicated no difference between the mean values.
In sum, male chimpanzees show clear and consistent biases in their social behavior toward maternal brothers, confirming the importance of maternal kinship in structuring patterns of behavior in a primate species where males rather than females routinely spend their lives in their natal group. But although kinship is important, its impact is not widespread; paternal brothers do not preferentially interact, and males in the majority of highly affiliative and cooperative dyads are unrelated or distantly related. These results add to a growing body of research indicating that direct as well as indirect fitness benefits are important in explaining cooperation among animals (18, 19).
Materials and Methods
Detailed descriptions of laboratory methods and analytical procedures for identifying maternal and paternal siblings are provided in SI.
Behavioral Observations.
Adolescent and adult male chimpanzees were observed by J.C.M. at the Ngogo study site (37) for a total of 20 months between 1999 and 2005. Observations of social behavior were conducted during hour-long samples of target males. During each observation period, scan samples were made at 10-min intervals to record which individuals were in proximity (≤5 min) or grooming (either giving or receiving) with the target. Associations between male subjects were recorded on a daily basis. Male participation in coalitions, meat-sharing, and boundary patrols was recorded ad libitum. Coalitions (n = 753) between two males were defined to occur whenever they directed aggression together toward others (16). Meat sharing (n = 421) was recorded during hunting episodes of mammalian prey (38). We also recorded adult male participation in territorial boundary patrols (n = 93) (39). Analyses of social behavior were based on 5,025 h of observations (median = 139 h, n = 41 males). Thirty-two males were present during the entire 7 years of study. Nine other males were included in observations after they matured and reached adolescence.
Assessing Age and Rank Similarity of Dyads.
We used standard morphological and behavioral criteria (13) to estimate the ages of individuals and classify dyads as belonging to the same (≤5 years age difference) or different (>5 years age difference) age categories. As most of the adolescent and adult males were born before study of the Ngogo chimpanzees began in 1995, many of their exact ages are unknown. Therefore, to determine how confident we could be in assigning dyads to either the same or a different age category, we assigned each individual a minimum and maximum possible age. For each dyad we examined the overlap in each of their age ranges, and found that we could confidently assign a dyad to the same or different age category a majority of times (592 of 820 dyads = 72%).
To assess the dominance rank similarity of dyads, we recorded the direction of pant grunts, a formal signal of submission given by low ranking individuals to high ranking animals (40, 41). We then used Version 1.0 of the MatMan software package (42) to assign dominance ranks to males in each of the 7 years of study. MatMan evaluates the linearity of a dominance hierarchy based on Landau's index (43) and implements an iterative procedure to rank individuals in a way to minimize the number and strength of inconsistencies between them (44, 45). We used yearly ranks to compute an average rank for each male over the seven-year study period and then ordered these average ranks from highest to lowest. We split this ordered list into thirds, classifying males as high-, medium-, or low-ranking. The rank similarity of male pairs was assessed by using these categories.
Statistical Analyses.
To assay whether individuals preferentially associate, maintain proximity, groom, form coalitions, share meat, and patrol their territory, we computed pairwise affinity indices between male dyads for each of these six social behaviors. Numerically this index is:
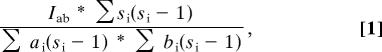 |
where Iab is the number of appearances of a and b together, ai is the number of appearances of a, bi is the number of appearances of b, and si is the size of group i. A particularly useful feature of this index is that it factors out each individual's general tendency to interact. As a result, it reflects only the interaction intensity that is specific to a particular dyad, rather than being generic to either individual's behavior (46).
Before using these observed indices, we normalized them by dividing by their expected values under the null hypothesis that social behaviors were generic rather than dyad-specific. We produced these expected values using a randomization technique. This procedure repeatedly reshuffled the membership of observed groups while retaining both the observed number of appearances of each individual and the observed distribution of group sizes. After each randomization, the pairwise affinity index was calculated for each dyad, and these randomized values were averaged to generate an expected value for each dyad. We performed 10,000 randomizations to generate null expectations. We log transformed the observed/expected ratios to ensure that dyadic interactions above and below expected levels would have equal weight. To avoid undefined values resulting from observed or expected values of zero, we truncated the range of the log transformed values to −2 ≤ x ≤ 2, corresponding to a floor of 0.01 and a ceiling of 100 for observed/expected ratios.
