Abstract
Several psychiatric disorders are associated with white matter defects, suggesting that oligodendrocyte (OL) abnormalities underlie some aspects of these diseases. Neuregulin 1 (NRG1) and its receptor, erbB4, are genetically linked with susceptibility to schizophrenia and bipolar disorder. In vitro studies suggest that NRG1-erbB signaling is important for OL development. To test whether erbB signaling contributes to psychiatric disorders by regulating the structure or function of OLs, we analyzed transgenic mice in which erbB signaling is blocked in OLs in vivo. Here we show that loss of erbB signaling leads to changes in OL number and morphology, reduced myelin thickness, and slower conduction velocity in CNS axons. Furthermore, these transgenic mice have increased levels of dopamine receptors and transporters and behavioral alterations consistent with neuropsychiatric disorders. These results indicate that defects in white matter can cause alterations in dopaminergic function and behavior relevant to neuropsychiatric disorders.
Keywords: dopamine, erbB receptor, neuregulin, schizophrenia, white matter
Neuregulin 1 (NRG1), a growth factor essential for brain development, and erbB4, one of its receptors, are genetically linked to schizophrenia and bipolar disorder (1–4). A role for NRG1-erbB receptor signaling in psychiatric diseases is also supported by studies showing that expression levels or function of NRG1, erbB3, and erbB4 are altered in patient tissues (1, 4, 5). Moreover, mice with reduced levels of NRG1 or erbB4 exhibit behavioral alterations relevant to mental illness (6–9). Although the evidence linking this pathway and psychiatric disorders is strong, the mechanisms by which it contributes to these diseases remain unknown. NRG1-erbB signaling is important in neurons, astrocytes, and oligodendrocytes (OLs), but the specific cell types through which altered NRG1-erbB signaling contributes to these disorders is undefined.
Significant alterations in white matter are found in schizophrenia, bipolar disorder, major depression, anxiety, and obsessive–compulsive disorder (10–14), and genes expressed by OLs have been linked with some of these diseases (15, 16). Interestingly, NRG1-erbB signaling regulates OL development in vitro (17), although this has not been shown in the intact organism.
To determine whether erbB signaling plays a role in CNS myelination and whether disruption of this pathway in OLs produces defects related to human psychiatric disorders, we analyzed mice in which erbB signaling in OLs is blocked by expression of a dominant negative erbB receptor (DN-erbB4) (18). We show that alterations in erbB signaling lead to changes in OL morphology, number, and function in vivo. Moreover, these transgenic (Tg) mice have increased levels of functional dopamine transporters (DAT) and D1 receptors and exhibit behavioral alterations suggestive of neuropsychiatric disorders. Together, these results indicate that altered NRG1-erbB signaling in OLs may be a potential contributor to the pathogenesis of mental illness.
Results
Tg Expression of DN-erbB4 in OLs.
We blocked erbB signaling in OLs by expression of DN-erbB4 under the control of the promoter for 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP), an enzyme expressed in OLs and myelinating Schwann cells (18, 19). DN-erbB4 is a truncated receptor lacking the tyrosine kinase domain and phosphorylation sites (20). DN-erbB4 expression completely blocks erbB2, erbB3, and erbB4 receptor signaling (18, 20, 21) without affecting signaling by other receptors, e.g., the EGF receptor, an erbB family member (21). Two Tg lines that express high levels of DN-erbB4, CNP3 and CNP48, were characterized and exhibited similar phenotypes.
