Abstract
MicroRNAs (miRNAs) are short 19- to 24-nt RNA molecules that have been shown to regulate the expression of other genes in a variety of eukaryotic systems. Abnormal expression of miRNAs has been observed in several human cancers, and furthermore, germ-line and somatic mutations in human miRNAs were recently identified in patients with chronic lymphocytic leukemia. Thus, human miRNAs can act as tumor suppressor genes or oncogenes, where mutations, deletions, or amplifications can underlie the development of certain types of leukemia. In addition, previous studies have shown that miRNA expression profiles can distinguish among human solid tumors from different organs. Because a single miRNA can simultaneously influence the expression of two or more protein-coding genes, we hypothesized that miRNAs could be candidate genes for cancer risk. Research in complex trait genetics has demonstrated that genetic background determines cancer susceptibility or resistance in various tissues, such as colon and lung, of different inbred mouse strains. We compared the genome positions of mouse tumor susceptibility loci with those of mouse miRNAs. Here, we report a statistically significant association between the chromosomal location of miRNAs and those of mouse cancer susceptibility loci that influence the development of solid tumors. Furthermore, we identified distinct patterns of flanking DNA sequences for several miRNAs located at or near susceptibility loci in inbred strains with different tumor susceptibilities. These data provide a catalog of miRNA genes in inbred strains that could represent genes involved in the development and penetrance of solid tumors.
Keywords: solid tumor, inbred strains, quantitative trait loci
The genetics of cancer penetrance is an evolving field that is leading to new strategies for cancer prevention and treatment. Susceptibility loci can influence not only cancer incidence and metastasis, but also the penetrance of other genetic disorders and susceptibility to infectious diseases (refs. 1 and 2 and www.informatics.jax.org). The penetrance of tumor phenotypes results from the interaction between genetics and environment. The variability of environmental factors and the genetic heterogeneity in human populations complicates the search for susceptibility loci. To control these factors, inbred mouse strains are useful models to use to search for allelic variants that confer susceptibility or resistance to different types of tumors (3). Quantitative trait loci analyses in mice have already identified chromosomal regions involved in cancer (3–5), and several protein-coding genes have a proven role as cancer susceptibility loci (6–10). We suggest that a newly discovered family of noncoding genes, the microRNA (miRNAs), could be responsible for susceptibility to solid tumors.
Several papers have shown that miRNAs play an important role in certain human cancers, including B cell chronic lymphocytic leukemias (11), and Burkitts lymphoma, colon cancer, lung cancer, and breast cancer (12–14). Furthermore, miRNA expression profiles can be used to classify human solid tumors and leukemias (10, 15). Some miRNAs can down-regulate large numbers of target mRNAs (16), and it is estimated that miRNAs could regulate 33% of the human genome (17). These targets may be oncogenes and tumor suppressor genes, supporting the idea that miRNAs could influence susceptibility and predispose individuals to different cancers. Because miRNAs are responsible for fine regulation of gene expression, “tuning” cellular phenotypes during delicate processes like development and differentiation, they represent a family of candidate genes for cancer susceptibility. Here, we evaluate the correlation of miRNAs with cancer susceptibility loci and identify miRNAs with a distinct pattern of flanking DNA regions in inbred mouse strains with diverse tumor phenotypes. Our work supports the hypothesis that miRNAs could represent a new family of susceptibility genes affecting the risk of cancer.
Results and Discussion
A genomewide approach was used to investigate associations between miRNAs and mouse cancer susceptibility loci. This type of investigation was previously successful in identifying a significant association between the genomic position of human miRNAs and that of fragile sites and cancer-associated genomic regions (18).
Compilation of Known Tumor Susceptibility and Modifier Loci.
We constructed a database that contained both susceptibility and modifier loci for eight types of solid tumors in the mouse: bladder, colon, liver, lung, mammary gland, ovary, small intestine, and skin. The list was built by locus symbol, peak marker, chromosome (Chr), tissue, and inbred strains. To obtain loci positions, we used the Mouse Genome Informatics Database (www.informatics.jax.org) and PubMed (www.pubmed.gov). Published data were evaluated to identify the peak location of each region (Fig. 1).