We conducted a series of pairwise comparisons to examine whether categories of genetic relationships affected patterns of male social behavior. We first calculated the mean log transformed observed/expected ratios of pairwise affinity indices for the maternal sibling, paternal sibling, and unrelated dyads. Because some of the younger males in our sample attained adolescence during the study period, we did not have sufficient data to characterize the pairwise affinity for all of the 820 possible dyads involving the 41 adolescent and adult males in our sample. For the most frequently observed social behaviors, association and patrolling, we calculated means using all 12 maternal sibling dyads, all 25 paternal sibling dyads, and 716 of 770 unrelated dyads. For the other four less-frequently observed social behaviors, we calculated means using 11 of 12 maternal sibling dyads, 18 of 25 paternal sibling dyads, and 460 of 770 unrelated dyads. The eight dyads involving RI and TY with BF, CT, DO, and WB were among those excluded when calculating the mean for the unrelated dyads for both sets of social behaviors, as we could not conclusively determine whether these dyads were paternal siblings or unrelated (see SI).
We computed the mean values of maternal siblings, paternal siblings, and unrelated dyads, using the difference between the means of related and unrelated dyads as test statistics. We evaluated test statistics relative to an expected frequency distribution generated by resampling the values in the original data. To produce these expected distributions, we re-shuffled pairwise affinity indices into the two categories of interest, maternal or paternal sibling dyads and unrelated pairs. Each resampled category included the same number of observations as there were in the original data. We computed the means of both categories in the resampled data and used the difference between these two values as a single data point. We repeated this process 10,000 times to produce an expected frequency distribution. We compared test statistics to these expected distributions to evaluate the null hypothesis that siblings did not show greater social affinity with each other than did individuals in unrelated dyads.
We used the resampling procedure to conduct four families of statistical tests comparing (i) maternal half-siblings to unrelated dyads, (ii) paternal half-siblings to unrelated dyads, (iii) maternal siblings to unrelated dyads controlling for age similarity, and (iv) maternal siblings to unrelated dyads controlling for rank similarity. The third and fourth families of tests were conducted through restricted randomization two-factor ANOVAs (26). This procedure is similar to that described above, but when creating the expected distribution through the reshuffling of pairwise affinity indices between maternal sibling and unrelated dyads, values were reshuffled in the same age or rank similarity category.
To determine the extent to which indirect and direct benefits influence patterns of affiliation and cooperation among male chimpanzees, we examined the number of related and unrelated pairs that engaged in each of the six social behaviors more than expected by chance. For each of the six social behaviors, we first calculated the top 2.5% of the distributions generated by group randomization to identify dyads that performed that behavior more frequently than chance expectation. We then computed the percentage of these dyads that were closely related (i.e., maternal siblings, paternal siblings, father–son pairs) and unrelated and distantly related. We then used a resampling procedure to test whether these percentages deviated significantly from 50/50. We also used the autosomal loci to calculate the mean genetic relatedness (47) between dyads that performed each of the six behaviors more frequently than chance expectation and between dyads that did not. We used the resampling approach described above to test whether these mean values differed. We used this same procedure to compare the mean genetic relatedness among dyads belonging to the same and different age cohorts. Because chimpanzees can presumably readily identify their maternal siblings (13), we excluded the 12 maternal sibling dyads from this analysis.
Each family of tests included six comparisons, involving associations, grooming, proximity, coalitions, meat sharing, and patrols. When making these multiple comparisons, we adjusted α levels with the sequential Bonferroni technique to correct for the increased probability of committing type I error (48).
This research was approved by the Unit for Laboratory Animal Medicine of the University of Michigan.
