Abstract
Targeting of the Hsp function in tumor cells is currently being assessed as potential anticancer therapy. An improved understanding of the molecular signals that trigger or attenuate the stress protein response is essential for advances to be made in this field. The present study provides evidence that the membrane fluidizer benzyl alcohol (BA), a documented nondenaturant, acts as a chaperone inducer in B16(F10) melanoma cells. It is demonstrated that this effect relies basically on heat shock transcription factor 1 (HSF1) activation. Under the conditions tested, the BA-induced Hsp response involves the up-regulation of a subset of hsp genes. It is shown that the same level of membrane fluidization (estimated in the core membrane region) attained with the closely analogous phenethyl alcohol (PhA) does not generate a stress protein signal. BA, at a concentration that activates heat shock genes, exerts a profound effect on the melting of raft-like cholesterol-sphingomyelin domains in vitro, whereas PhA, at a concentration equipotent with BA in membrane fluidization, has no such effect. Furthermore, through the in vivo labeling of melanoma cells with a fluorescein labeled probe that inserts into the cholesterol-rich membrane domains [fluorescein ester of polyethylene glycol-derivatized cholesterol (fPEG-Chol)], we found that, similarly to heat stress per se, BA, but not PhA, initiates profound alterations in the plasma membrane microdomain structure. We suggest that, apart from membrane hyperfluidization in the deep hydrophobic region, a distinct reorganization of cholesterol-rich microdomains may also be required for the generation and transmission of stress signals to activate hsp genes.
Keywords: molecular chaperones, stress signaling, membrane defects, rafts, cancer therapy
There is a growing body of evidence linking the cellular response to heat stress to changes in the lipid composition and architecture of membranes. This “membrane sensor” hypothesis predicts that, besides protein denaturation, a stress protein (Hsp) signal may also originate from the cellular membranes (1). It has been proposed that, rather than the overall changes in the physical state of membranes per se, the appearance of specific microdomains, locally formed nonbilayer structures, or changes in the composition of particular lipid molecular species involved directly in specific lipid–protein interactions are potentially and equally able to furnish stimuli for the activation or attenuation of heat shock genes (2, 3).
As recently reviewed, a membrane-associated Hsp-response-refining signal can be related to the altered operation of various membrane-localized receptor proteins, transmitters, lipases, or other molecules (4). The plasma membrane, which is the barrier to the external environment and well suited for sensing thermal stress, may thereby act as an important regulatory interface. Hence, even subtle abnormalities in the lipid phase of surface membranes, caused by aging or pathophysiological conditions, could seriously influence membrane-initiated signaling processes related to Hsp expression (5). In line with this concept, pharmacological methods are applicable for the correction of membrane defects and hence for normalization of the dysregulated stress protein response in such prominent disease states as type 2 diabetes or cancer (4, 5). Therapies that can up-regulate the formation and the concomitant surface membrane expression of Hsp70, with the simultaneous down-regulation of Hsp25/27, might result in the suppression of tumor growth and abrogate the metastatic potential of the tumor in melanoma (6).
Our understanding of the plasma membrane has changed considerably as our knowledge of lipid microdomains has deepened. Favorable interactions between cholesterol and saturated lipids are known to result in the formation of patches of liquid-ordered (Lo) or raft domains within the liquid-disordered (Ld) membranes (7, 8). Specific signaling proteins are targeted to or concentrated in rafts in consequence of the greater solubility of their lipid anchors in Lo than in Ld compartments. Because the raft structure is also dependent on the thermally controlled lipid-phase behavior, we assumed that even mild changes in temperature could result in a fundamentally altered solubility and consequently in the redistribution and activity of potential stress-sensing/signaling proteins in the rafts (4, 5). We recently furnished evidence that the drug candidate bimoclomol, its analogs, and the widely used membrane fluidizer benzyl alcohol (BA) are all capable of activating cellular Hsp formation without causing measurable protein denaturation (9–13).
The precise mechanism of BA-induced stress protein signaling through membrane perturbation, however, remained unclear. It was shown that various membrane intercalators possessing a small polar head group as a common denominator, such as palmitoyl ceramide or hexadecanol (14), not only give rise to bulk membrane hyperfluidization but also possess the ability to displace cholesterol from preexisting sterol/sphingomyelin microdomains. It is noteworthy that a membrane intercalator molecule such as deoxycholic acid was also found to activate raft-associated growth factor receptors in a ligand-independent manner (15). Overall, the importance of cholesterol as a key component of the regulation of signal transduction through membrane lipid-ordered microdomains is well established (5, 8).
In the present work, we show that the induction of hsp genes by BA in B16(F10) mouse melanoma cells is mediated by heat shock transcription factor 1 (HSF1). Studies on the transcriptional regulation of selected Hsps demonstrate that heat stress and BA stress probably operate through both common and different sensing and signaling mechanisms. The membrane fluidity elevation attained with phenethyl alcohol (PhA), which is closely related in structure to BA, revealed that hyperfluidization at the core membrane domain is not sufficient for the generation of a stress protein signal in B16(F10) cells. When a fluorescence quenching method was used (16), it was observed that BA, at a concentration activating heat shock genes, profoundly affects the properties of raft-like cholesterol-sphingomyelin domains in vitro. We have provided direct evidence that both heat stress and BA treatment induce a characteristic reorganization of cholesterol-rich membrane domains detectable with the nontoxic fluorescein ester of polyethylene glycol-derivatized cholesterol (fPEG-Chol) probe (17) in vivo. We suggest that disruption and concomitant reorganization of the cholesterol-lipid interactions may induce the formation of plasma membrane domains which play a critical role in the generation of a primary membrane-associated stress signal.