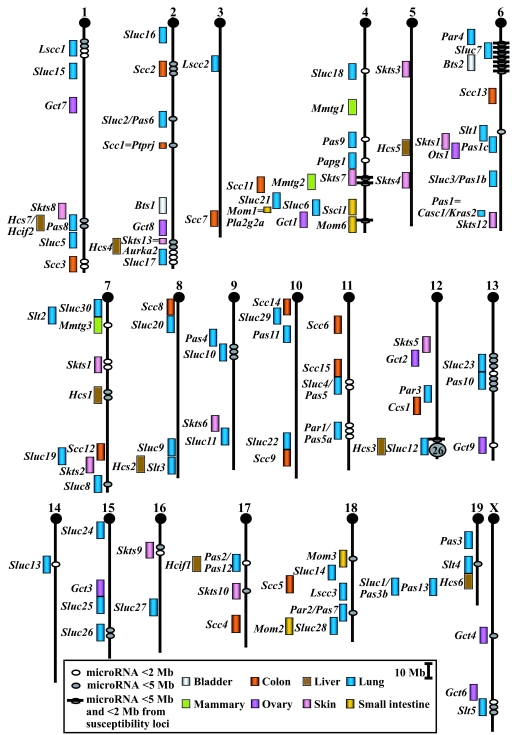
Fig. 1.
Chromosomal positions of mouse tumor susceptibility loci and associated miRNAs. The MUSMIRSUS database was used to identify miRNAs at or near the peak of mouse tumor susceptibility loci (www.kimmelcancercenter.org/siracusa/musmirsus.htm). Mouse Chrs are shown with centromeres at the top. The map positions of mouse miRNAs (ovals) are compared with those of susceptibility loci (rectangles) for eight types of solid tumors. MiRNAs <5 Mb from the peak of a susceptibility locus are shown. A large cluster of 28 miRNAs on distal Chr 12 includes tissue-specific miRNA genes (mir-127, mir-136, mir-134, mir-154) that map to an imprinted region (19); most of these miRNAs reside near the lung Sluc12 locus.
Several observations were derived from this analysis. First, Chr 4 had the largest number of susceptibility loci (13 total), followed by Chr 6 (12 loci). Second, Chr Y had no susceptibility loci, followed by Chr 14 (1 locus). The frequency distribution [supporting information (SI) Fig. 4] suggests that genes predisposing to solid tumors are not distributed randomly within the genome, but tend to be concentrated on certain Chrs. Third, Fig. 1 shows that several susceptibility loci identified for the same organ overlap (such as for lung, Pas6 and Sluc2 on Chr 2, Pas1b and Sluc3 on Chr 6, Par1 and Pas5a on Chr 11, Pas2 and Pas12 on Chr 17, and Pas3b and Sluc1 on Chr 19). Because these tumor susceptibility loci were discovered by using different test conditions and strains in different laboratories and yet, reside in close proximity, they may be caused by the same gene(s). Fourth, several susceptibility loci identified for different organs overlap (such as Hcs7/Hcif2 and Pas8 on Chr 1, Hcs3 and Sluc12 on Chr 12, and the 9 loci on distal Chr 4). These findings suggest that genes controlling the integrity and/or homeostasis of multiple solid organs may be concentrated within the genome; there may be only one or multiple, closely linked genes in each of these regions. Alternatively, future studies that define more susceptibility loci in these and other organs may prove otherwise.
Construction of the MUSMIRSUS Database.
A total of 229 mouse miRNA gene locations were downloaded from the July 2005 miRNA registry web site (http://microrna.sanger.ac.uk). We combined and compared the Mb positions of mouse miRNAs with those of known susceptibility and modifier loci (www.ensembl.org); this ordered list was used to identify miRNAs that mapped at or near the peak of tumor loci (see Table 1 as an example). The database was named MUSMIRSUS, for Mus miRNA susceptibility and is contained in its entirety at www.kimmelcancercenter.org/siracusa/musmirsus.htm.
Table 1.