Acknowledgments
We thank J. Lwanga, G. Isabirye-Basuta, J. Kasenene, and the staff of the Makerere University Biological Field Station for logistical support; A. Magoba, G. Mbabazi, L. Ndagizi, H. Sherrow, A. Tumusiime, M. Wakefield, and D. Watts for help with sample collection at Ngogo; R. W. Wrangham (Harvard University, Cambridge, MA) and M. N. Muller (Boston University, Boston, MA) for providing Kanyawara samples; A. Abraham, M. Arandjelovic, R. Bergl, B. Bradley, C. Lang, C. Rowney, and H. Siedel for assistance in the laboratory; and C. Boesch, D. Lukas, S. Pääbo, M. Sobolewski, and A. Widdig for discussions and comments on earlier versions of this manuscript. Field research in Uganda was sponsored by the Uganda Wildlife Authority and the Uganda National Council of Science and Technology. Research at Ngogo was funded by U.S. National Science Foundation Grants BCS-0215622 and IOB-0516644, the National Sciences and Engineering Research Council of Canada, the L. S. B. Leakey Foundation, the Wenner–Gren Foundation, the Max Planck Society, the University of Michigan, and the Detroit Zoological Institute. Funding for collection of Kanyawara samples was provided by U.S. National Science Foundation Grant BCS-0416125 and a grant from the L. S. B. Leakey Foundation (to R. Wrangham).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611449104/DC1.
References
- 1.Sachs J, Mueller U, Wilcox T, Bull J. Q Rev Biol. 2004;88:135–160. doi: 10.1086/383541. [DOI] [PubMed] [Google Scholar]
- 2.Dugatkin L. Cooperation Among Animals. New York: Oxford Univ Press; 1997. [Google Scholar]
- 3.Nowak M. Science. 2006;314:1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boesch C, Boesch-Achermann H. The Chimpanzees of the Tai Forest: Behavioural Ecology and Evolution. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 5.Muller MN, Mitani JC. Adv Study Behav. 2005;35:275–331. [Google Scholar]
- 6.Hamilton WD. J Theoret Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 7.Boyd R. Science. 2007;314:1555–1556. doi: 10.1126/science.1136841. [DOI] [PubMed] [Google Scholar]
- 8.Fehr E, Fischbacher U. Trends Cogn Sciences. 2004;8:185–190. doi: 10.1016/j.tics.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Henrich J, Henrich N. Cogn Sys Res. 2006;7:220–245. [Google Scholar]
- 10.Silk JB. Int J Primatol. 2002;23:849–875. [Google Scholar]
- 11.Mitani JC, Watts DP, Muller MN. Evol Anthropol. 2002;11:9–25. [Google Scholar]
- 12.Morin PA, Moore JJ, Chakraborty R, Jin L, Goodall J, Woodruff DS. Science. 1994;265:1193–1201. doi: 10.1126/science.7915048. [DOI] [PubMed] [Google Scholar]
- 13.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard Univ Press; 1986. [Google Scholar]
- 14.Goldberg TL, Wrangham RW. Anim Behav. 1997;54:559–570. doi: 10.1006/anbe.1996.0450. [DOI] [PubMed] [Google Scholar]
- 15.Mitani JC, Merriwether DA, Zhang C. Anim Behav. 2000;59:885–893. doi: 10.1006/anbe.1999.1389. [DOI] [PubMed] [Google Scholar]
- 16.Mitani JC, Watts DP, Pepper J, Merriwether DA. Anim Behav. 2002;64:727–737. [Google Scholar]
- 17.Mitani JC. In: Cooperation in Primates: Mechanisms and Evolution. Kappeler P, van Schaik C, editors. Berlin: Springer; 2005. pp. 101–113. [Google Scholar]
- 18.de Waal F, Brosnan S. In: Cooperation in Primates: Mechanisms and Evolution. Kappeler P, van Schaik C, editors. Berlin: Springer; 2005. pp. 85–105. [Google Scholar]
