Abstract
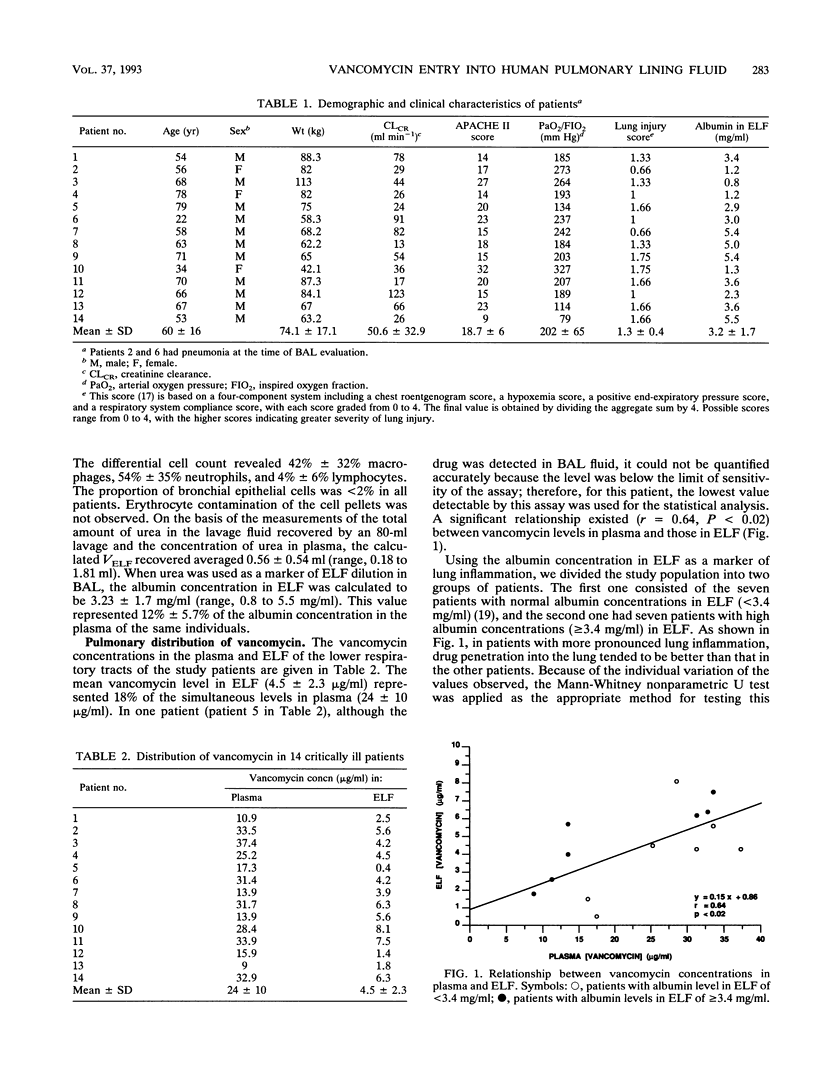
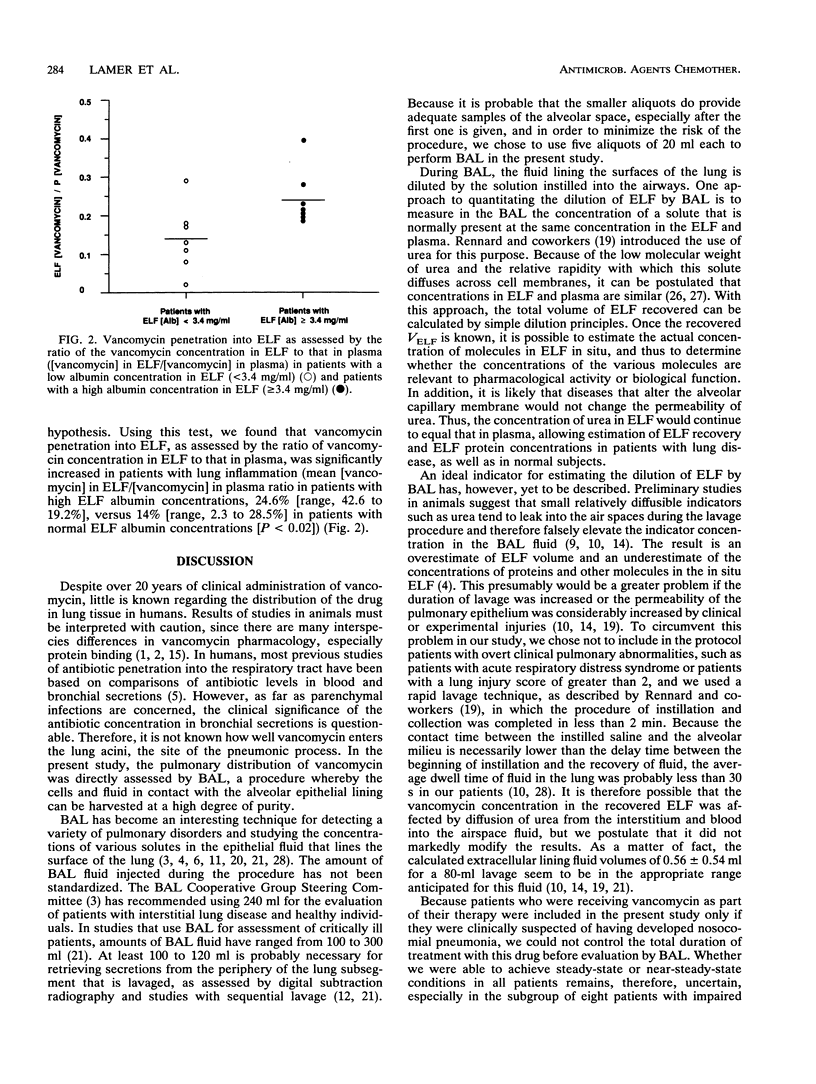
Vancomycin penetration into the fluid lining the epithelial surface of the lower respiratory tract was studied by performing fiberoptic bronchoscopy with bronchoalveolar lavage on 14 critically ill, ventilated patients who had received the drug for at least 5 days. The apparent volume of epithelial lining fluid (ELF) recovered by bronchoalveolar lavage was determined by using urea as an endogenous marker. Vancomycin levels in ELF ranged from 0.4 to 8.1 micrograms/ml (mean, 4.5 micrograms/ml), while the mean simultaneous level of the drug in plasma was 24 micrograms/ml (range, 9 to 37.4 micrograms/ml). There was a significant relationship (r = 0.64, P < 0.02) between vancomycin levels in plasma and those in ELF, with a correlation whose slope (0.15) indicated that the blood-to-ELF ratio of drug penetration was 6:1. Using the albumin concentration in ELF as a marker of lung inflammation, we found that vancomycin penetration was higher in patients with ELF albumin values of > or = 3.4 mg/ml than in patients with normal values (< 3.4 mg/ml) (P < 0.02). These results suggest that the vancomycin distribution includes the ELF of the lower respiratory tract at a concentration that is dependent upon the levels in blood and the alveolar capillary membrane protein permeability. These concentrations were well above the MICs for most staphylococci and enterococci.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht L. M., Rybak M. J., Warbasse L. H., Edwards D. J. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP. 1991 Jul-Aug;25(7-8):713–715. doi: 10.1177/106002809102500701. [DOI] [PubMed] [Google Scholar]
- Bailey E. M., Rybak M. J., Kaatz G. W. Comparative effect of protein binding on the killing activities of teicoplanin and vancomycin. Antimicrob Agents Chemother. 1991 Jun;35(6):1089–1092. doi: 10.1128/aac.35.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D. R., Honeybourne D., Wise R. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob Agents Chemother. 1992 Jun;36(6):1171–1175. doi: 10.1128/aac.36.