Examples of miRNAs located near solid tumor susceptibility loci
| Locus/marker | Chr | Mb | Tissue | Resistant strains | Susceptible strains | Distance in bp* | miRNA homolog in human cancers† |
|---|---|---|---|---|---|---|---|
| mmu-mir-30a | 1 | 23.5 | 791,353 | Low lung; high pancreas | |||
| mmu-mir-30c-2 | 1 | 23.5 | 771,919 | High prostate, colon, pancreas; low breast (miR-30c) | |||
| Lscc1/D1Mit169 | 1 | 24.3 | Lung | AKR/J, C57BL/6J, 129X1/SvJ | A/J, SWR/J, NIH Swiss | ||
| mmu-mir-194–1 | 1 | 185.0 | 169,212 | Human FRA1H | |||
| mmu-mir-215 | 1 | 185.0 | 168,950 | Human FRA1H; high lung, stomach | |||
| Scc3/D1Mit208 | 1 | 185.1 | Colon | BALB/cHeA | STS/A | ||
| Sluc17/D2Mit200 | 2 | 179.5 | Lung | B10.O20 | O20 | ||
| mmu-mir-1–1 | 2 | 180.0 | 597,348 | ||||
| mmu-mir-133a-2 | 2 | 180.0 | 606,679 | ||||
| mmu-mir-31 | 4 | 87.2 | 402,170 | High breast | |||
| Pas9/D4Mit77 | 4 | 87.6 | Lung | BALB/c | A/J | ||
| Mmtg2/D4Mit200–308 | 4 | 118.6 | Mammary | FVB/Nmul-TgN | FVB/NJ | ||
| mmu-mir-30c-1 | 4 | 119.1 | 469,491 | High prostate, colon, pancreas; low breast (miR-30c) | |||
| mmu-mir-30e | 4 | 119.1 | 472,563 | ||||
| Mom6/D4Mit64 - Tel | 4 | 146.7 | Small intestine | AKR/J | C57BL/6J | ||
| mmu-mir-34a | 4 | 148.0 | 855,896 | Human CAGR, high lung, prostate, solid cancer vs. normal | |||
| Sluc4/Pas5/D11Mit15 | 11 | 69.6 | Lung | B10.O20 | O20 | ||
| mmu-mir-324 | 11 | 69.6 | 63,439 | ||||
| mmu-mir-195 | 11 | 69.8 | 286,438 | High lung, prostate, solid cancer vs. normal | |||
| mmu-mir-196a-1 | 11 | 95.9 | 20,475 | High lung, prostate (miR-196–1) | |||
| Par1/Pas5a/D11Mit54 | 11 | 95.9 | Lung | C57BL/6J, BALB/c, SM/J, M. spretus | A/J | ||
| mmu-mir-10a | 11 | 96.0 | 31,526 | High colon, lung, solid cancer vs. normal | |||
| mmu-mir-152 | 11 | 96.5 | 564,754 | Low pancreas, solid cancer vs. normal | |||
| mmu-mir-219–1 | 17 | 32.5 | 719,176 | Low lung | |||
| Pas2/Pas12/D17Mit34 | 17 | 33.2 | Lung | C57BL/6J | A/J |
miRNAs located < 1 Mb from the peak marker of susceptibility loci on Chrs 1, 2, 4, 11, 17 are shown. For a complete list, see the MUSMIRSUS Database (www.kimmelcancercenter.org/siracusa/musmirsus.htm).
*The base pairs (bp) represent the distance between a miRNA and the closest susceptibility locus.
A Significant Number of miRNAs Are Located Close to Tumor Susceptibility Loci.
To test hypotheses about the relationship of the incidence of miRNAs and their association with tumor susceptibility loci, we used the random effect poisson regression model (18). For this analysis, the random effect used was Chr, in that data within a Chr is assumed correlated. Under this model, the number of miRNAs defined “events” and nonoverlapping lengths of susceptibility regions defined “time.” The “length” of a susceptibility region was ±0.5 Mb if the gene is known or estimated as ±5 Mb from the peak marker. Although a quantitative trait locus need not be at the peak marker, the critical distance selected here covers the most likely interval. A miRNA was considered within a susceptibility region if it was located ±5 Mb from the peak location of a given locus. The fixed effect in the model consisted of an indicator variable for the presence/absence of each susceptibility region. We report the incidence rate ratio (IRR), two-sided 95% confidence interval of the IRR, and two-sided P values for testing the hypothesis that the IRR is 1.0. An IRR significantly >1 indicates an increase in the number of miRNAs within a region. All statistical computations were completed by using STATA version 7.0.