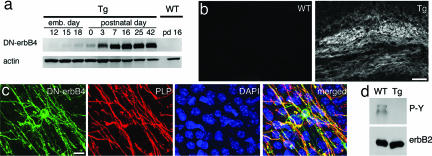
Western blot analysis showed that DN-erbB4 is expressed in the pattern expected from the CNP promoter (19). DN-erbB4 expression is first detected on embryonic day 15, and expression increases dramatically at early postnatal ages (Fig. 1a). Immunostaining showed DN-erbB4 expression in white matter tracts including the corpus callosum (Fig. 1b), anterior, and hippocampal commissures. No immunostaining was found in WT (corpus callosum shown in Fig. 1b). DN-erbB4+ cells were also seen in gray matter (cingulate cortex shown in Fig. 1c) and exhibited typical OL morphology (small round or oval soma of 10-μm diameter with a number of branching processes). These cells also express the OL-specific markers proteolipid protein (PLP) (Fig. 1c) and CC1 [supporting information (SI) Fig. 5].
Fig. 1.
Transgene expression is specific to OL lineage cells. (a) Western blot analysis of spinal cord lysates shows that the time course of DN-erbB4 expression closely resembles that of myelination. Actin was used as a loading control. (b) Immunostaining for DN-erbB4 protein in coronal sections of adult corpus callosum shows expression only in white matter of Tg mice. (c) Immunostaining of cingulate cortex in adult Tg mice for DN-erbB4 (green) and PLP (red) shows overlap. Blue shows DAPI-stained nuclei. (d) Immunoprecipitation of erbB2 from corpus callosum followed by phosphotyrosine Western blot shows that erbB2 phosphorylation is reduced in the white matter of adult Tgs. (Scale bars: 100 μm in b and 20 μm in c.)
Because some CNP promoter-driven Tg lines express GFP in multipotent precursors (22), we verified that DN-erbB4 expression is restricted to the OL lineage in our lines using a battery of cell-specific markers. DN-erbB4 immunostaining was observed in all PDGFαR+ OL precursors cells (SI Fig. 5), which also express CNP (23). Antibodies for NG2, a chondroitin sulfate proteoglycan expressed by multipotent precursors, including those for OLs (24), labeled only a subset of DN-erbB4+ cells (SI Fig. 5). Importantly, the morphology of NG2+/DN-erbB4+ cells was consistent with OL lineage cells (SI Fig. 5). OL lineage specific expression of DN-erbB4 was supported by the lack of overlap with markers for immature (Tuc-4 and doublecortin) or mature (Tuj1 and tyrosine hydroxylase) neurons (SI Fig. 5) or astrocytes (GFAP) (data not shown). Thus, the DN-erbB4 transgene is targeted exclusively to all cells of the OL lineage.
To determine whether DN-erbB4 effectively blocks normal erbB receptor function in white matter, we examined the phosphorylation state of endogenous erbB receptors in the corpus callosum of adult mice. Although erbB2 was immunoprecipitated equally from WT and Tg tissues, its phosphorylation was greatly reduced in Tg mice (Fig. 1d). Similar results with erbB3 and erbB4 (data not shown) indicate that erbB receptors are active in adult WT OLs and that this activation is dramatically reduced in Tg mice.
Loss of erbB Signaling in OLs Leads to Alterations in Myelin Thickness and OL Numbers.
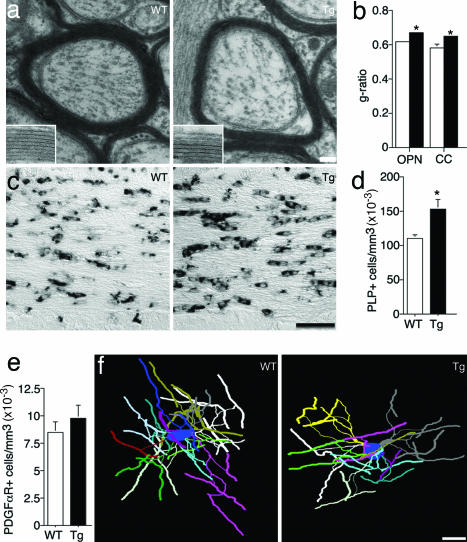
EM analysis showed no differences in the absolute number of myelinated and unmyelinated axons in the optic nerve of adult Tg mice (data not shown). Similarly, the density of myelinated and unmyelinated axons in the corpus callosum was normal (data not shown). However, the thickness of the myelin sheath was clearly altered in Tg mice. Measurement of the g-ratio (axon area/total fiber area) in cross-sections of optic nerve and corpus callosum in adult mice (P56) showed that the myelin sheath is significantly thinner when erbB signaling is abolished in OLs, although myelin structure is normal (Fig. 2 a and b).