Results
Heat Shock Genes Are Differentially Induced in a Stimulus-Specific Manner.
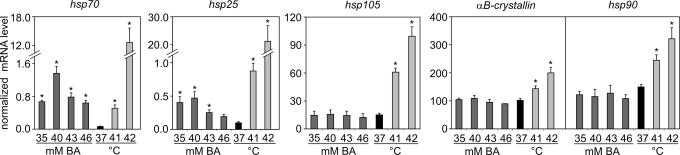
It is known from our previous work that membrane fluidization by BA enhances the de novo synthesis of Hsp70 protein in K562 cells (12). In the present study, the effects of BA on the expression of hsp genes were studied in the melanoma cell line B16(F10). To follow the initial expression changes, the mRNA levels of hsp70, hsp25, αB-crystallin, hsp90, and hsp105 were determined immediately after exposure of the cells to increasing concentrations of BA (35–46 mM) or subjection to heat (41°C or 42°C) (Fig. 1). BA treatment elevated the levels of hsp70 and hsp25 mRNAs, which peaked at 40 mM. The amount of hsp70 was higher than that of hsp25 in BA-treated cells, which was the opposite after mild (41°C) heat exposure (i.e., less hsp70 than hsp25). Interestingly, the expressions of other hsp family members were not increased in the BA-treated samples under the same conditions. Overall, the membrane fluidizer BA provoked a distinct stress protein response at the growth temperature, manifested by elevations of the mRNA levels of only two hsp classes. It should be mentioned that both heat and BA priming gave rise to the development of cellular thermotolerance (E.N., G.B., A.M., I.H., and L.V., unpublished work).
Fig. 1.
Effects of the membrane fluidizer BA on hsp gene expression. B16(F10) cells were treated with BA at the indicated concentrations and were exposed to heat or left untreated (37°C) for 1 h. Hsp expression was followed immediately after the treatments by quantitative real-time RT-PCR. The amounts of hsp70, hsp25, hsp105, αB-crystallin, and hsp90 mRNAs were determined and normalized to 103 β-actin. The data shown are mean values ± SEM. ∗, P < 0.05 compared with control as analyzed by Student's unpaired t test with the Bonferroni adjustment, n = 3−15.
Activation of HSF1 by Heat and BA Treatment.
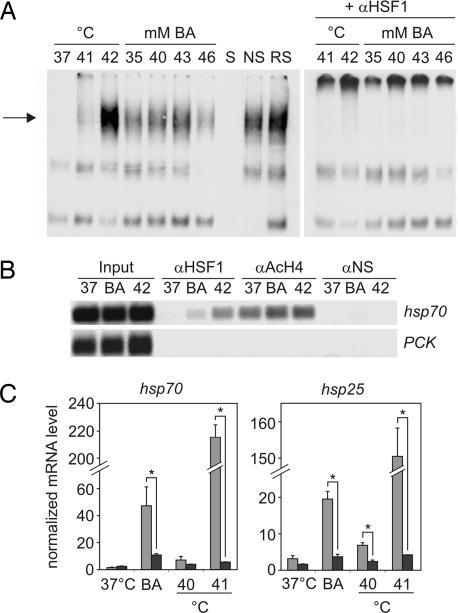
Because HSF1 is the major transcription factor regulating hsp transcription (18), we examined the involvement of HSF1 in BA-triggered hsp expression. The heat shock element (HSE)-binding activity was analyzed by EMSA in extracts of cells treated with different concentrations of BA or exposed to heat stress (Fig. 2A). Similarly to heat, BA treatment stimulated formation of the HSE–HSF complex. Perturbation with HSF1 antibody supershifted the complexes formed in both the BA- and heat-treated samples, but HSF2 (data not shown) did not exhibit such an effect. This finding indicated that HSF1 acquired DNA-binding ability upon the administration of BA. The in vivo binding of HSF1 to the mouse proximal hsp70.1 promoter was studied by ChIP in B16(F10) cells treated with BA (43 mM) or heat-stressed at 42°C (Fig. 2B). After BA treatment, HSF1 was detected on the hsp70 promoter, but a more marked binding was observed during heat treatment. This finding is in agreement with the hsp expression data showing that heat stress at 42°C induces a much higher level of hsp70 mRNA than does 43 mM BA treatment (Fig. 1).
Fig. 2.