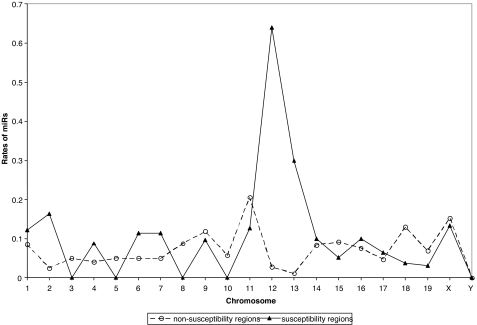
Overall, 96 miRNAs (41.9%) were located <5 Mb from the peak of the closest locus (or loci), representing a highly significant association between the incidence of miRNAs and tumor susceptibility regions. Specifically, the relative incidence of miRNAs occurred at a rate 1.5 times higher in susceptibility regions than nonsusceptibility regions [IRR = 1.63; 95% confidence interval (1.25, 2.14); P < 0.001]. Therefore, miRNAs were found more often in (or near) susceptibility regions than in other areas of the genome (Fig. 2). The estimated rate of miRNAs is moderate in most Chrs, but quite large in Chrs 2, 6, 7, 13, and especially 12. Thus, a larger number of miRNAs than expected by chance occurs in these five Chrs. Regardless of location, the model indicates that miRNAs are significantly more likely to reside within tumor susceptibility regions than outside of them. In addition, a recent report suggests an association between the locations of mouse miRNAs and known sites of retroviral integration in mouse cancers (20).
Fig. 2.
Rates of miRNAs in tumor-susceptibility regions of mouse Chrs. The plot illustrates the estimated rate of miRNAs in susceptibility regions versus nonsusceptibility regions by Chr. For plotting purposes, rates of miRNAs within a region are computed as number of miRNAs per length of region (within each Chr). Rates of miRNAs are plotted by Chr and susceptibility region status (susceptibility region versus not). The estimated length in Mb of the genome was based on Ensembl version 33 (September 2005). There is a significant association (P < 0.001) of the incidence of miRNAs near the peak of tumor-susceptibility regions.
Specific miRNAs as Candidates for Tumor Susceptibility Loci.
Thirty-five of the 229 miRNAs (15.3%) are located in close proximity (<2 Mb) to 24 susceptibility loci, some of which overlap with the same miRNA. For example, mir-30c-1 and mir-30e are 0.47 Mb distal to the mammary Mmtg2 locus, <2.5 Mb proximal to the colon Scc11 locus, and <3.6 Mb distal to the skin Skts7 locus on Chr 4. Similarly, mir-34a is 0.86 Mb distal to the small intestine Mom6 locus and <4.6 Mb distal to the ovary Gct1 locus on Chr 4. In addition, the bladder Bts2 locus and the lung Sluc7 locus overlap on Chr 6 and reside 0.4–4.8 Mb from a cluster of six miRNAs. These miRNAs are all <1 Mb from at least one susceptibility locus and also reside <5 Mb from a second susceptibility locus for a different organ.
Eighteen mouse miRNAs (7.9%) localized near (<5 Mb) susceptibility loci correspond to human miRNAs located in human cancer-associated regions and fragile sites as reported (18). Some of these 18 miRNAs are members of the let family (let-7a-1, let-7a-2, let-7d, let-7f-1), and reduced expression of let-7 miRNAs is associated with shortened postoperative survival in human lung cancer (21). Because the expression of let-7 and RAS protein are opposite in lung tumors versus normal tissue, RAS was shown to be regulated by the let-7 family (22). In fact, members of let-7 are located within 4 Mb of loci predisposing to lung cancer: let-7a-2 at Sluc10 on Chr 9, let-7f-2 at Slt5 on Chr X, and the cluster let-7d, let-7f-1, and let7a-1 at Sluc23 on Chr 13. The list of miRNAs at or near sites commonly altered in human cancers includes mir-215 and mir-194-1, which are <0.17 kb from D1Mit208, a marker that defines the peak of the colon Scc3 locus on Chr 1. Homologous human miRNAs are located inside the FRA1H at Chr 1q42.1; this fragile site is an HPV16 integration site involved in cervical cancer (18). In addition, mir-181b-2 and mir-199b are 3.3 Mb from the peak of the colon Scc2 locus on Chr 2; homologous human miRNAs are located inside a cancer-associated genomic region at Chr 9q33–34.1, which is a region commonly deleted in bladder cancer (18).
