Abstract
Plants have evolved multiple mechanisms to selectively suppress pathogens by production of secondary metabolites with antimicrobial activities. Therefore, direct selections for antiviral compounds from plants can be used to identify new agents with potent antiviral activity but not toxic to hosts. Here, we provide evidence that a class of compounds, seco-pregnane steroid glaucogenin C and its monosugar-glycoside cynatratoside A of Strobilanthes cusia and three new pantasugar-glycosides of glaucogenin C of Cynanchum paniculatum, are effective and selective inhibitors to alphavirus-like positive-strand RNA viruses including plant-infecting tobacco mosaic virus (TMV) and animal-infecting Sindbis virus (SINV), eastern equine encephalitis virus, and Getah virus, but not to other RNA or DNA viruses, yet they were not toxic to host cells. In vivo administration of the compounds protected BALB/c mice from lethal SINV infection without adverse effects on the mice. Using TMV and SINV as models, studies on the action mechanism revealed that the compounds predominantly suppress the expression of viral subgenomic RNA(s) without affecting the accumulation of viral genomic RNA. Our work suggested that the viral subgenomic RNA could be a new target for the discovery of antiviral drugs, and that seco-pregnane steroid and its four glycosides found in the two medicinal herbs have the potential for further development as antiviral agents against alphavirus-like positive-strand RNA viruses.
Keywords: antiviral drugs, Strobilanthes cusia, Cynanchum atratum
Viruses replicate in host cells by hijacking the host-cell metabolic machinery. It is therefore difficult for antiviral therapies to inhibit only viral functions but spare host cellular processes. Nevertheless, great progress has been achieved over the past decades in antiviral research and therapy to reduce morbidity and mortality in virus-infected individuals (1, 2), and many antiviral drugs have been approved in recent years, most of which targeted either viral proteins or host cellular proteins. However, some of these compounds might induce the resistance of viruses to them and cause cell toxicity (3–5). The continuing emergence of highly pathogenic viruses like avian influenza viruses and severe acute respiratory syndrome coronavirus underscores the importance of advancing the search for effective antiviral agents. Plants have evolved constitutive and inducible defense mechanisms by producing a vast array of secondary metabolites against various microbial pathogens (6). Many herbaceous plants have been used in traditional Chinese medicine to treat viral diseases (7). It is conceivable that antiviral compounds would occur in plants as part of their innate defense arsenal, and the vast assortment of secondary metabolites could serve as a large pool for screening for previously undescribed antiviral compounds with a selective target spectrum and nontoxic to the host plants. Here, we provide evidence that a class of compounds, the seco-pregnane steroid and its glycosides from Strobilanthes cusia and Cynanchum paniculatum, are effective and selective inhibitors of several members of the positive-strand RNA-containing alphavirus-like supergroup, not only of plant origin but also including animal-infecting viruses. We also show that these compounds exert their antiviral activity with a previously undescribed mechanism by specifically suppressing the expression of viral subgenomic (sg)RNA without affecting the viral genomic RNA expression.
Results
Identification of Small-Molecule Compounds Inhibiting Tobacco Mosaic Virus (TMV) Infection.
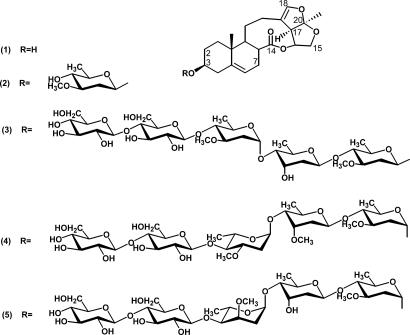
Although S. cusia and C. paniculatum have long been used in traditional Chinese medicine to treat some human diseases, including those caused by infection from several viruses (8–10), the plant constituents responsible for the antiviral activities remain unknown. To screen for active antiviral compounds, TMV was chosen as a model target and the conventional local lesion assay was used to examine plant extract fractions containing virus-inhibitory materials. By anti-TMV activity-guiding fractionations, glaucogenin C (1) and cynatratoside A (2) were purified from S. cusia (Fig. 1) and identified as potential inhibitors of TMV multiplication after a series of chromatographic separations. Interestingly, compounds 1 and 2 were previously isolated from other herb species, Cynanchum glaucescens (11) and Cynanchum atratum (12), respectively. This fact prompted our efforts to search for more seco-pregnane steroid analogs with anti-TMV activity in C. paniculatum, which led to the isolation of three new seco-pregnane steroidal glycosides paniculatumoside C (3), paniculatumoside D (4), and paniculatumoside E (5) [Fig. 1; supporting information (SI) Tables 1–3]. These three glycosides all contain five sugar units in their oligosaccharide moieties linked to the hydroxyl group at C-3 of the same aglycone (glaucogenin C, 1) but differ in the configuration of the first sugar and in the structures of the second and third sugars (Fig. 1). Our results show that the seco-pregnane steroid glaucogenin C and its glycosidal derivatives isolated from S. cusia and C. paniculatum possess antiviral activity.
Fig. 1.
Structures of the antiviral seco-pregnane steroid and its glycosides isolated from S. cusia (1 and 2) and C. paniculatum (3–5).
Dose-Dependent Inhibition of TMV Infection by the Seco-Pregnane Steroid and Its Glycosides.