- 19.Clutton-Brock T. Science. 2002;296:69–72. doi: 10.1126/science.296.5565.69. [DOI] [PubMed] [Google Scholar]
- 20.Altmann J. Behav Ecol Sociobiol. 1979;6:161–164. [Google Scholar]
- 21.Widdig A, Nürnberg P, Krawkzac M, Streich WJ, Bercovitch FB. Proc Natl Acad Sci USA. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widdig A, Nürnberg P, Krawkzac M, Streich WJ, Bercovitch FB. Behaviour. 2002;139:371–391. [Google Scholar]
- 23.Smith K, Alberts SC, Altmann J. Proc R Soc London Ser B. 2003;270:503–510. doi: 10.1098/rspb.2002.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silk JB, Altmann J, Alberts SC. Behav Ecol Sociobiol. 2006;61:183–195. [Google Scholar]
- 25.Csilléry K, Johnson T, Beraldi D, Clutton-Brock T, Coltman D, Hansson B, Spong G, Pemberton JM. Genetics. 2006;173:2091–2101. doi: 10.1534/genetics.106.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgington ES. Randomization Tests. New York: Dekker; 1995. [Google Scholar]
- 27.Holmes WG, Sherman PW. Am Sci. 1983;71:46–55. [Google Scholar]
- 28.Alberts SC. Proc R Soc London Ser B. 1999;266:1501–1506. doi: 10.1098/rspb.1999.0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchan JC, Alberts SC, Silk JB, Altmann J. Nature. 2003;425:179–181. doi: 10.1038/nature01866. [DOI] [PubMed] [Google Scholar]
- 30.Parr LA, de Waal FBM. Nature. 1999;399:647–648. doi: 10.1038/21345. [DOI] [PubMed] [Google Scholar]
- 31.Charpentier MJE, Peignot P, Hossaert-McKey M, Wickings EJ. Anim Behav. 2007;73:37–45. [Google Scholar]
- 32.Widdig A, Bercovitch FB, Jürgen Streich W, Sauermann U, Nürnberg P, Krawczak M. Proc R Soc London Ser B. 2004;271:819–826. doi: 10.1098/rspb.2003.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altmann J, Alberts SC, Haines SA, Dubach J, Muruthi P, Coote T, Geffen E, Cheesman DJ, Mututua RS, Saiyalel SN, et al. Proc Natl Acad Sci USA. 1996;93:5797–5801. doi: 10.1073/pnas.93.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constable J, Ashley M, Goodall J, Pusey A. Mol Ecol. 2001;10:1279–1300. doi: 10.1046/j.1365-294x.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- 35.Vigilant L, Hofreiter M, Siedel H, Boesch C. Proc Natl Acad Sci USA. 2001;98:12890–12895. doi: 10.1073/pnas.231320498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boesch C, Kohou G, Nene H, Vigilant L. Am J Phys Anth. 2006;130:103–115. doi: 10.1002/ajpa.20341. [DOI] [PubMed] [Google Scholar]
- 37.Struhsaker T. Ecology of an African Rainforest. Gainesville, FL: Univ Press of Florida; 1997. [Google Scholar]
- 38.Mitani JC, Watts DP. Anim Behav. 2001;61:915–924. [Google Scholar]
- 39.Mitani JC, Watts DP. Anim Behav. 2005;70:1079–1086. [Google Scholar]
- 40.Bygott JD. In: The Great Apes. Hamburg D, McCown E, editors. Menlo Park, CA: Benjamin Cummings; 1979. pp. 405–427. [Google Scholar]
- 41.Hayaki H, Huffman M, Nishida T. Primates. 1989;30:187–197. [Google Scholar]
- 42.de Vries H, Netto W, Hanegraaf P. Behaviour. 1993;125:157–175. [Google Scholar]
- 43.Landau HG. Bull Math Biophys. 1951;13:1–19. [Google Scholar]
- 44.de Vries H. Anim Behav. 1995;50:1375–1389. [Google Scholar]
- 45.de Vries H. Anim Behav. 1998;55:827–843. doi: 10.1006/anbe.1997.0708. [DOI] [PubMed] [Google Scholar]
- 46.Pepper JW, Mitani JC, Watts DP. Int J Primatol. 1999;20:613–632. [Google Scholar]
- 47.Wang J. Mol Ecol. 2004;13:3169–3178. doi: 10.1111/j.1365-294X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 48.Holm S. Scand J Stat. 1979;6:65–70. [Google Scholar]