Some miRNAs can act as oncogenes or tumor suppressor genes (12, 14). If our hypothesis that miRNAs are candidates for tumor susceptibility genes is true, it is expected that some miRNAs will act cell autonomously and be abnormally expressed in cancer cells, whereas others will act noncell autonomously to influence cancer development (4). The expression of human homologs of candidate miRNAs for mouse tumor susceptibility loci was extensively reported. Nineteen of the 35 (54.3%) miRNAs located <2 Mb from the peak of a tumor susceptibility locus are abnormally expressed in at least one type of human solid cancer, with expression being significantly different from normal controls (23, 24).
Direct Sequence Screen of Inbred Strains.
Discrete sequence alterations are frequently found in human cancers, and variation in the sequence of precursor miRNAs were identified in the normal human population (25) and the germ line of cancer patients (26). It was shown that a C-to-A polymorphism in the mature mir-30c-2 sequence may alter target selection and thus exert profound biological effects (25). According to the miRanda algorithm, 10 of 12 predicted targets were not considered likely for the A-type variant mir-30c-2. Interestingly, the mouse homolog is located <1 Mb from the peak marker of the lung Lscc1 locus on Chr 1.
We sequenced several miRNAs to determine whether inbred strains with different tumor susceptibilities possessed unique alleles. Of 229 miRNAs, 10% are located <1 Mb from the peak marker that defines 15 solid tumor susceptibility loci (see Table 1 as an example). In fact, eight of these loci have more than one miRNA that lies <1 Mb away from their peak marker. Selection of miRNAs was based on criteria that included their proximity to susceptibility loci, the same miRNA being present in human cancer-associated genomic regions, and the available inbred strains. We sequenced 13 miRNAs (mir-1-1, mir-7-1, mir-10a, mir-30c-2, mir-31, mir-34a, mir-133a-2, mir-196a-1, mir-207, mir-219-1, mir-324, mir-346, and mir-448) comparing susceptible, resistant, and/or common inbred strains.
Genomic regions corresponding to each precursor miRNA were amplified, including flanking 5′ and 3′ ends, because these regions are needed for miRNA expression (27). A highly conserved motif ≈200 bp upstream of the miRNA stem loop was found in most independently (intergenic) transcribed nematode miRNAs (28). Moreover, independent transcription units in which the precursor miRNA is sufficient for processing have been found in plants, with their promoter elements being located upstream of the stem loop (29).
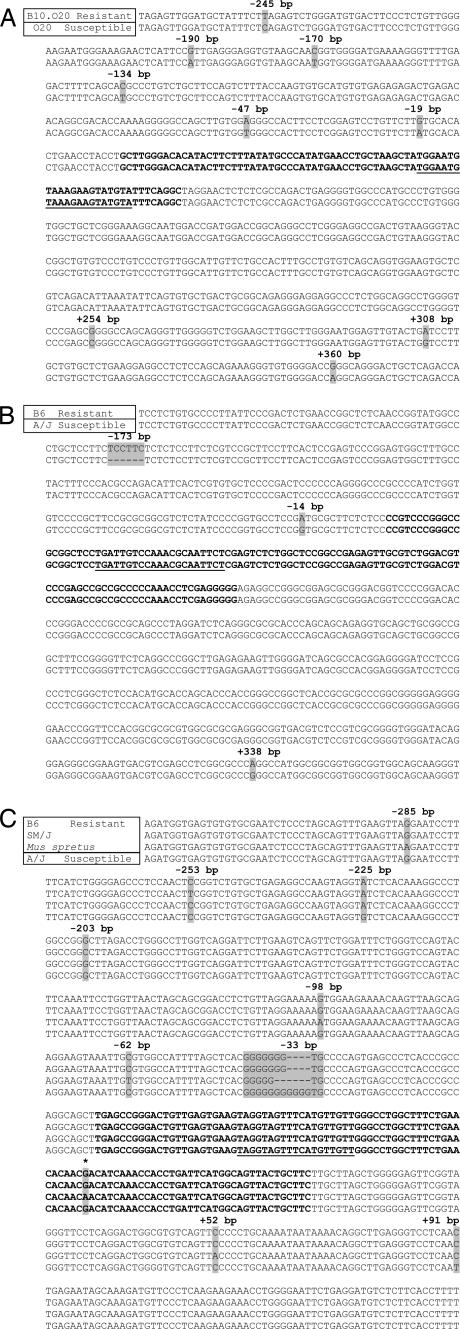
Seven of the 13 miRNAs showed sequence differences between inbred strains (Fig. 3 and SI Figs. 5–8). Fig. 3A shows that the flanking region of mir-1-1 (near Sluc17) has six substitutions upstream of the stem loop in the susceptible O20 compared with the resistant B10.O20 strain. Fig. 3B shows a deletion of TCCTTC for mir-219-1 (near Pas2/Pas12) in the susceptible A/J compared with the resistant B6 strain. Furthermore, A/J carries a base substitution upstream and downstream of the stem loop. Fig. 3C shows a dramatic difference upstream of the mir-196a-1 stem loop (near Par1/Pas5a). The susceptible A/J carries the longest stretch of guanines compared with the resistant B6, SM/J, and Mus spretus strains. Together with substitutions, the data show that there are four mir-196a-1 alleles. In addition, M. spretus has the only substitution found within a stem-loop structure.