To further characterize the inhibitory effect of the compounds on TMV infection, TMV solutions premixed with different concentrations of each purified compound were used as inocula to infect tobacco BY-2 protoplasts and the level of TMV multiplication was assayed on the local lesion host Chenopodium quinoa. As shown in SI Table 4, the numbers of TMV-induced local lesions were inversely proportional to the concentrations of each compound in the inocula, and at 50 nM all tested compounds completely blocked TMV replication. The calculated IC50 value for compound 1 is ≈17 nM, and the IC50 values of its four glycosidal derivatives are each ≈25 nM. These results imply that the aglycon per se is responsible for the inhibitory effect, and the linked oligosaccharide chains make little contribution to antiviral activity. Furthermore, the size of local lesions was reduced gradually with increasing amount of the compounds in the inoculum (SI Table 4).
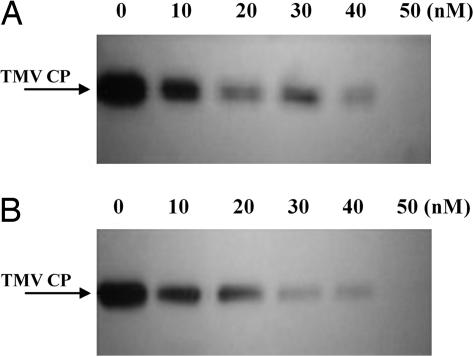
The level of accumulation of TMV coat protein (CP) serves as a good molecular marker for the extent of virus multiplication. The TMV CP levels in BY-2 protoplasts infected with TMV together with different concentrations of each compound were determined by Western blot analysis. As shown in Fig. 2, the level of TMV CP gradually decreased with increasing concentration of compound 1 (Fig. 2A) or 3 (Fig. 2B), and at 50 nM, the synthesis of TMV CP was completely abolished. Compounds 2, 4, and 5 exhibited similar inhibitory effects on the synthesis of TMV CP when present in the inocula (data not shown). These molecular data are consistent with those obtained from the bioassay described in the preceding paragraph. Collectively, these results indicated that the seco-pregnane steroid and its glycosides inhibited TMV replication in a dose-dependent manner.
Fig. 2.
Effects of glaucogenin C and paniculatumoside C on accumulation of TMV CP in protoplasts. (A and B) Total protein was extracted from 5 ml of protoplasts (1.5 × 106/ml) infected with TMV pretreated with different concentrations of glaucogenin C or paniculatumoside C, respectively. An equal amount (10 μg) of total protein was electrophoresed in each lane for Western blot analysis.
Seco-Pregnane Steroid Inhibited TMV Replication in Intact Plants.
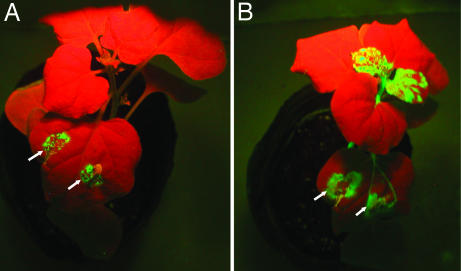
We further investigated whether seco-pregnane steroid and its glycosides could inhibit TMV replication in intact plants. Two approaches were used. First, Nicotiana tabacum cv. K326 plants, a systemic host of TMV, were inoculated with TMV alone or TMV plus 50 nM of compound 3, 4, or 5. The results showed that in the plants infected with TMV in the presence of the compounds, infection was limited to the inoculated leaves, which developed necrotic lesions at 5 days postinfection (dpi). Notably, no symptoms were observed on uninoculated leaves during the whole life span of the plants. By contrast, plants infected by TMV alone developed severe disease symptoms in inoculated and uninoculated leaves (data not shown). Second, a modified TMV expression vector (30B:GFP) carrying a GFP gene downstream of the TMV CP sgRNA promoter, which can systemically infect and express GFP in Nicotiana benthamiana plants (13), was used to facilitate monitoring the replication of the virus vector in whole plants (see SI Text for assay of systemic movement of TMV). N. benthamiana plants were infected with 30B:GFP through agroinfiltration. When an Agro35S-30B:GFP suspension containing 50 nM compound 3 was used to infect the plants, green fluorescent spots were visible under long-wave UV light in inoculated leaves at 5 dpi. However, green fluorescence was observed only around the infiltrated areas and not in upper uninoculated leaves (Fig. 3A). Tests with the Agrobacterium inoculum incorporating 1, 4, or 5 gave similar results (data not shown). As a control, infection by Agro35S-30B:GFP without the compounds generated green fluorescence not only in the inoculated leaves but also in upper systemic leaves (Fig. 3B). Together, these results suggest that the seco-pregnane steroid and its glycosides can inhibit replication of TMV in its host plants, resulting in the failure of systemic virus infection.
Fig. 3.
Effect of paniculatumoside C (3) on TMV spread in N. benthamiana plants. (A) A N. benthamiana plant inoculated on one of the lower leaf with Agro35S-30B:GFP containing 50 nM of the compound. (B) A N. benthamiana plant inoculated with Agro35S-30B:GFP without the compound. The plants were photographed under long-wave UV light at 5 dpi. Arrows indicate the inoculation sites.
Seco-Pregnane Steroid and Glycosides Selectively Inhibit the Accumulation of TMV sgRNAs.