Fig. 2.
OL structure and number are altered in the absence of erbB signaling. (a) Representative EM of myelinated axons in the corpus callosum. (Insets) Higher magnification of myelin in WT and Tg animals. (b) Quantification of the g-ratio in WT (open bars) and Tg (filled bars) optic nerve (OPN) and corpus callosum (CC). Tg mice have thinner myelin sheaths than WTs (P = 0.031). (c) Representative images of PLP in situ hybridization in corpus callosum sections. (d) Quantification of PLP+ cells in corpus callosum shows a higher density of OLs in Tg mice (P = 0.007). (e) The density of PDGFαR+ OL progenitors is not altered in Tg mice (P = 0.36). (f) Three-dimensional reconstructions of representative WT and Tg frontal cortex OLs. For quantification of OL morphology see Table 1. Error bars represent SEM. (Scale bars: 80 nm in a, 26 nm in Insets, 50 μm in c, and 20 μm in f.)
Because NRG1-erbB signaling has been suggested to promote OL lineage cell proliferation and survival, we counted mature OLs and OL precursors in adult corpus callosum using in situ hybridization for cell-specific markers and unbiased stereology. Surprisingly, the number of cells expressing the differentiated OL marker PLP was significantly increased by 40% in Tg mice (Fig. 2 c and d). Similar results were obtained in cingulate cortex (data not shown). In contrast, the number of PDGFαR+ OL progenitors (Fig. 2e) and BrdU+ proliferating PDGFαR+ cells was normal in adult CNP-DN-erbB4 mice (data not shown). Thus, alterations in OL number do not seem to be a consequence of abnormal proliferation by OL progenitors in the adult.
OL Morphology Is Altered in CNP-DN-erbB4 Mice.
To assess OL morphology, we crossed WT and CNP-DN-erbB4 mice to animals expressing eGFP under the control of the PLP promoter (25) and then traced and quantified the morphology of frontal cortex OLs of adult mice as described in ref. 26 (Fig. 2f). Although the number of primary processes emerging from each OL was unaltered, the number of branch points, the maximum branch order, and total process length per OL were significantly reduced in Tg mice (Table 1). Interestingly, even though the number of internodes per OL was significantly reduced, average internode length was unchanged (Table 1). Thus, in the absence of erbB signaling, the brain contains a larger number of smaller OLs, each myelinating less axonal surface. Importantly, many aspects of brain structure were normal in Tg mice, including brain volume, neuronal density in the cingulate cortex and striatum, and number of tyrosine hydroxylase+ neurons in the substantia nigra and ventral tegmental area (data not shown).
Table 1.
OL morphology in CNP-DN-erbB4 mice is less complex
| Mice | Total process length, μm | No. of primary processes | No. of internodes | Internode length, μm | No. of branch points | Maximum branch order | Maximum shell radius, μm |
|---|---|---|---|---|---|---|---|
| WT | 1,635 ± 92 | 13.3 ± 0.6 | 33.3 ± 1.9 | 30.8 ± 0.9 | 46.5 ± 3.0 | 5.5 ± 0.3 | 68 ± 2.0 |
| Tg | 1,157 ± 45 | 12.4 ± 0.7 | 24.1 ± 0.9 | 30.6 ± 1.0 | 31.3 ± 1.5 | 4.7 ± 0.2 | 53.3 ± 2.5 |
| P value | 0.0001 | 0.31 | 0.0002 | 0.90 | 0.0002 | 0.02 | 0.001 |
The morphology of eGFP-expressing OLs in the frontal cortex of Tg and WT mice was analyzed (n = 15 cells per genotype).