Fig. 3.
MiRNA gene sequences differ between resistant and susceptible inbred strains. The nucleotide sequences of mir-1-1 on Chr 2 (near the lung Sluc17 locus) (A), mir-219-1 on Chr 17 (near the lung Pas2/Pas12 locus) (B), and mir-196a-1 on Chr 11 (near the lung Par1/Pas5a locus) (C) are shown in the strains indicated in the top left boxes. Gray shading highlights sequence variants (substitutions and/or in-dels). The miRNA stem-loop sequences are in bold, and the mature miRNA sequences are underlined. The tumor-susceptibility loci that map close to each miRNA are shown in parentheses. For mir-1-1, several substitutions were found between the resistant B10.O20 and the susceptible O20 strain. The sequence of B10.O20 is identical to that of the B6 strain. For mir-219-1, a 6-bp in-del and two substitutions were found between the resistant B6 and the susceptible A/J strains. For mir-196a-1, four alleles were detected; the sequence of B6 is identical to that of the evolutionarily divergent CAST strain. The asterisk indicates the position of a substitution found within the mir-196a-1 stem loop. Mir-196a-2 was also sequenced, because it has the same mature sequence as mir-196a-1, but is located on a different Chr; mir-196a-2 had two substitutions upstream of the stem loop in M. spretus, but no difference was found between susceptible and resistant strains (data not shown).
For mir-30c-2 (near Lscc1), the resistant AKR and B6 strains had different alleles, with six substitutions and one insertion–deletion (in-del). The susceptible A/J strain has a third allele (SI Fig. 5). For mir-133a-2 (near Sluc17), a deletion of TATATGTA was found in the susceptible O20 compared with the resistant B10.O20 strain. Furthermore, there are four substitutions upstream and two substitutions downstream of the mir-133a-2 stem loop (SI Fig. 6). For mir-34a (near Mom6), six substitutions and one in-del was found between the susceptible B6 and the resistant AKR strains. AKR and C3H had the same allele, B6, BALB and A/J had a second allele, and CAST had a third allele (SI Fig. 7). For mir-10a (near Par1/Pas5a), an in-del upstream of the stem loop was detected between the susceptible A/J versus the resistant B6 and SM/J strains (as well as BALB). M. spretus and CAST had additional unique alleles (SI Fig. 8).
Computational Prediction of miRNA Promoters.
Because only a few miRNA promoters have been identified experimentally (30–32), we used an automated computational pipeline to determine miRNA promoters, depending on their relative genomic location to the annotated protein-coding genes. The annotation system combines the genomic alignments of mRNAs and miRNAs with ab initio polymerase II promoter predictions of FirstEF (33). The genomic sequences and alignments of RefSeq mRNAs and known mRNAs were downloaded from the University of California, Santa Cruz web site (http://genome.ucsc.edu). Genomic alignments of miRNAs were determined with the BLAT program (34).