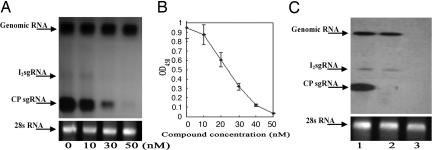
It is known that TMV requires CP for long-distance movement (14, 15), and the 30-kDa movement protein (MP) is responsible for the cell-to-cell spread of TMV (16). Having demonstrated that the seco-pregnane steroid and its glycosides can restrict the long-distance movement and, to a lesser extent, cell-to-cell spread of TMV in its host plants, we sought to investigate the molecular mechanisms underlying these inhibitory effects. TMV CP and MP are translated, respectively, from a sgRNA, originally called low molecular mass component (17, 18) and from the I2 sgRNA (19), both produced during virus replication and 3′-coterminal with the TMV genomic RNA. The accumulation level of the CP sgRNA or I2 sgRNA is thus a key determinant for the amount of CP or MP synthesized. The steady-state levels of the CP sgRNA and I2 sgRNA as well as the genomic RNA were determined after infection by TMV supplemented with varying concentrations of compound 3 by Northern blot analysis using the CP gene sequence as a probe. In BY-2 protoplasts, the three viral RNAs responded to the action of the compound differentially. At 4 hours postinfection (hpi), accumulation of the CP sgRNA was markedly reduced along with the increase in concentration of the compound and completely vanished at 50 nM (Fig. 4A). At the same time point, decrease in the level of the I2 sgRNA correlating to increasing concentration of the compound was not as sharp as that of the CP sgRNA and surprisingly, the level of the genomic RNA remained unchanged even at the highest concentration tested (50 nM) (Fig. 4A). In parallel, we determined the CP level in infected protoplasts for each concentration of 3 by the ELISA. The results showed that the CP level decreased with increasing concentration of the compound at a slope similar to that of the reduction of the CP sgRNA level (Fig. 4B), suggesting that the translational efficiency of the CP sgRNA and the stability of CP were not affected by the compound.
Fig. 4.
Effect of paniculatumoside C (3) on accumulation of TMV RNAs and the CP. (A) Northern blot analysis of viral RNAs with the CP gene sequence as a probe. 28S rRNA stained with ethidium bromide was used as an RNA loading control. Total RNA from BY-2 protoplasts 4 h after infection with TMV treated with different concentrations of the compound. (B) ELISA analysis of TMV CP. For each concentration of the compound, the assay was repeated three times. Error bars indicate SD. (C) Effect of paniculatumoside C on expression of TMV RNAs in N. tabacum cv. K326 plants. Total RNA was isolated from leaves inoculated with TMV in the absence (lane 1) or presence (lane 2) of 50 nM of 3 and analyzed by Northen blot hybridization by using the CP gene sequence as a probe. Total RNA from the upper uninoculated leaves of the plant infected by TMV treated with 50 nM compound was analyzed in parallel (lane 3). Equal RNA loading was monitored by ethidium bromide staining of 28S rRNA.
We also analyzed the accumulation levels of the three viral RNAs in the inoculated leaves of N. tabacum cv. K326 plants infected by TMV with or without 50 nM compound 3. As shown in Fig. 4C (lanes 1 and 2), the CP sgRNA was almost completely wiped out by the compound, whereas the abundance of the I2 sgRNA was only slightly reduced and that of the genomic RNA apparently unaffected. These results are in perfect accordance with the data obtained from the experiments carried out in the infected protoplasts (Fig. 4A). Furthermore, none of the TMV RNAs was detected in the uninoculated leaves of the tobacco plant infected with TMV containing 50 nM compound 3 (Fig. 4C, lane 3), confirming this compound inhibited systemic TMV infection in the host plant. Tests with the other four compounds gave similar results (data not shown).
Taken together, our data suggest that glaucogenin C and cynatratoside A isolated from S. cusia, and paniculatumosides C, D and E isolated from C. paniculatum exert their inhibitory effects on TMV infection mainly through selective suppression of the CP sgRNA expression in infected plant cells, which in turn diminishes the synthesis of CP and impairs production of the TMV virions and the systemic spread of the virus in host plants.
Suppression of Sindbis Virus (SINV)-Induced Cytopathy and SINV Propagation in BHK-21 Cells by the Compounds.
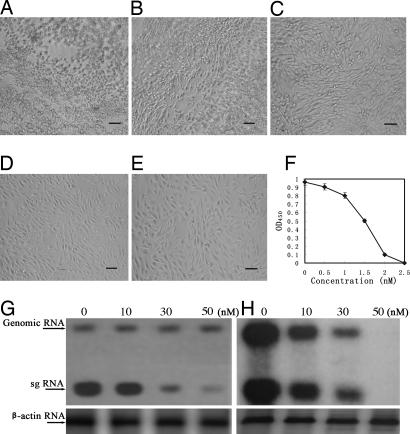
Having demonstrated that glaucogenin C and its glycosides inhibited TMV replication by specifically impairing the expression of viral sgRNAs, we were interested to see whether the compounds would exert inhibitory effects on SINV, an alphavirus that resembles TMV in genome organization and replication strategy, especially in exploiting sgRNAs to express their structural proteins. We first examined the influence of the compounds on the cytopathic effects of SINV infection in BHK-21 cells. As an example, Fig. 5A–D show the dose-dependent inhibition of SINV-induced cytopathy by compound 3. Tests with compounds 1, 2, 4, or 5 showed similar results (data not shown). None of the compounds was inhibitory to the growth of BHK-21 cells after a 48-h incubation at concentrations of 100 nM (e.g., see Fig. 5E), 40 times the concentration (2.5 nM) at which the SINV-induced cytopathic effect was nearly abolished.