Reduced Conduction Velocity in Tg Mice.
Decreased myelin thickness led to changes in action potential propagation in 5-week-old mice. Optic nerve conduction velocity was 18% slower in Tg mice (WT, 3.23 ± 0.13 m/s; Tg, 2.63 ± 0.16 m/s; P = 0.009) indicating altered information flow in the CNS. Slower conduction could impact timing of neuronal signals, causing defects in behavior. Therefore, we compared adult WT and Tg mice in several behavioral tests.
Tg Mice Exhibit Behavioral Alterations.
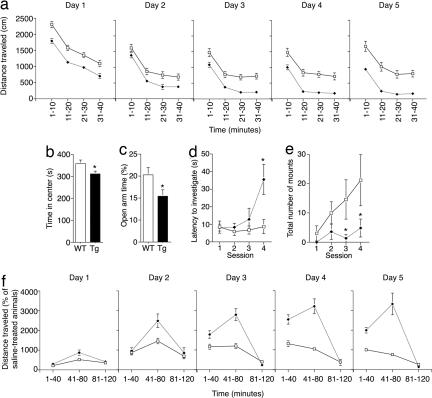
When exploring an open field, Tg mice were hypoactive, both moving less quickly (SI Fig. 6a) and stopping more often (data not shown) than WTs. Overall, Tg mice covered much less distance than WTs during their first exposure to the open field (SI Fig. 6b). Vertical exploration (rearing) was similarly reduced in Tg mice (SI Fig. 6c). Interestingly, exposure to the same open-field chamber daily for five consecutive days exacerbated the hypoactivity, indicating that Tg mice undergo enhanced habituation over the sessions (days × genotype F1,4 = 6.28; P < 0.0001) (Fig. 3a). These results suggest that aspects of experience-dependent behavioral plasticity are altered in CNP-DN-erbB4 mice.
Fig. 3.
Behavioral alterations in Tg mice. (a) Locomotor activity in the open field as a function of time in WT (open squares) and Tg (black diamonds) mice. Tg animals habituate to a greater extent (F1,4 = 6.28; P < 0.0001). (b) Tg mice spend less time in the center of the open-field chamber (P = 0.0004). (c) Tg mice spend less time on the open arms of an elevated plus maze (P = 0.033). (d) Tg mice take longer to investigate an intruder after repeated exposure in a social investigation test (F1,3 = 3.68; P = 0.02). (e) Tg mice attempt to mount intruder mice less frequently than WTs (F1,3 = 2.73; P = 0.05). (f) Tg mice sensitize more robustly to amphetamine than WT mice (F4,176 = 7.54; P < 0.001). Error bars represent SEM.
Tg mice also spent significantly less time in the center region and more in the periphery of the open field arena than WTs (Fig. 3b), a behavior believed to reflect increased levels of anxiety (27). The possibility that Tg mice have heightened anxiety was supported by their behavior in an elevated plus maze, as they spent less time exploring the open arms (Fig. 3c and SI Fig. 6 d and e) and performed fewer head dips (SI Fig. 6f).
The increased habituation and anxiety-like behavior displayed by CNP-DN-erbB4 mice suggested that they might have abnormal responses to novel stimuli or context, raising the question of whether there would be defects in social interactions. This was an intriguing possibility, because patients with and mouse models of psychiatric disorders display altered social behaviors (9, 28). We therefore subjected WT and Tg mice to a social interaction test. An initially unfamiliar intruder WT male was placed into the cage of a resident WT or Tg male four times (5-min trial with an intertrial interval of 15 min), and the number and type of interactions between the mice were recorded. Resident WTs quickly sought out and initiated social investigation of the intruder with the same latency in each session (Fig. 3d). In contrast, the latency to investigate a novel intruder increased significantly over the test sessions in Tg animals (Fig. 3d). Once investigation had been initiated, the interaction frequency between resident and intruder was the same for WTs and Tgs (data not shown). Interestingly, in the case of mounting, a behavior that reflects dominance, WTs showed an increase with repeated exposure whereas Tgs did not (Fig. 3e). Frequency of fighting was variable and not significantly different between genotypes, suggesting that aggression was not significantly altered. Because these types of social interactions are influenced by impaired olfactory function, we performed an olfactory discrimination test and a buried food test. Olfaction was normal in Tg mice in the tests used (data not shown). Together, these data indicate that Tg mice have abnormal social behavior.