For intragenic miRNAs that overlap with protein-coding genes and in the sense strand, the promoter of the host protein-coding gene is considered as the promoter of the miRNA, because the “host” transcript and miRNAs usually portray similar expression profiles, indicating that these miRNAs are transcribed as part of long host transcription units (35–37). For the rest, we ran the FirstEF program on the 5′ upstream region of all miRNAs, with cut-off values for posterior probabilities of donor, promoter, and first exon of 0.4, 0.3, and 0.4, respectively, to predict the polymerase II promoters. Values of the posterior probabilities range from 0 to 1, where higher probabilities indicate a greater confidence that the corresponding predictions are correct (SI Table 2).
Of seven miRNAs that showed sequence differences between tumor-susceptible and tumor-resistant strains, five miRNAs (71.4%) had changes within their predicted promoter regions (SI Table 2). The majority of alterations resided in the 5′ flanking and promoter regions (Fig. 3 and SI Figs. 5–8). Most variants were substitutions and in-dels; the in-dels were mostly in simple repeats. Because control of miRNA expression levels resides primarily in the 5′ flanking regions, the implications of these findings are that subtle changes in the nucleotide sequence of miRNA promoters between inbred strains could significantly affect expression of miRNA transcripts.
miRNAs in Development, Differentiation, and Cancer.
miRNAs have significant roles in development and differentiation (38–40). Because a single miRNA can simultaneously target multiple genes, alterations in the amount and/or sequence of a mature miRNA can have significant effects on the expression of target genes. In addition, mutations in the promoter regions of miRNAs could affect the spatial, temporal, and/or absolute levels of miRNAs, without changing the sequence of the mature miRNA. The downstream consequences of such alterations would be manifest as changes in cellular physiology and phenotype. For example, if the fine-tuning of gene expression controlled by miRNAs was subtly altered, leading to enhanced rates of proliferation and/or decreased rates of apoptosis, such changes could significantly influence an individual's risk of developing cancer over time. Our studies have shown that there is a statistically significant correlation between the map locations of mouse tumor susceptibility loci and the map locations of miRNAs. Furthermore, substitutions and in-dels were found predominantly in promoter regions of several miRNAs between tumor-susceptible and tumor-resistant inbred strains. Taken together, this report supports the idea that miRNAs should be considered candidates for tumor susceptibility loci. MiRNAs could represent a new family of genes involved in the penetrance of human cancer and other diseases. Identification of such genes would be important for clarifying the mechanisms involved in tumor initiation, growth, and progression and revealing new markers for molecular diagnosis and novel targets for drug therapy.
Materials and Methods
Mice.
The A/J, AKR/J (AKR), BALB/cJ (BALB), C57BL/6J (B6), C3H/HeJ (C3H), CAST/Ei (CAST), M. spretus, and SM/J inbred strains were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at the Thomas Jefferson University Animal Facility (Philadelphia, PA). O20/ADem and B10.O20/Dem mice were maintained at the Department of Laboratory Animal Resources of Roswell Park Cancer Institute. Both facilities are accredited by the American Association for the Accreditation of Laboratory Animal Care.
Sequencing.
High molecular weight kidney DNA was isolated by using the DNeasy Tissue Kit (Qiagen, Valencia, CA). The genomic region of each miRNA was amplified and sequenced by using primers listed in SI Table 3. Sequence analyses were performed on genomic DNA from two mice from each strain.
Acknowledgments
G.A.C. was supported by a Kimmel Foundation Award and a Chronic Lymphocytic Leukemia Global Research Foundation Grant. S.C.N. is supported by a National Cancer Institute Minority Supplement Award. This research was supported by National Cancer Institute Grants P01CA76259 and P01CA81534 (to C.M.C.) and RO1CA72027 (to L.D.S.).
Abbreviations
- miRNA
microRNA
- Chr
chromosome
- in-del
insertion–deletion
- IRR
incidence rate ratio.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702177104/DC1.