Analysis of the role of HSF1 in BA-triggered hsp induction. (A) HSF–HSE-binding activity was studied by EMSA and by HSF1 antibody perturbation assay in extracts of B16(F10) cells treated with BA subjected to heat or left untreated (37°C) for 1 h as indicated. The arrow indicates the position of the HSE–HSF complex. To ascertain the specificity of bindings, extracts of 40 mM BA-treated cells were coincubated with cold specific (S) or nonspecific (NS) oligonucleotides or with normal rabbit serum (RS). The data are representative of three independent experiments with similar results. (B) ChIP analysis of the recruitment of HSF1 (αHSF1) to the hsp70.1 promoter during control (37), 43 mM BA (BA), or 42°C heat shock (42) treatments. Nonspecific antibody (αNS) served as negative control, whereas acetylated histone H4 (αAcH4) served as positive control. The input represents 1% of the material used in the ChIP assay. HSF1 was not recruited to the phosphoenolpyruvate carboxykinase (PCK) promoter. Data are representatives of three independent experiments. (C) Hsf1+/+ (light gray bars) and Hsf1−/− (dark gray bars) MEF cells were treated with 30 mM BA and exposed to heat (40°C or 41°C) or left untreated (37°C) for 1 h. The levels of expression of hsp70 and hsp25 mRNAs were analyzed by quantitative real-time RT-PCR, and mRNA quantities were normalized to 103 β-actin. Mean values ± SD are presented. ∗, P < 0.05, as analyzed by Student's unpaired t test, n = 3.
Induction of hsp Genes by BA Is Mediated by HSF1.
To verify the essential role of HSF1 in the BA-induced stress response, hsp induction was compared in Hsf1−/− and Hsf1+/+ mouse embryonic fibroblast (MEF) cells (19). The expressions of the hsp70 and hsp25 genes were determined in 30 mM BA- or heat- (40°C or 41°C) treated samples by quantitative real-time RT-PCR (Fig. 2C). Both hsp70 and hsp25 were induced by BA treatment in Hsf1+/+ cells. However, in Hsf1−/− cells, the induction of hsp70 and hsp25 was severely impaired after both treatments. It is noteworthy that a small but definite increase of mRNAs was still present in Hsf1−/− cells after both treatments. Taken together, these data indicate that, as shown for heat stress, the BA-induced hsp expression is basically HSF1-mediated.
Comparative Analysis of the Promoter Region of Heat-Inducible hsp70 Gene.
In a search for potential BA-specific regulatory elements in BA-activated hsp70 transcription, chloramphenicol acetyltransferase (CAT) reporter plasmids containing different fragments of the rat hsp70.1 promoter (20) were transiently transfected to B16(F10) cells [supporting information (SI) Methods]. CAT activities were determined after 40 mM BA or 42°C heat shock treatment (SI Fig. 6). Deletion of the different parts of the hsp70 promoter region affected the BA-triggered transcriptional activation in a manner closely similar to heat stress. When either HSE1 or HSE2 deletion constructs were used, BA treatment (similarly to heat) greatly reduced the expression of the reporter gene, further confirming the role of the HSF1–HSE interaction in the BA-triggered stress response.
Membrane Hyperfluidization in the Hydrophobic Core Does Not Necessarily Reflect the Concomitant Generation of a Heat Shock Gene-Inducing Signal.
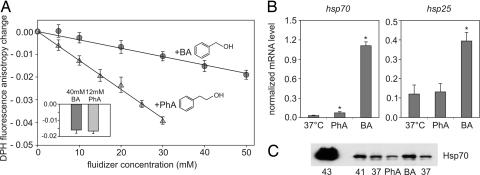
The change in membrane order is not uniform across the depth of the membrane but is position-dependent. Fluorescence polarization is one of the most sensitive and convenient means of probing the fluidity and organization of membranes. 1,6-diphenylhexatriene (DPH) is a probe known to be incorporated inside the hydrophobic core of membranes (21). The addition of increasing amounts of BA to a total membrane fraction isolated from B16(F10) cells resulted in a linear increase of membrane fluidity (Fig. 3A). Compared with BA, the slightly more hydrophobic PhA (22) proved to be a more effective membrane fluidizer (Fig. 3A). Thus, 40 mM BA, which confers high inducibility for hsp70 mRNA, appeared to be equipotent in membrane fluidization with ≈12 mM PhA. The levels of the changes in membrane fluidity induced by these concentrations of the two membrane fluidizers measured in vivo by DPH on B16(F10) cells proved to be remarkably identical (Fig. 3A Inset).
Fig. 3.
BA and PhA-induced membrane fluidization and heat shock gene activation in B16(F10) cells. (A) Membrane fluidization by BA and PhA tested by monitoring DPH anisotropy. The total membrane fraction was incubated with increasing amounts of BA or PhA at 37°C, and the steady-state DPH fluorescence anisotropy was monitored. (Inset) Equipotent concentrations of BA and PhA (40 mM and 12 mM, respectively), determined in vitro, were added to prelabeled cells, and the subsequent changes in DPH fluorescence anisotropy were monitored. The data shown are mean ± SD, n = 3. The structural formulas of BA and PhA are also presented in the figure. (B) Effects of equipotent membrane fluidization on hsp gene expression. Cells were treated with 12 mM PhA or 40 mM BA for 1 h or left untreated (37°C), the expression levels of hsp70 and hsp25 mRNAs were measured by quantitative real-time RT-PCR, and the mRNA quantities were normalized to 103 β-actin. The data show mean values ± SD. ∗, P < 0.05 compared with control as analyzed by Student's unpaired t test with the Bonferroni adjustment, n = 3. (C) Hsp70 protein expression shown by Western blotting after 6-h recovery in cells treated with 40 mM BA, 12 mM PhA, subjected to heat at 41°C or 43°C or left at 37°C for 1 h. Data are representative of three independent experiments.