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastre J., Brun P., Fourtillan J. B., Soler P., Basset G., Manuel C., Trouillet J. L., Gibert C. Pulmonary disposition of roxithromycin (RU 28965), a new macrolide antibiotic. Antimicrob Agents Chemother. 1987 Sep;31(9):1312–1316. doi: 10.1128/aac.31.9.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastre J., Viau F., Brun P., Pierre J., Dauge M. C., Bouchama A., Akesbi A., Gibert C. Prospective evaluation of the protected specimen brush for the diagnosis of pulmonary infections in ventilated patients. Am Rev Respir Dis. 1984 Nov;130(5):924–929. doi: 10.1164/arrd.1984.130.5.924. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Feng N. H., Mason G., Sietsema K., Silverman P., Hukkanen J. Solute concentrations of the pulmonary epithelial lining fluid of anesthetized rats. J Appl Physiol (1985) 1990 Jan;68(1):275–281. doi: 10.1152/jappl.1990.68.1.275. [DOI] [PubMed] [Google Scholar]
- Feng N. H., Hacker A., Effros R. M. Solute exchange between the plasma and epithelial lining fluid of rat lungs. J Appl Physiol (1985) 1992 Mar;72(3):1081–1089. doi: 10.1152/jappl.1992.72.3.1081. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Kelly C. A., Kotre C. J., Ward C., Hendrick D. J., Walters E. H. Anatomical distribution of bronchoalveolar lavage fluid as assessed by digital subtraction radiography. Thorax. 1987 Aug;42(8):624–628. doi: 10.1136/thx.42.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- Marcy T. W., Merrill W. W., Rankin J. A., Reynolds H. Y. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jun;135(6):1276–1280. doi: 10.1164/arrd.1987.135.6.1276. [DOI] [PubMed] [Google Scholar]
- May D. G., Stratton C. W., Denney W. D., Watts F. L., Bernard G. R., Branch R. A. Vancomycin entry into lung lymph in sheep. Antimicrob Agents Chemother. 1987 Nov;31(11):1689–1691. doi: 10.1128/aac.31.11.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans A. A model of cefoperazone tissue penetration: diffusion coefficient and protein binding. Antimicrob Agents Chemother. 1992 Feb;36(2):295–298. doi: 10.1128/aac.36.