Fig. 5.
Effect of paniculatumoside C (3) on SINV-induced cytopathic effect, SINV propagation, and viral RNAs expression in BHK-21 cells. (A–D) Cell morphology was observed under a light microscope 24 h after infection with SINV containing 0, 1.0, 1.5, or 2.5 nM of 3 or (E) after mock infection in the presence of 100 nM compound. The protective effects against SINV infection are indicated by: + (weak, B), + + (moderate, C), + + + (obvious, D). Bars in the images represent 50 μm. (F) SINV accumulation quantified by ELISA assays as described in Materials and Methods. For each concentration of the compound presented in the inoculum, nine repeated assays were performed and their OD450 values (after subtraction of the blank readings) averaged. (G and H) Northern blot analysis of SINV RNA expression in the presence of different concentrations of paniculatumoside C. BHK-21 cells were infected with SINV with or without the compound. Total RNA was extracted at 3 (G) and 24 (H) hpi, and viral RNAs were probed with the 32P-labeled DNA sequence corresponding to the SINV capside protein gene. The β-actin RNA served as a control to allow comparison of amount of viral RNAs between lanes.
The anti-SINV activity of the compounds was also revealed by suppression of SINV accumulation in BHK-21 cells when each of the compounds was present in the inocula. ELISA assays showed that the amount of SINV in BHK-21 cells decreased along with the increased concentrations of compound 3 and was reduced to an undetectable level at 2.5 nM with an estimated EC50 of 1.5 nM (Fig. 5F). The concentration of the compounds that inhibited uninfected BHK-21 cell proliferation by 50% (CC50) was ≈16.5 μM (data not shown). This resulted in a selectivity index value of 11000 (CC50/EC50). Tests with the other four compounds gave similar results (data not shown).
Seco-Pregnane Steroid and Glycosides Preferentially Inhibit the Expression of SINV sgRNA.
In the SINV replication cycle, two viral mRNAs are produced and can be readily detected in infected cells: the 49S genomic RNA, which is translated into the viral nonstructural proteins, and the 26S sgRNA, which corresponds to the 3′ third of the genomic RNA and encodes the viral structural proteins. To determine whether the inhibition of SINV multiplication by the compounds is because of the selective inhibition of the sgRNA expression, like the mechanism of inhibition of TMV replication, Northern blot hybridizations were carried out to analyze the synthetic kinetics of the SINV RNAs in BHK-21 cells infected by SINV containing different concentrations of the compounds. Fig. 5 shows the effects of compound 3 (the other four compounds exhibited similar activities; data not shown). At the early stage of infection (3 hpi), the level of the 26S sgRNA decreased almost linearly with an increase in the concentration of compound 3, and no obvious change was observed in the level of the genomic RNA even at the highest concentration tested (50 nM) (Fig. 5G). At the late stage of infection (24 hpi), the level of SINV genomic RNA and that of 26S sgRNA reduced at a similar rate on increase in compound 3 concentration (Fig. 5H), possibly because at that stage, the active synthesis of both viral RNAs ceased. Nevertheless, these results demonstrate that the compounds preferentially suppressed the expression of SINV sgRNA in the virus replication process.
Effect of Compound 3 on the Mortality of SINV-Infected Newborn BALB/c Mice.
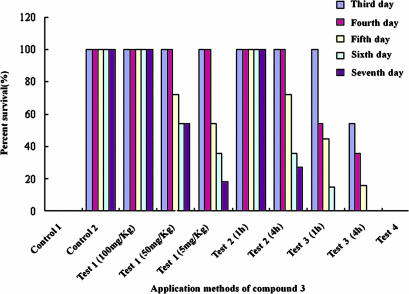
The in vitro potency of the compounds against SINV replication was an encouraging finding, but a further validation of its antiviral activity required use of an animal infection model. To do this, we studied the effects of compound 3 against lethal SINV infection using different drug administration approaches. Compound 3 proved particularly effective in reducing the mortality rate of challenged newborn BALB/C mice when administered at a dose of 5, 50, or 100 mg/kg body weight (BW) mixed with SINV before inoculation: at these dosages, 18%, 54%, and 100% of the mice were recorded as long-term survivors, respectively (Fig. 6). Moreover, no evidence of disease was observed in surviving mice after 3 weeks, indicating that virus was cleared and that compound 3 not only suppressed virus replication but also delayed disease onset. When mice were treated with compound 3 at a dose of 100 mg/kg BW 1 h before inoculation with SINV, survival was up to 100% at 7 dpi. When SINV was inoculated 4 h after treatment with compound 3, survival was reduced to 30% at 7 dpi. However, when compound 3 was administrated 1 or 4 h after SINV infection, no mice survived, although delayed mortality was observed. Furthermore, when compound-treated mice that survived infection were subsequently inoculated with a lethal dose (100 × LD50) of SINV on day 7 after the initial viral challenge, no mice survived the observation period (Fig. 6). These results suggest that compound 3 protected mice from SINV lethal infection by directly inhibiting SINV multiplication rather than mounting a protective immune response to SINV infection.