To test whether the elevated membrane fluidity at a specific membrane depth per se is sufficient to provoke a stress protein response in B16(F10) cells, the effects of PhA on hsp expression were studied at the concentration causing an identical increase in membrane fluidity in the DPH-detectable region with that due to 40 mM BA. Cells were treated with 12 mM PhA or with 40 mM BA, and hsp70 and hsp25 mRNA levels were measured (Fig. 3B). Surprisingly, in contrast with BA, no increase at all in hsp25 expression was detected after PhA treatment. However, there was a minor but significant rise in hsp70 mRNA levels applying 12 mM PhA, which was however not comparable to that induced by BA. These findings were confirmed by measuring Hsp70 protein levels by Western blotting (Fig. 3C). Thus, if an equal increase of membrane fluidity monitored by DPH is achieved with different membrane perturbers, this is not necessarily coupled with similar levels of induction of heat shock genes.
BA, but Not PhA, Results in Destabilization of Cholesterol/Sphingomyelin Domains in Fluid Bilayer Membranes.
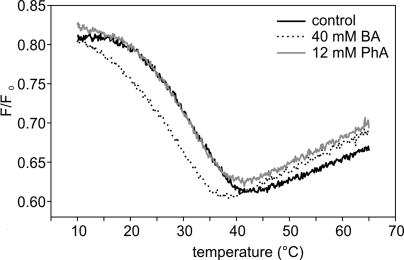
Analogously to the effects of BA, shear stress or hydrostatic pressure, while causing membrane hyperfluidization, regulates the expression of many genes, including hsp genes (see references in ref. 5). The cholesterol-sensitive compartments in the plasma membrane play an essential role in the primary activation of the signaling cascade(s). It appears likely that, on the basis of their chemical structures, the currently used membrane fluidizers are capable of interfering with membrane cholesterol and subsequently modifying its miscibility within the ordered membrane microdomains (rafts). To test this assumption, we used the fluorescence-quenching assay to monitor the association of sterol with membrane domains as a function of temperature (14, 16) by using unilamellar vesicles in which Lo [d-erythro-N-palmitoyl-sphingomyelin (PSM) and cholesterol/ cholestatriene-3β-ol (CTL)] and Ld [1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-stearoyl-(7-doxyl)-sn-glycero-3-phosphocholine (7SLPC)] phases coexist. The melting of ordered domains is seen as a decrease in the fluorescence intensity of the probe (CTL) when it comes into closer contact with the quencher (7SLPC) because of a more homogenous bilayer. As shown in Fig. 4, 40 mM BA was able to shift the melting of the Lo domains to a significantly lower temperature. By contrast, administration of 12 mM PhA did not destabilize the Lo domains at all, and this system behaved like the control system.
Fig. 4.
Melting of sterol-rich ordered domains as a consequence of BA or PhA treatment in a fluid bilayer as examined by fluorescence quenching of CTL. The F samples were composed of POPC/7SLPC/PSM/cholesterol/CTL at a ratio of 30:30:30:9:1. In F0 samples, POPC replaced 7SLPC. Only seconds before the measurements were started, 12 mM PhA and 40 mM BA were introduced to the samples, and the temperature was raised at 5°C/min. Fluorescence emission intensities were measured, and the ratio F/F0 was calculated. The data are representative of three independent experiments.
Heat and BA Treatments Caused a Similar Redistribution of Cholesterol-Rich Membrane Domains in B16(F10) Cells.
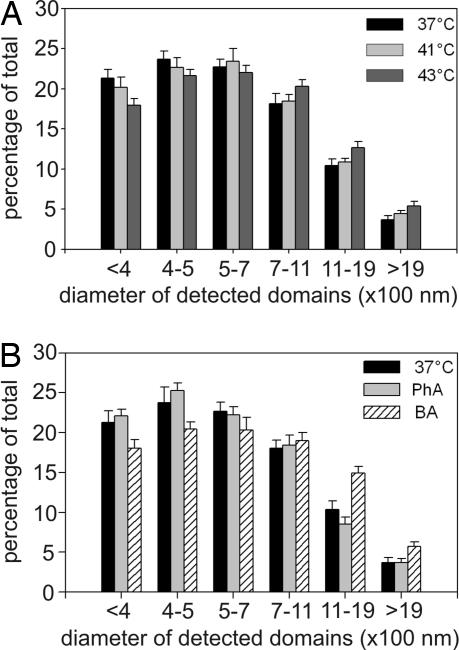
It was recently demonstrated that a nontoxic fluorescent probe, fPEG-Chol, specifically recognizes sterol-rich membrane domains and is colocalized with various raft markers (17). Unlike filipin and other cholesterol probes, this molecule could be applied as an aqueous dispersion to various samples. When added to live mammalian cells, fPEG-Chol was distributed exclusively within the plasma membrane (data not shown). In the present study, B16(F10) cells treated with 40 mM BA or 12 mM PhA, or exposed to heat stress (41 or 43°C) were incubated with fPEG-Chol, and the labeled “spots” were visualized by confocal microscopy. As revealed by Fig. 5, on the basis of the diameter, the detected domains were separated into six classes. A characteristic rearrangement of the membrane microdomains was observed when the cells were exposed to heat (Fig. 5A). Whereas the number of smaller domains decreased in response to more severe heat stress (43°C), the larger domains piled up. The amplitude of the effects observed was proportional to the temperature: The effect of mild heat (41°C) showed the same tendency, however these changes were not significant. A very similar result was obtained after the addition of BA (Fig. 5B), with the exception of a domain classified into the interval 1,100–1,900 nm, where BA induced a pronounced elevation. The pattern of fPEG-Chol-labeled domain distribution was remarkably unchanged after 12 mM PhA treatment.