Amphetamine Sensitization Is Increased in CNP-DN-erbB4 Mice.
The dopamine system plays a role in locomotion, anxiety, and social interactions. Defects in dopamine function can lead to hypoactivity and altered social responses (29, 30), and dopaminergic alterations are found in neuropsychiatric disorders (31). Therefore, we tested whether the response to the dopamine-releasing psychostimulant amphetamine was affected in Tgs. Mice were injected with amphetamine (2 mg/kg) or saline once a day for five consecutive days, and their locomotor behavior was measured in an open field. Both WT and Tg mice responded to an injection of saline with increased locomotion compared with uninjected animals (F1,49 = 66.17; P < 0.0001), but this reaction was more pronounced in Tg animals (F1,49 = 13.73; P < 0.005), indicating that they were hyperresponsive to injections. As expected, locomotion after the first exposure to amphetamine was larger than in saline-injected mice of both genotypes, and the increase appeared to be larger in Tg mice (Fig. 3f). Repeated treatment with amphetamine increased activity in both genotypes, but the intensity of the effects was altered in Tg mice (day × genotype F4,176 = 7.54; P < 0.0001). In WT mice, the second amphetamine injection produced a large increase in activity compared with the first day, indicating sensitization to the drug, but effects of amphetamine leveled off thereafter. In Tg mice, enhancement of locomotor activity by repeated injections was significantly greater than in WTs and continued to increase for several days (Fig. 3f). Notably, amphetamine-induced hyperactivity seems to overcome the augmented habituation observed in CNP-DN-erbB4 mice (Fig. 3a). Together, these results indicate that CNP-DN-erbB4 mice have enhanced sensitization to amphetamine.
Tg Mice Have Alterations in the Levels of Dopamine Receptors and Transporters.
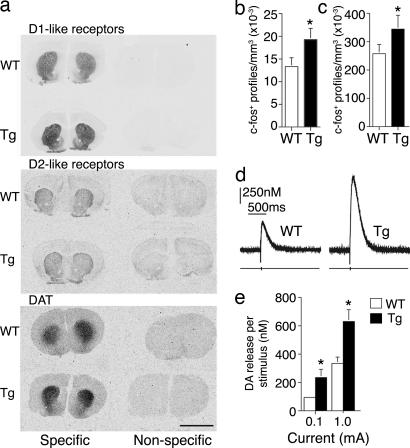
The disrupted amphetamine response suggested that dopamine signaling might be altered in Tg mice. Therefore, we used quantitative autoradiography to examine the levels of expression of DAT, a target of amphetamine, as well as dopamine D1- and D2-like receptors in brain regions implicated in cognition, reward, and movement (Fig. 4a). Levels of DAT and D1-like binding in the cortex, nucleus accumbens, and striatum of CNP-DN-erbB4 mice were consistently and significantly increased. D2-like receptor binding was not significantly different, but showed an increasing trend (Fig. 4a and SI Table 2). To confirm that the enhanced ligand binding represented increased levels of functional transporters and receptors, we tested whether activation of DAT or D1-like receptors produced larger physiological responses in intact Tg animals. Animals were injected with amphetamine or the D1 agonist dihydrexidine (DHX), and effects on striatal expression of the immediate early gene c-fos, a marker of neural activity, were measured. Basal levels of c-fos expression were similar whereas both amphetamine and DHX induced greater c-fos expression in Tg mice (Fig. 4 b and c). Moreover, stereotypic behaviors such as repetitive grooming, digging, and sniffing induced by DHX occurred more often in Tg mice (data not shown). Finally, we measured evoked dopamine release in brain slices containing the nucleus accumbens using continuous amperometry with carbon fiber electrodes. As expected from increased DAT expression, which may lead to increased vesicular dopamine, the evoked dopamine signal was significantly larger in Tg mice (Fig. 4 d and e). The increase in release could indicate that more dopamine is released per stimuli although we cannot exclude the possibility that the number of terminals is also increased.