Fig. 6.
Protection of BALB/c mice from SINV infection by paniculatumoside C (3) treatments. Mice inoculated with 20 μl of SINV (100 × LD50) alone and treated with 20 μl of 3 alone at a dose 100 mg/kg BW were served as control 1 and control 2, respectively. Mice were inoculated with 20 μl (100 × LD50) of SINV mixed with 20 μl of 3 at a dose of 5, 50, or 100 mg/kg BW (Test 1). Mice were treated with 3 at a dose of 100 mg/kg BW 1 and 4 h before inoculation with 100 × LD50 of SINV (Test 2). Mice were administrated with 3 at a dose 100 mg/kg BW 1 or 4 h after SINV infection (Test 3). Compound 3-treated mice that survived infection were inoculated with a lethal dose (100 × LD50) of SINV on day 7 after the initial viral challenge (Test 4). For each treatment, 11 mice were tested and percent survivals calculated based on the number of survival mice counted at 3, 4, 5, 6, and 7 days after the treatment.
Taken together, the results demonstrate that these plant-derived compounds inhibit the multiplication of SINV in vitro and in vivo.
Antiviral Potency, Selectivity, and Activity Spectrum of Compound 3.
As a representative of the seco-pregnane steroidal glycoside analogs, compound 3 proved to be an effective inhibitor of several RNA viruses, including SINV, Getah virus (GETV), and eastern equine encephalitis virus (EEEV), belonging to the alphavirus-like supergroup (Table 1). The compound inhibited the replication of these viruses in various animal and human cells. The EC50 of the compound 3 for inhibition of SINV, GETV, and EEEV were 1.5, 1, and 2 nM, respectively. The selectivity index (50% cytotoxicity concentration/EC50) for the inhibition of SINV, GETV, and EEEV was calculated to be 11,000, 16,500, and 8,250, respectively. On the other hand, compound 3 showed no activity against other RNA or DNA viruses, i.e., Japanese encephalitis virus (JEV), hepatitis C virus (HCV), HIV, measles virus, influenza virus A, Reovirus, and Adenovirus (HAdV-4), at a concentration of >20 μM (Table 1).
Table 1.
Compound 3 antiviral spectrum of activity and selectivity
| Virus | Family | Classification | EC50, nM* |
|---|---|---|---|
| SINV | Togaviridae | Positive single-strand RNA | 1.5 |
| GETV | Togaviridae | Positive single-strand RNA | 1 |
| EEEV | Togaviridae | Positive single-strand RNA | 2 |
| JEV | Flaviviridae | Positive single-strand RNA | >25,000 |
| HCV | Flaviviridae | Positive single-stranded RNA | >25,000 |
| HIV | Retroviridae | Positive single-stranded RNA | >25,000 |
| Measles virus | Paramyxoviridae | Negative single-stranded RNA | >25,000 |
| Influenza virus A | Orthomyxoviridae | Negative single-stranded RNA | >20,000 |
| Reovirus | Reoviridae | Double-stranded RNA | >25,000 |
| HAdV-4 | Adenoviridae | Double-stranded DNA | >25,000 |
*Compound concentration required to achieve 50% inhibition of SINV, EEEV, GETV-induced cytopathogenicity in (BHK21), JEV-induced cytopathogenicity in C6 cells, HCV-induced cytopathogenicity in B cells, HIV-induced cytopathogenicity in H9 cells, measles virus-induced cytopathogenicity in Veto cells, influenza virus-induced cytopathogenicity in Madin–Darby canine kidney cells, Reovirus-induced cytopathogenicity in CIK cells, and adenovirus-induced cytopathogenicity in 983A cells. EC50 values are the averages of at least two independent determinations.
Discussion
One of the major challenges in designing ideal antiviral agents is to confer on them the ability to distinguish target viruses from host cells, notwithstanding that viruses use the host's biosynthetic machinery for multiplication. In this regard, plants could be a rich source for finding potential antiviral agents. Plants have evolved multiple layers of defense mechanisms against microbial infection by producing >100,000 low-molecular-mass secondary metabolites, many of which might have the potency to selectively inhibit virus replication but no toxicity to the plant itself (6, 20, 21). Such examples have been provided in the anti-HIV research: a large variety of natural products have been described as anti-HIV agents (22), such as ingenol derivatives (23), betulinic acid and its derivatives (24, 25), and hypericin (26, 27). On the contrary, few natural inhibitors against plant-infecting viruses were reported except the anti-TMV agent pyrroloisoquinoline alkaloids (28).