Fig. 5.
Redistribution of the cholesterol-rich membrane domains on the surface of B16(F10) cells monitored by a fPEG-Chol probe. (A) Cells were heat-stressed at 41°C or 43°C or left at 37°C for 1 h, then labeled with fPEG-Chol for 20 min and imaged by a Olympus Fluoview 1000 confocal microscope. The domain size was analyzed with ImageJ software, and the detected domains were separated into six classes. (B) Cells were treated with 40 mM BA or 12 mM PhA or left untreated (37°C) for 1 h and the domain size was analyzed as in A. The data shown are mean values ± SEM, n = 3. Kolmogorov–Smirnov test was performed to analyze the equality of distributions. Samples treated at 43°C and with BA differed from the control (37°C) significantly (P < 0.05).
Discussion
The identification of pharmacological agents that potentially normalize the dysregulated expression (23) and cellular localization (6) of Hsps is currently a major area of investigation. The nonproteotoxic Hsp coinducer hydroxylamines (9, 24) interact specifically with and significantly alter the mobility and organization of distinct classes of membrane lipids and may thereby activate specific signaling cascades linking membranes to heat shock genes (10). The mode of action of nonproteotoxic Hsp-inducing membrane perturbants (represented by BA in the present study) is still unresolved. The influence of BA resembles the membrane effects of hemodynamic shear stress, and its underlying mechanism may be analogous to mild, “fever-like” heat, at least in respect to its nonproteotoxicity. Severe heat shock causes proteins to undergo unfolding and causes nascent polypeptides to undergo misfolding, which triggers the expression of hsp genes. By contrast, mild heat stress is not coupled with protein denaturation, and a hitherto unidentified change in the plasma membrane organization and/or composition has been suggested to act as a cellular thermosensor (4, 5, 25). BA has been shown to up-regulate heat shock genes in Escherichia coli (11), cyanobacteria (3), yeast cells (26), plant cells (27), and mammalian cells (12). Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct MAPK pathways (28). BA is known to affect several membrane-based signaling events, ranging from the activation of ERK (28) to phospholipase D (29). Both mechanical perturbation of the plasma membrane and fluidization by BA led to ligand-independent conformational transitions in a G protein-coupled receptor in endothelial cells (30).
Whereas heat caused up-regulation of the overall array of heat shock genes, the induction attained with BA was notably selective. As indicated by EMSA and ChIP assays, HSF1 bound to the hsp70.1 promoter upon BA addition, similarly to that for heat stress. Different parts of the hsp70 promoter region affected the BA-triggered transcriptional activation in a closely similar manner to heat stress. Although the treatment of cells with heat or BA stress resulted in a basically HSF1-mediated Hsp response, we observed a residual inducibility for hsp70 and hsp25 in Hsf1−/− MEF cells. Accordingly, the existence of alternative hsp-signaling pathways that may operate independently from HSF1 cannot be excluded.
Besides sublethal heat stress or various concentrations of BA, B16(F10) cells were exposed to the slightly more hydrophobic analog of BA, PhA (22). Surprisingly, if applied at the concentration equipotent in membrane fluidization with BA, monitored at a distinct membrane depth, PhA was shown to be ineffective as an hsp activator. To unravel the possible mechanisms underlying the different capabilities of BA and PhA for heat shock gene activation, we followed the effects of BA and of PhA on the stability of sphingomyelin/cholesterol-rich domains in bilayer membranes of unilamellar vesicles, employing an Lo domain-selective fluorescent probe (16). It emerged that BA, but not PhA, considerably destabilized the sphingomyelin/cholesterol-rich domains during temperature-induced melting. In a search for further differences, we used the fPEG-Chol as a probe to monitor the size changes of cholesterol-rich membrane domains in vivo (17). Similarly to heat treatment, the cholesterol-rich surface membrane microdomains fused into larger platforms upon the administration of BA. In contrast, PhA at a concentration equipotent in DPH-detectable membrane fluidization with BA caused no such effect. Use of the extreme oxidation-sensitive membrane-localized fluorophore C11-BODIPY581/591 (31) in vivo we demonstrated that such a membrane microdomain reorganization was unrelated to lipid peroxidation (Z.B., E.N., and L.V., unpublished data). How cholesterol homeostasis is regulated during heat or BA stress and also during the subsequent adaptation phase is currently under investigation in our laboratory. The increased susceptibility (chemical potential) of cholesterol displaced from plasma membrane lipids may also be linked to the formation of cholesterol glucoside, a known lipid mediator that rapidly activates HSF1 and induces the formation of Hsp70 (32).