Fig. 4.
Dopaminergic neurotransmission is altered in Tg mice. (a) Autoradiography of D1-like and D2-like receptors and DAT binding on brain sections. For quantification see SI Table 2. (Scale bar: 4 mm.) (b) c-fos induction in the striatum in response to amphetamine is significantly higher in Tg mice (P = 0.04). (c) c-fos induction in the striatum in response to DHX is significantly higher in Tg mice (P = 0.01). (d) Representative traces of evoked dopamine release in nucleus accumbens in response to a 1-mA current pulse to the medial forebrain bundle. (e) Amplitude of evoked dopamine release is enhanced in Tg compared with WT mice (n = 10; 0.1 mA, P = 0.044; 1 mA, P = 0.007). Error bars represent SEM.
Discussion
These studies provide in vivo evidence that erbB signaling regulates OL maturation and myelin production in the CNS. Importantly, our results also indicate that erbB signaling is not essential for the survival of OLs in the adult. Proliferation and survival of OLs depend on axonally derived factors, and competition for these factors regulates OL number in the adult CNS (32). Our results suggest that erbB signaling contributes to OL survival indirectly by regulating the size of OL arbors. It is possible that, in the normal brain, the first OLs to contact axons have access to NRG1, which promotes process outgrowth, enabling more effective competition for survival factors while other OLs die. In the absence of erbB signaling, OLs produce shorter processes or grow them more slowly, allowing more OLs to establish axonal contact. Thus, more OLs survive with simpler processes. Although OLs in Tg mice make fewer internodes, internode size is normal, suggesting that internode length is controlled via other mechanisms. This differs from Schwann cells, where internode length and myelin thickness are clearly linked and regulated by erbB signaling, because both are altered in peripheral nerves of CNP-DN-erbB4 mice (18).
Our results have implications for psychiatric disorders given that a significant fraction of patients have white matter defects (10–14). It is worth noting that some of the human findings, reductions in the numbers of OLs and damage to the myelin sheath (13), are different from those obtained in the CNP-DN-erbB4 mice. It is possible that OL abnormalities progress through different phases in psychiatric disorders. Postmortem human brains may reflect the latest phases, e.g., OL loss, whereas our findings in mice reflect an earlier dysfunction. Other factors (medication, drug addiction, and diabetes) may also contribute to the pattern of OL degeneration seen in patients. Examination of white matter alterations in patients close to the onset of symptoms could help to clarify the contributions of OL defects to such diseases.
Our findings indicate that defects in OL structure/function can cause alterations in neurotransmission that are relevant to psychiatric diseases. It is possible that alterations in information flow due to defective myelin could lead to changes in dopaminergic neurotransmission, which produce compensatory changes in the levels of dopamine receptors and transporters. NRG1-erbB signaling regulates NMDA, GABA, and ACh receptor expression (1), which are implicated in psychiatric disorders. Although other neurotransmitter systems may be affected in CNP-DN-erbB4 mice, it is noteworthy that the phenotypes of these mice are consistent with alterations in the dopamine system. Mice overexpressing DAT display increased habituation and increased response to cocaine (which blocks DAT) (33). Mice overexpressing D1 receptors are hypolocomotive, whereas mice lacking D1 receptors exhibit less habituation and do not sensitize to amphetamine (30). Thus, altered behaviors of CNP-DN-erbB4 mice could be explained by the observed changes in the dopamine system. Although CNP-DN-erbB4 mice display peripheral hypomyelination (18), the defects in behavioral plasticity described here are clearly due to CNS defects.