The mechanisms of plant chemical defenses imply that the plant metabolites against plant viruses may have low toxicities to the plant hosts themselves. Furthermore, it is reasonable to predict that plant chemical agents against plant viruses might also be effective against animal-infecting viruses with similar replication mechanisms. Therefore, we should be able to discover new agents against animal viruses from plant antiviral defenses with low toxicities to both plant and animal host cells. Guided by this idea, we selected S. cusia as a source in our primary experiments to investigate antiviral agents. We have shown that S. cusia extracts exhibited anti-TMV activities (data not shown). In this research, by anti-TMV activity-guiding isolation, we found that seco-pregnane steroids glaucogenin C (1) and cynatratoside A (2) from S. cusia, and later, three chemical analogs, paniculatumoside C (3), paniculatumoside D (4), and paniculatumoside E (5) from C. paniculatum, were metabolites with potent antiviral activities and nontoxic to hosts. These compounds were found to be specifically active against the alphavirus-like supergroup of positive-strand RNA viruses, including both plant-infecting TMV and animal viruses SINV, EEEV, and GETV, suggesting that the compounds inhibit a conserved viral target essential for replication of these viruses. The specificity of the compounds' antiviral activity was supported by the observations that the compounds were inactive against unrelated RNA- and DNA-containing viruses.
Using TMV and SINV as models, study of the mechanism of action revealed that the compounds predominantly suppressed the expression of sgRNA without affecting accumulation of viral genomic RNA, implying that the compounds selectively target the viral sgRNA expression machinery. The alphavirus-like supergroup members share similarities in genome organization and expression strategy, including production of sgRNA(s), which direct(s) synthesis of the viral structural proteins and some nonstructural proteins (29). It is generally believed that the transcription machinery for expression of the sgRNA of both TMV and SINV mainly consists of a transcriptase complex formed by the viral RNA-dependent RNA polymerase (RdRp) and some cellular factors (and other viral nonstructural proteins in the case of SINV), and a stretch of nucleotides on the negative-strand genome-length RNA surrounding the beginning of the sgRNA, called the sgRNA promoter to which the transcriptase binds. Apparently the viral RdRp is not a target of the compounds, because replication of the viral genomic RNA is also catalyzed by the same RdRp. Our results imply that the compounds might alter the structure of the sgRNA promoter, affect the function of the cellular factors associated with the transcriptase complex or interfere with the binding of the transcriptase to the sgRNA promoter. At present, we have no experimental data to define in which of the above-mentioned ways the compounds impair the expression of the viral sgRNA. Comparison of the core sequences of the sgRNA promoters from the alphavirus-like supergroup members revealed a high similarity not only among the animal-infecting viruses but also extending to several plant-infecting members in a number of nucleotides crucial for promoter activity (30, 31). Thus compounds targeting the TMV and SINV sgRNA promoters might also target sgRNA promoters of other members of the alphavirus-like supergroup. In support of this hypothesis, compound 3 has been shown to be active against two other tested members (i.e., EEEV and GETV) of the alphavirus-like supergroup but not against other RNA and DNA viruses, which do not produce sgRNA during their life cycle. Taken together, these results suggest that the compounds target conserved factors essential for expression of viral sgRNA.
We have also shown that compound 3 protected mice from challenge with lethal dose of SINV when the compound was present in the inoculum or administrated before virus inoculation. These results indicate that the compound directly inhibits virus multiplication rather than induces an immune response. Unlike other saponins such as triterpenoid saponins or C-27 steroidal saponins, compounds 2 and 3 at the concentrations of 20–100 μg/ml did not cause hemolysis and agglutination of rabbit erythrocytes in our experiments (data not shown). Thus, maximum therapeutic benefit requires the continued presence of the compound to inhibit virus multiplication.
Taken together, the potent anti-alpha-like virus activity of the compounds in vitro and in vivo makes them lead candidates for development as antiviral drugs to prevent alphavirus superfamily and other positive-strand RNA virus infections in humans and in plants. In conclusion, our work suggests that suppressing of the viral sgRNA expression is a valid antiviral strategy, and our results pave the way for the discovery of more potential antiviral compounds from plant sources.
Materials and Methods
Extraction and Structure Determination of C21-Steroidal Glycosides.
Compounds 1, 2 and 3, 4, and 5 were isolated from S. cusia and C. paniculatum, respectively, as described in SI Text. The NMR data assignments for the three steroidal glycosides (3, 4, and 5) are presented in SI Tables 1, 2, and 3, respectively.
Quantification of TMV CP.
BY-2 protoplasts (see SI Text for preparation of tobacco BY-2 protoplasts) were inoculated with TMV pretreated with different concentrations of the compounds as described above. Total protein was extracted at 4 or 12 hpi from 5 ml of infected protoplasts (32) and quantified by the Coomassie dye-binding assay (Bio-Rad, Hercules, CA) (33). The accumulation levels of TMV CP in the protoplasts were determined by ELISA (34) and Western blot analysis (35).
Analysis of TMV RNAs.
Infections of BY-2 protoplasts and N. tabacum cv. K326 plants by TMV with or without compound 3 are as described in SI Text (local lesion assay of TMV). Portions of infected protoplasts were collected at 4 hpi and the inoculated and upper uninoculated leaves collected at 5 dpi. Total RNA was extracted from the protoplasts and from the tobacco leaves by using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Hybridization, washing, and autoradiography were performed by using standard methods (35).
Cytopathic Effect Assay of SINV.