References
- 1.Hunter K, Welch DR, Liu ET. Nat Genet. 2003;34:23–24. doi: 10.1038/ng0503-23b. [DOI] [PubMed] [Google Scholar]
- 2.Siracusa LD, Silverman KA, Koratkar R, Markova M, Buchberg A. In: Oncogenomics: Molecular Approaches to Cancer. Brenner C, Duggan D, editors. Hoboken, NJ: Wiley-Liss; 2004. pp. 255–290. [Google Scholar]
- 3.Mao J-H, Balmain A. Curr Opin Genet Dev. 2003;13:14–19. doi: 10.1016/s0959-437x(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 4.Demant P. Nat Rev Genet. 2003;4:721–734. doi: 10.1038/nrg1157. [DOI] [PubMed] [Google Scholar]
- 5.Dragani T. Cancer Res. 2003;63:3011–3018. [PubMed] [Google Scholar]
- 6.Cormier RT, Hong KH, Halberg RB, Hawkins TL, Richardson P, Mulherkar R, Dove WF, Lander ES. Nat Genet. 2000;17:88–91. doi: 10.1038/ng0997-88. [DOI] [PubMed] [Google Scholar]
- 7.Ruivenkamp CA, van Wezel T, Zanon C, Stassen AP, Vlcek C, Csikos T, Klous AM, Tripodis N, Perrakis A, Boerrigter L, et al. Nat Genet. 2002;31:295–300. doi: 10.1038/ng903. [DOI] [PubMed] [Google Scholar]
- 8.Ewart-Toland A, Briassouli P, de Koning JP, Mao JH, Yuan J, Chan F, MacCarthy-Morrogh L, Ponder BAJ, Nagase H, Burn J, et al. Nat Genet. 2003;34:403–412. doi: 10.1038/ng1220. [DOI] [PubMed] [Google Scholar]
- 9.Youngren KK, Coveney D, Peng X, Bhattacharya C, Schmidt LS, Nickerson ML, Lamb BT, Deng JM, Behringer RR, Capel B, et al. Nature. 2005;435:360–364. doi: 10.1038/nature03595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P, Wang Y, Vikis H, Maciag A, Wang D, Lu Y, Liu Y, You M. Nat Genet. 2006;38:888–895. doi: 10.1038/ng1849. [DOI] [PubMed] [Google Scholar]
- 11.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al. Proc Natl Acad Sci USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esquela-Kerscher A, Slack FJ. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 13.Sevignani C, Calin GA, Siracusa LD, Croce CM. Mamm Genome. 2006;17:189–202. doi: 10.1007/s00335-005-0066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 16.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 17.Bartel DP. Cell. 2004;23:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, et al. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seitz H, Royo H, Lin SP, Youngson N, Ferguson-Smith AC, Cavaille J. Biol Chem. 2004;385:905–911. doi: 10.1515/BC.2004.118. [DOI] [PubMed] [Google Scholar]
- 20.Huppi R, Volfovsky N, Mackiewicz M, Runfola T, Jones TL, Martin SE, Stephens R, Caplen NA. Semin Cancer Biol. 2007;17:65–73. doi: 10.1016/j.semcancer.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, et al. Cancer Res. 2004;64:3753–3756. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamaoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et al. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 24.Volinia S, Calin GA, Liu C-G, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, et al. Proc Natl Acad Sci USA. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwai N, Naraba H. Biochem Biophys Res Commun. 2005;331:1439–1444. doi: 10.1016/j.bbrc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 26.Calin GA, Ferracin M, Cimmino A, Di Leva G, Shimizu M, Wojcik S, Iorio MV, Visone R, Sever NI, Fabbri M, et al. N Engl J Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 27.Chen CZ, Li L, Lodish HF, Bartel DP. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 28.Ohler U, Yekta S, Lim LP, Bartel DP, Burge CB. RNA. 2004;10:1309–1322. doi: 10.1261/rna.5206304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parizotto EA, Dunoyer P, Rahm N, Himber C, Voinnet O. Genes Dev. 2007;18:2237–2242. doi: 10.1101/gad.307804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai X, Hagedorn CH, Cullen BR. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim VN. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 33.Davuluri RV, Grosse I, Zhang MQ. Nat Genet. 2001;29:412–417. doi: 10.1038/ng780. [DOI] [PubMed] [Google Scholar]
- 34.Kent WJ. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau NC, Lim LP, Weinstein EG, Bartel DP. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 36.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ambros V. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 39.Costa FF. Gene. 2005;357:83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Mendell JT. Cell Cycle. 2005;4:1179–1184. doi: 10.4161/cc.4.9.2032. [DOI] [PubMed] [Google Scholar]