We suggest that the mode of action of the hsp inducer BA may be analogous to shear stress. Hemodynamic shear stress, an important determinant of vascular remodeling and atherogenesis, is able to increase Hsp expression under isothermal conditions in response to acute hypertension, balloon angioplasty, or advanced lesions of atherosclerosis. The mechanical stress-induced Hsp70 expression has been demonstrated to be regulated by the small G proteins Ras and Rac through phosphatidylinositol 3-kinase (PI3K) (33). Active Rac1 binds preferentially to cholesterol-rich membranes (rafts) and this binding step is specifically determined by the composition and physical state of the membrane lipids (34). Analogously, moderate heat stress has been observed to induce the membrane translocation of Rac1 and membrane ruffling in a Rac1-dependent manner, in parallel with the activation of HSF1 and an increased Hsp expression (35).
Overall, our data suggest that, apart from membrane hyperfluidization in the deep hydrophobic region, a distinct reorganization of cholesterol-rich microdomains may also be required for the generation and transmission of sufficient stress signals to activate hsp genes. Because the BA-targeted reshaping of specific membrane microdomains in the currently used melanoma model was shown to be coupled with a modulation of the activities of certain hsp genes, our approach alone or in combination with other interventions (such as hyperthermia) may serve as a potential mode of therapeutic strategy.
Materials and Methods
Materials.
POPC was from Avanti Polar Lipids (Alabaster, AL). PSM was purified as described in ref. 36. fPEG-Chol (17) was a generous gift from Toshihide Kobayashi (RIKEN Discovery Research Institute, Saitama, Japan). All other chemicals were from Sigma (St. Louis, MO) unless otherwise indicated.
Cell Culturing and Treatments.
B16(F10) mouse melanoma cells were cultured in RPMI medium 1640 supplemented with 10% FBS and 4 mM l-glutamine. MEFs derived from an Hsf1−/− or Hsf1+/+ mouse (19) were maintained as in ref. 37. For heat shock treatments, the plates were immersed in a water bath set to the indicated temperature (±0.1°C). For BA and PhA treatments, the growth medium was changed to fresh growth medium containing BA or PhA at the indicated concentrations.
Quantitative Real-Time RT-PCR.
RNA was isolated by using the NucleoSpin RNA II kit (Macherey-Nagel, Duren, Germany). Two micrograms of RNA was reverse-transcribed through use of the RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas, St. Leon-Rot, Germany). Hsp70 (Mm01159846s1), hsp25 (Mm00834384_g1), hsp90 (Mm00658568_gH), hsp105 (Mm00442865_m1), αB-crystallin (Mm00515567_m1), and β-actin (Mm00607939s1) primers with TaqMan probes were purchased from Applied Biosystems (Foster City, CA). TaqMan Universal PCR Master Mix (Applied Biosystems) was used to prepare the reaction mixes. The PCR runs were performed in a Rotor-Gene 3000 instrument (Corbett Research, Sydney, Australia). Relative quantities of mRNAs were normalized to β-actin.
EMSA and Antibody Perturbation.
EMSA was performed as described in ref. 38. For the perturbation assay, the cell lysates were incubated with HSF1 antibody (39) or rabbit serum in a dilution of 1:200 and 1:10, respectively. The specificity of the binding was ascertained by adding unlabeled specific or nonspecific (NF-κB binding site 5′-AGCTTCAGAGGGGACTTTCCGAGAGG-3′) oligonucleotides to the samples in a 100-fold molar excess.
ChIP.
The ChIP protocol is described by Östling et al. (40). Sonicated chromatin was immunoprecipitated with HSF1 (SPA-901; Stressgen, Victoria, Canada), acetylated histone H4 (06-866; Upstate Biotechnologies, Lake Placid, NY) antibodies or normal rabbit serum (NS; Jackson ImmunoResearch Laboratory, Bar Harbor, ME). The following primers were used in the PCR amplifications: mHsp70.1 US, 5′-CAC CAG CAC GTT CCC CA-3′; DS, 5′-CGC CCT GCG CCT TTA AG-3′; mPCK (mouse phosphoenolpyruvate carboxykinase) US, 5′-GAG TGA CAC CTC ACA GCT GTG G-3′, 5′-GGC AGG CCT TTG GAT CAT AGC C-3′ (41).
Western Blotting.
Cells were lysed in 2× Laemmli buffer, and equal amounts of proteins were run on SDS/PAGE and then transferred to PVDF membrane. Membranes were probed with anti-Hsp70 (SPA-810; Stressgen) antibody.
Membrane Fluidity Measurements by DPH.
The total membrane fraction of B16(F10) cells was isolated essentially as described in ref. 42 and labeled in PBS (OD360 = 0.1) with 0.2 μM DPH for 10 min. DPH-labeled membranes were incubated with increasing concentrations of BA or PhA for 5 min at 37°C, and steady-state fluorescence anisotropy was measured as in ref. 21.
For measuring membrane fluidity of living cells, B16(F10) cells were labeled with 0.2 μM DPH for 10 min in PBS (OD360 = 0.1). BA (40 mM) and PhA (12 mM) were added to prelabeled cells, and subsequent changes in DPH fluorescence anisotropy were monitored. The changes caused by BA and PhA were calculated from the data gained 5 min after fluidizer administration.
Steady-State Fluorescence-Quenching Measurements.
Sample preparation and fluorescence measurement details were exactly as recently described in ref. 36. Vesicles were prepared to yield a final lipid concentration of 50 μM. The F samples consisted of POPC/7SLPC/PSM/cholesterol/CTL in a molar ratio of 30:30:30:9:1. In the F0 samples, POPC replaced 7SLPC. BA (40 mM) and PhA (12 mM) were added to the samples only seconds before the measurements were started. The fluorescence intensities F and F0 were compared to reveal the fraction of CTL quenched by 7SLPC.
fPEG-Chol Labeling, Confocal Microscopy, and Domain Size Analysis.