For testing the antiviral activity of the compounds, 2 × 105 pfu/ml of thrice-plaque-purified SINV strain XJ-160 (36) was treated with different concentrations of each compound for 15 min and adsorbed to BHK-21 cells at a multiplicity of infection of 0.01 pfu (induced 100% cytopathogenicity at the third day after infection) per cell in 96-well plates (in quadruplicate). After a 1-h adsorption period, the inoculum (0.1 ml) was removed, and cell monolayers were washed thoroughly with serum-free DMEM. Fresh DMEM (0.1 ml) supplemented with 5% FBS, 10 units/ml of penicillin, and 10 μg/ml of streptomycin was added to each well. Cells were maintained in a humidified 5% CO2 incubator at 37°C. At 24 hpi, the cytopathic morphology of the cells was examined by using a light microscope (see SI Text for cytotoxicity test of the compounds on BHK-21 cells).
Quantification of SINV.
Inoculation of BHK-21 cells with SINV (XJ-160) mixed with different concentrations of the compounds was as described above. The accumulation of SINV was quantified by ELISA (37).
Analysis of SINV RNA Expression.
To study the effects of compounds on accumulation of SINV RNAs, BHK-21 cells were infected at a multiplicity of 10 pfu per cell in 96-well plates (in quadruplicate). The accumulation of SINV RNAs in BHK-21 cells infected by SINV mixed with different concentrations of the compounds was quantified by Northern blot hybridization (38).
Effects of Compound 3 on the Survival of SINV-Infected Mice.
BALB/c mice (2–3 days old, weighing ≈2 g) were intracerebrally inoculated each with 20 μl of maintenance medium containing 100 × LD50 of SINV simultaneously with, before, or after administration of compound 3. In experiments with mixed SINV and compound 3 as inocula, 20 μl of compound 3 corresponding to 5, 50, or 100 mg/kg BW was included in the inoculum. In treatments where compound 3 was administrated 1 or 4 h before or after inoculation with SINV, 20 μl of compound 3 at 100 mg/kg BW was used. In another treatment, the mice surviving 7 days after inoculation with mixed SINV and compound 3 were reinoculated with a lethal dose (100 × LD50) of SINV. Three additional groups of mice, naive, killed, and received only compound 3 (100 mg/kg BW) were included as controls in this study to assess the toxicity of compound 3 and the immune status of mice that surviving infection as a result of compound treatment. Mice in the naive group were left untreated, whereas mice in the killed group received a lethal dose of SINV. For each treatment, 11 mice were inoculated, and the survived mice were counted 3, 4, 5, 6, and 7 days after inoculation. All animals were housed in filter-top microisolator cages and fed commercial mouse chow. Animal husbandry and experimental procedures were in accordance with China Public Health Service policy and were approved by the Institutional Animal Care and Use Committee or by the Ethical Committee on Vertebrate Animal Experiments of Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention.
Antiviral Spectrum of Compound 3.
SINV, GETV, EEEV, JEV, HCV, HIV, measles virus, influenzavirus A, Reovirus, and Adenovirus (HAdV-4) were obtained from the Chinese Center for Disease Control and Prevention, and EEEV, JEV, and HCV were obtained from John Hopkins Medical University (Baltimore, MD). All assays were carried out in the appropriate medium. Ninety-six-well cell culture plates were seeded 24 h before use with 2 × 105 cells of BHK-21 (for SINV and GETV), 2 × 105 cells of C6/36 (for EEEV and JEV), 2 × 105 cells of B (for HCV), 2 × 105 cells of Madin–Darby canine kidney (for influenza virus A), 2 × 105 cells of H9 (for HIV), 2 × 105 cells of Veto (for measles virus), 2 × 105 cells of CIK (for Reovirus), or 2 × 105 cells of 983A (for HAdV-4) cell line per well. Cells were infected at the cell culture infectious dose causing 100% cytopathic effects at 3 dpi for these viruses. Compound 3 was mixed with the viruses at final concentrations of 100, 50, 10, 3, 2, 1.5, 1, and 0 nM before virus inoculation. As controls, uninfected cells and cells receiving these viruses without compound were included on each assay plate. EC50 was defined as the concentration of the compound that inhibited 50% virus-induced cytopathic cells.
Acknowledgments
We are grateful to Linqi Wang and Yuqun Piao for assistance in the tests of EEEV, JEV, and HCV. We also thank Shihong Fu for analyses of SINV, JEV, Reovirus, and GETV; Zhen Zhu, Aili Cui, and Liuying Tang for analyses of measles virus and Adenovirus; and Jie Dong for analysis of influenza virus. This work was supported by the National Natural Science Foundation of China (Grant 30370957) and the Natural Science Foundation of Yunnan Provence (Grant 2003C0061M).
Abbreviations
- BW
body weight
- CP
coat protein
- dpi
days postinfection
- EEEV
eastern equine encephalitis virus
- GETV
Getah virus
- HCV
hepatitis C virus
- hpi
hours postinfection
- JEV
Japanese encephalitis virus
- SINV
Sindbis virus
- TMV
tobacco mosaic virus
- sg
subgenomic.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702398104/DC1.