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. F., Matthay M. A., Luce J. M., Flick M. R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988 Sep;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Nagarajan R. Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob Agents Chemother. 1991 Apr;35(4):605–609. doi: 10.1128/aac.35.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Crystal R. G. Fibronectin in human bronchopulmonary lavage fluid. Elevation in patients with interstitial lung disease. J Clin Invest. 1982 Jan;69(1):113–122. doi: 10.1172/JCI110421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jan;135(1):250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenzer K. S., Wang C. H., Anhalt J. P. Automated fluorescence polarization immunoassay for monitoring vancomycin. Ther Drug Monit. 1983;5(3):341–345. doi: 10.1097/00007691-198309000-00017. [DOI] [PubMed] [Google Scholar]
- Stevens R. M., Teres D., Skillman J. J., Feingold D. S. Pneumonia in an intensive care unit. A 30-month experience. Arch Intern Med. 1974 Jul;134(1):106–111. [PubMed] [Google Scholar]
- TAYLOR A. E., GUYTON A. C., BISHOP V. S. PERMEABILITY OF THE ALVEOLAR MEMBRANE TO SOLUTES. Circ Res. 1965 Apr;16:353–362. doi: 10.1161/01.res.16.4.353. [DOI] [PubMed] [Google Scholar]
- Tawara S., Matsumoto S., Kamimura T., Goto S. Effect of protein binding in serum on therapeutic efficacy of cephem antibiotics. Antimicrob Agents Chemother. 1992 Jan;36(1):17–24. doi: 10.1128/aac.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore J., Robin E. D., Gaudio R., Acevedo J. Transalveolar transport of large polar solutes (sucrose, inulin, and dextran). Am J Physiol. 1975 Oct;229(4):989–996. doi: 10.1152/ajplegacy.1975.229.4.989. [DOI] [PubMed] [Google Scholar]
- Valeyre D., Soler P., Basset G., Loiseau P., Pre J., Turbie P., Battesti J. P., Georges R. Glucose, K+, and albumin concentrations in the alveolar milieu of normal humans and pulmonary sarcoidosis patients. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1096–1101. doi: 10.1164/ajrccm/143.5_Pt_1.1096. [DOI] [PubMed] [Google Scholar]