Tg lines in which DN-erbB4 is expressed in astrocytes (21) did not show the same anatomical or behavioral defects (data not shown), indicating that the phenotypes described here are not due to nonspecific effects of DN-erbB4 expression but to specific alteration of OL–neuron interactions. The behavioral alterations found in mice with disrupted NRG1-erbB signaling in the entire organism or in all brain cells (6–9) are similar but not identical to those in CNP-DN-erbB4 mice, suggesting that defects in erbB signaling in different cell types may contribute to different aspects of psychiatric symptoms.
Although NRG1 and erbB4 are genetically linked to psychiatric disorders (1, 2, 4, 9), the nature of the alterations in the expression or function of these proteins (either gain or loss of function) is undefined and controversial. Our results indicate that loss of NRG1-erbB signaling in OLs may contribute to these diseases. Thus, the possibility that the reported increases in NRG1 or erbB4 expression (4, 5, 34, 35) or erbB4 activation (36) reflect a compensatory response to a loss/reduction in function in this signaling pathway needs to be considered. This signaling pathway may also contribute to neuropsychiatric disorders through nongenetic mechanisms because both NRG1 and erbB4 expression have been shown to be altered by environmental insults (37–39).
Without losing sight of the gap between rodent and human behavior, it is interesting to explore parallels between some of the behavioral changes described in the Tgs and schizophrenia patients. Our results suggest that defects in erbB signaling in OLs may contribute to positive and negative symptoms of this disease. Reduced locomotion and social dysfunction observed in CNP-DN-erbB4 are reminiscent of negative symptoms of chronic schizophrenia patients who exhibit motor retardation and social withdrawal (40). Approximately 40% of schizophrenia patients have increased sensitivity to amphetamine during an acute schizophrenic episode. Thus, the increased amphetamine sensitization observed in CNP-DN-erbB4 mice suggests that erbB signaling in white matter may also underlie some positive symptoms of schizophrenia (41). Furthermore, CNP-DN-erbB4 mice also exhibit heightened, anxiety-like behavior, a symptom found in schizophrenia as well as bipolar patients (42, 43).
In summary, this study shows that erbB signaling plays a critical role in regulating OL morphology and number in adult brain and that alterations in white matter cause defects in the dopaminergic system and result in behavioral alterations that are consistent with psychiatric disorders.
Materials and Methods
Animals and Housing.
Mice were generated from homozygous crosses of WT or Tg mice and genotyped as previously described (18).
Immunoprecipitation and Western Blot.
Western blots with antibodies recognizing flag (1:1,000; Sigma, St. Louis, MO), actin (1:20,000; Oncogene Research Products, Cambridge, MA), phosphotyrosine (4G10, 1:5,000; gift of Thomas M. Roberts, Dana–Farber Cancer Institute, Boston, MA), erbB2, erbB3, or erbB4 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) and immunoprecipitation of erbB receptors were performed as in ref. 44. For detailed methods see SI Methods.
Immunohistochemistry.
Immunohistochemical detection of the DN-erbB4 transgene was performed as described in ref. 45. The same conditions were used for doublecortin antibodies (1:100; Santa Cruz Biotechnology). Immunostaining with antibodies against TUC-4 (1:100; Chemicon, Temecula, CA) and NeuN (1:250; Chemicon) was performed as described in ref. 24. Immunostainings for NG2 (1:500; gift from Joel Levine, Stony Brook University, Stony Brook, NY), PDGFαR (1:200; Pharmingen, Torrey Pines, CA), and CC1 (1:250; Oncogene Research Products) were performed as described in ref. 46. Immunostainings were performed on four animals of each genotype.