References
- 1.De Clercq E. Curr Opin Microbiol. 2005;8:552–560. doi: 10.1016/j.mib.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crumpacker C. In: Fields Virology. Knipe DM, Howley PM, editors. Philadephia: Williams & Wilkins; 2001. pp. 393–433. [Google Scholar]
- 3.De Clercq E. J Pharmacol Exp Ther. 2001;297:1–10. [PubMed] [Google Scholar]
- 4.De Clercq E. Nat Rev Drug Discov. 2002;1:13–25. doi: 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- 5.De Clercq E. Nat Rev Microbiol. 2004;2:704–720. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon RA. Nature. 2001;411:843–847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- 7.Chinese Pharmacopoeia Commission; Chinese Pharmacopoeia Commission, editor. Chinese Pharmacopoeia. Beijing, China: Chemical Industry Press; 2005. [Google Scholar]
- 8.College NM; College NM, editor. Zhong Yao Da Cidian. Shanghai, China: Shanghai Scientific and Technological Press; 1979. pp. 1250–1252.pp. 1894–1895. and. [Google Scholar]
- 9.Mak NK, Leung CY, Wei XY, Shen XL, Wong RN, Leung KN, Fung MC. Biochemical Pharmacology. 2004;67:167–174. doi: 10.1016/j.bcp.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Ko HC, Wei BL, Chiou WF. J Ethnopharmacol. 2006;107:205–210. doi: 10.1016/j.jep.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa T, Hayashi K, Mitsuhashi H. Chem Pharm Bull. 1983;31:870–878. doi: 10.1248/cpb.31.3971. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZX, Zhou J, Hayashi K, Mitsuhashi H. Chem Pharm Bull (Tokyo) 1985;33:1507–1514. doi: 10.1248/cpb.33.1507. [DOI] [PubMed] [Google Scholar]
- 13.Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO. Virology. 1999;255:312–323. doi: 10.1006/viro.1998.9579. [DOI] [PubMed] [Google Scholar]
- 14.Dawson WO, Bubrick P, Grantham GL. Phytopathology. 1988;78:783–789. [Google Scholar]
- 15.Saito T, Yamanaka K, Okada Y. Virology. 1990;176:329–336. doi: 10.1016/0042-6822(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 16.Deom CM, Oliver MJ, Beachy RN. Science. 1987;237:389–394. doi: 10.1126/science.237.4813.389. [DOI] [PubMed] [Google Scholar]
- 17.Hunter TR, Hunt T, Knowland J, Zimmern D. Nature. 1976;260:759–764. doi: 10.1038/260759a0. [DOI] [PubMed] [Google Scholar]
- 18.Siegel A, Hari V, Montgomery I, Kolacz K. Virology. 1976;73:363–371. doi: 10.1016/0042-6822(76)90397-4. [DOI] [PubMed] [Google Scholar]
- 19.Beachy RN, Zaitlin M. Virology. 1977;81:160–169. doi: 10.1016/0042-6822(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 20.Thomas CM, Tang S, Hammond-Kosack K, Jones JD. Mol Plant–Microbe Interact. 2000;13:465–469. doi: 10.1094/MPMI.2000.13.4.465. [DOI] [PubMed] [Google Scholar]
- 21.Roger H. Matthews' Plant Virology. London, UK: Academy Press; 2002. [Google Scholar]
- 22.De Clercq E. Med Res Rev. 2000;20:323–349. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara M, Ijichi K, Tohuhisa K, Katsuura K, Wang G-Y-S, Uemura D, Shigeta S, Konno K, Yokota T, Baba M. Antiviral Chem Chemother. 1996;7:230–236. doi: 10.1128/aac.40.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH. J Nat Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 25.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. J Med Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 26.Meruelo D, Lavie G, Lavie D. Proc Natl Acad Sci USA. 1988;85:5230–5234. doi: 10.1073/pnas.85.14.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie G, Valentine F, Levin B, Mazur Y, Gallo G, Lavie D, Weiner D, Meruelo D. Proc Natl Acad Sci USA. 1989;86:5963–5967. doi: 10.1073/pnas.86.15.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An T, Huang RQ, Yang Z, Zhang DK, Li GR, Yao YC, Gao J. Phytochemistry. 2001;58:1267–1269. doi: 10.1016/s0031-9422(01)00382-x. [DOI] [PubMed] [Google Scholar]
- 29.Goldbach R, Olivier Le Gall O, Wellink J. Semin Virol. 1991;2:19–25. [Google Scholar]
- 30.Siegel RW, Adkins S, Kao CC. Proc Natl Acad Sci USA. 1997;94:11238–11243. doi: 10.1073/pnas.94.21.11238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wielgosz MM, Raju R, Huang HV. J Virol. 2001;75:3509–3519. doi: 10.1128/JVI.75.8.3509-3519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe Y, Kishibayashi N, Motoyoshi F, Okada Y. Virology. 1987;161:527–532. doi: 10.1016/0042-6822(87)90147-4. [DOI] [PubMed] [Google Scholar]
- 33.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Wu CY, Jan JT, Ma SH, Kuo CJ, Juan HF, Cheng YS, Hsu HH, Huang HC, Wu D, Brik A, et al. Proc Natl Acad Sci USA. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 36.Liang GD, Li L, Zhou GL, Fu SH, Li QP, Li FS, He HH, Jin Q, He Y, Chen BQ, et al. J Gen Virol. 2000;81:1347–1351. doi: 10.1099/0022-1317-81-5-1347. [DOI] [PubMed] [Google Scholar]
- 37.Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, Beadle JR, Wan WB, Hostetler KY, Kern ER. Antimicrob Agents Chemother. 2005;49:3724–3733. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wielgosz MM, Huang HV. J Virol. 1997;71:9108–9117. doi: 10.1128/jvi.71.12.9108-9117.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]