B16(F10) cells were treated with 40 mM BA or 12 mM PhA or heat stressed at 41°C or 43°C for 60 min, then incubated with 0.2 μM fPEG-Chol for 20 min as in ref. 17. After fPEG-Chol staining, the cells were washed and imaged with an Olympus (Melville, NY) Fluoview 1000 microscope by using the 488-nm argon–ion laser line for the excitation of fluorescein. Pictures were taken with a resolution of 1,600 × 1,600; the final magnification was ×300. The domain size was analyzed with the freeware ImageJ software (www.uhnresearch.ca/facilities/wcif/imagej), with its fast Fourier transform (FFT) bandpass filter and the nucleus counter plug-in. The domains that we found were sorted into classes according to diameter. Diameters were calculated as 2X(Npix/π)−2, where X is the size of a pixel in nanometers, and Npix is the number of pixels covering the actual domain.
Acknowledgments
We thank Toshihide Kobayashi for kindly providing fPEG-Chol and Ivor J. Benjamin (University of Utah Health Sciences Center, Salt Lake City, UT) for Hfs1+/+ and Hfs1−/− fibroblasts. This work was supported by Agency for Research Fund Management and Research Exploitation Grant RET OMFB 00067/2005, Marie Curie Host Fellowship MTKI-CT-2004-003091, the Academy of Finland, the Åbo Akademi University Centre of Excellence in Cell Stress, and the Sigrid Jusélius Foundation.
Abbreviations
- 7SLPC
1-palmitoyl-2-stearoyl-(7-doxyl)-sn-glycero-3-phosphocholine
- BA
benzyl alcohol
- CTL
cholestatriene-3β-ol
- DPH
1,6-diphenylhexatriene
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- fPEG-Chol
fluorescein ester of poly(ethyleneglycol)-derivatized cholesterol ether
- HSE
heat shock element
- HSF
heat-shock transcription factor
- Ld
liquid-disordered phase
- Lo
liquid-ordered phase
- MEF
mouse embryonic fibroblast
- PhA
phenethyl alcohol
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- PSM
d-erythro-N-palmitoyl-sphingomyelin
- Tm
midtemperature of the gel-to-liquid-crystalline phase transition.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702557104/DC1.
References
- 1.Vigh L, Maresca B, Harwood J. Trends Biochem Sci. 1998;23:369–373. doi: 10.1016/s0968-0004(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 2.Vigh L, Maresca B. In: Cell and Molecular Responses to Stress. Storey KB, Storey JM, editors. Amsterdam: Elsevier; 2002. pp. 173–188. [Google Scholar]
- 3.Horváth I, Glatz A, Varvasovszki V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benkő S, Joó F, Vigh L. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigh L, Escriba P, Sonnleitner A, Sonnleitner M, Piotto S, Maresca B, Horváth I, Harwood LJ. Prog Lipid Res. 2005;44:303–344. doi: 10.1016/j.plipres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Vigh L, Török Z, Balogh G, Glatz A, Piotto S, Horváth I. In: Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks. Csermely P, Vigh L, editors. New York: Springer; 2007. pp. 114–131. [Google Scholar]
- 6.Bausero MA, Page DT, Osinaga E, Asea A. Tumor Biol. 2004;25:243–251. doi: 10.1159/000081387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vereb G, Szöllösi J, Matkó J, Farkas T, Vigh L, Mátyus L, Waldmann TA, Damjanovich S. Proc Natl Acad Sci USA. 2003;100:8053–8058. doi: 10.1073/pnas.1332550100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeyda M, Stulnig TM. Prog Lipid Res. 2006;45:187–202. doi: 10.1016/j.plipres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Vigh L, Literáti NP, Horváth I, Török Z, Balogh G, Glatz A, Kovács E, Boros I, Ferdinándy P, Farkas B, et al. Nature Med. 1997;3:1150–1154. doi: 10.1038/nm1097-1150. [DOI] [PubMed] [Google Scholar]
- 10.Török Z, Tsvetkova NM, Balogh G, Horváth I, Nagy E, Pénzes Z, Hargitai J, Bensaude O, Csermely P, Crow JH, et al. Proc Natl Acad Sci USA. 2003;100:3131–3136. doi: 10.1073/pnas.0438003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigapova N, Török Z, Balogh G, Goloubinoff P, Vigh L, Horváth I. Biochem Biophys Res Comm. 2005;328:1216–1223. doi: 10.1016/j.bbrc.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 12.Balogh G, Horváth I, Nagy E, Hoyk Z, Benkő S, Bensaude O, Vigh L. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 13.De Marco A, Vigh L, Diamant S, Goloubinoff P. Cell Stress Chaperones. 2005;10:329–339. doi: 10.1379/CSC-139R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alanko MK, Halling KK, Maunula S, Slotte JP, Ramstedt B. Biochim Biophys Acta. 2005;1715:111–121. doi: 10.1016/j.bbamem.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Jean-Louis S, Akare S, Ali MA, Mash EA, Meuillet E, Martinez JD. J Biol Chem. 2006;281:14948–14960. doi: 10.1074/jbc.M506710200. [DOI] [PubMed] [Google Scholar]
- 16.Heczkova B, Slotte PJ. FEBS Lett. 2006;580:2471–2476. doi: 10.1016/j.febslet.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 17.Sato SB, Ishi K, Makino A, Iwabuchi K, Yamaji-Hasegawa A, Senoh Y, Nagaoka I, Sakuraba H, Kobayashi T. J Biol Chem. 2004;279:23790–23796. doi: 10.1074/jbc.M313568200. [DOI] [PubMed] [Google Scholar]
- 18.Anckar J, Sistonen L. In: Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks. Csermely P, Vigh L, editors. New York: Springer; 2007. pp. 78–88. [Google Scholar]
- 19.McMillan DR, Xiao X, Shao L, Graves K, Benjamin IJ. J Biol Chem. 1998;273:7523–7528. doi: 10.1074/jbc.273.13.7523. [DOI] [PubMed] [Google Scholar]
- 20.Fiszer-Kierzkowska A, Wysocka A, Jarzab M, Lisowska K, Krawczyk Z. Biochim Biophys Acta. 2003;1625:77–87. doi: 10.1016/s0167-4781(02)00592-4. [DOI] [PubMed] [Google Scholar]
- 21.Török Z, Horváth I, Goloubinoff P, Kovács E, Glatz A, Balogh G, Vigh L. Proc Natl Acad Sci USA. 1997;94:2192–2197. doi: 10.1073/pnas.94.6.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penfold J, Staples E, Tucker I, Soubiran L, Thomas RK. J Coll Interface Sci. 2002;247:397–403. doi: 10.1006/jcis.2001.8041. [DOI] [PubMed] [Google Scholar]
- 23.Sőti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P. Br J Pharmacol. 2005;146:769–780. doi: 10.1038/sj.bjp.0706396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieran D, Kalmar B, Dick JRT, Riddoch-Contreras J, Burnstock G, Greensmith L. Nature Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 25.Park HG, Han SI, Oh SY, Kang HS. Cell Mol Life Sci. 2005;62:10–23. doi: 10.1007/s00018-004-4208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carratu L, Franceschelli S, Pardini C, Kobayashi GS, Horvath I, Vigh L, Maresca B. Proc Natl Acad Sci USA. 1997;93:3870–3875. doi: 10.1073/pnas.93.9.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saidi Y, Finka A, Chakhporanian M, Zrud JP, Schaefer DG, Goloubinoff P. Plant Mol Biol. 2005;59:697–711. doi: 10.1007/s11103-005-0889-z. [DOI] [PubMed] [Google Scholar]
- 28.Sangwan V, Dhindsa RS. FEBS Lett. 2002;531:561–564. doi: 10.1016/s0014-5793(02)03626-8. [DOI] [PubMed] [Google Scholar]
- 29.Vaultire MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, Benghassaine-Kesri G, Cicek D, Zachowski A, Ruelland E. FEBS Lett. 2006;580:4218–4223. doi: 10.1016/j.febslet.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 30.Chachisvilis M, Zhang YL, Frangos JA. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pap EHW, Drummen GPC, Winter VJ, Kooij TWA, Rijken P, Wirtz KWA, Op den Kamp JAF, Hage WJ, Post JA. FEBS Lett. 1999;453:278–282. doi: 10.1016/s0014-5793(99)00696-1. [DOI] [PubMed] [Google Scholar]
- 32.Kunimoto S, Murofushi W, Kai H, Ishida Y, Uchiyama A, Kobayashi T, Kobayashi S, Murofushi H, Murakami-Murofushi K. Cell Struct Funct. 2002;27:157–162. doi: 10.1247/csf.27.157. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q, Schett G, Li C, Hu Y, Wick G. Circ Res. 2000;86:1124–1130. doi: 10.1161/01.res.86.11.1122. [DOI] [PubMed] [Google Scholar]
- 34.Del Pozo MA, Aldreson NB, Kiosses WB, Chiang HH, Andreson RGW, Schwartz MA. Science. 2004;303:839–842. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- 35.Han SI, Oh SY, Woo SH, Kim KH, Kim JH, Kang HS. J Biol Chem. 2001;276:1889–1895. doi: 10.1074/jbc.M006042200. [DOI] [PubMed] [Google Scholar]
- 36.Bjorkqvist YJ, Nyholm TKM, Slotte JP, Ramstedt B. Biophys J. 2005;88:4054–4063. doi: 10.1529/biophysj.104.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Mol Cell Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosser DD, Theodorakis NG, Morimoto RI. Mol Cell Biol. 1988;8:4736–4744. doi: 10.1128/mcb.8.11.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarge KD, Murphy SP, Morimoto RI. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Östling P, Bjork JK, Roos-Mattjus P, Mezger V, Sistonen L. J Biol Chem. 2007;282:7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- 41.Cissell MA, Zhao L, Sussel L, Henderson E, Stein R. J Biol Chem. 2003;278:751–756. doi: 10.1074/jbc.M205905200. [DOI] [PubMed] [Google Scholar]
- 42.Maeda M, Doi O, Akamatsu Y. Biochim Biophys Acta. 1980;597:552–563. doi: 10.1016/0005-2736(80)90227-8. [DOI] [PubMed] [Google Scholar]