In Situ Hybridization and Stereology.
In situ hybridization for PLP and PDGFαR was performed as in ref. 47. Quantification of labeled cells was performed, blind to genotype, by using the optical dissector method (Stereo Investigator software; MicroBrightField, Williston, VT) as described in ref. 48. Proliferation of OLs in adult mice was measured by double PDGFαR in situ hybridization and anti-BrdU (1:400; DakoCytomation, Glostrup, Denmark) immunostaining as described in ref. 47. Cell density was compared between genotypes with a Student t test (n = 4 per genotype).
Quantification of g-Ratio.
EM and quantification of axon numbers were done blind to genotype as in ref. 45. Images of myelinated axons were digitized and the g-ratio was calculated as axon/axon+myelin sheath cross-sectional area and compared by using a Student t test (n = 8 per genotype, 195–265 axons per mouse). For detailed methods see SI Methods.
OL Morphology.
Quantification of OL morphology in the frontal cortex of adult mice was done as in ref. 26 while blind to genotype. Each parameter was compared between genotypes with a Student t test (n = 15 cells per genotype). For details see SI Methods.
Conduction Velocity.
Optic nerves were dissected from P35 mice (n = 8 per genotype) and immersed in saline (125 mM NaCl/26 mM NaHCO3/25 mM glucose/5 mM KCl/1.25 mM NaH2PO4/2 mM CaCl2/1 mM MgCl2). A pair of saline-filled stimulation electrodes was placed at one end of the nerve, and an extracellular recording electrode (5–10 MΩ) filled with 3 M KCl was placed at the other end. One hundred stimuli (0.2 Hz) were applied, and responses were measured in current clamp with an Axopatch 200B amplifier (output gain ×100, digitized at 20 kHz, filtered at 5 kHz). Conduction velocity was calculated as time from stimulus artifact to action potential peak divided by the distance between stimulating and recording electrodes. All tests were performed blind to genotype. Conduction velocity was compared between genotypes with a Student t test.
Behavioral Tests.
Researchers blind to the genotype of the animals performed all behavioral tests. Maternal behavior did not differ between Tg and WT mice.
Open Field.
Adult mice (P84) were placed in an open-field chamber (14 in × 14 in) equipped with infrared sensors (Med Associates, St. Albans, VT) for 120 min. Total distance traveled (in centimeters), time spent in, and number of rears were compared in the center and periphery by using a one-way ANOVA (n = 14–15 per genotype). A separate set of mice (P112) was tested in the open field daily for five consecutive days. Habituation, measured as total distance traveled during the first 40 min over the repeated sessions, was compared by using a three-way ANOVA [genotype, day (repeated measure), and time (repeated measure); n = 26–27 per genotype].
Elevated Plus Maze.
An animal was placed in the center of the maze and allowed to explore for 5 min. A one-way ANOVA compared total entries, number of open and closed arm entries, and number of rears and head dips (n = 34–37 per genotype).
Social Interaction Test.
This test was performed between two adult male mice (P112; n = 8 per genotype) as described (28). Latency to start and time spent in olfactory investigation, mounting, and fighting were scored and analyzed with two-way ANOVA [genotype and session (repeated measures)] (n = 8–10 per genotype).
Amphetamine Sensitization.
This test was performed as described for rats (49). Briefly, adult male mice (P112) were placed in a holding cage for 30 min, then placed in the open-field chamber, and baseline behavior was recorded for 40 min. The animal was then injected with a dose of amphetamine (2 mg/kg per 10 ml of free d-amphetamine, s.c.) or an equal volume of saline (10 ml/kg, s.c.) and placed back in the chamber for 120 min. Each animal was treated identically for five consecutive days. After each session, the animals were returned to their home cage (group-housed). Effect of injection was measured as locomotor counts for 20 min after saline injection relative to the 40-min baseline period and compared with activity of uninjected animals during the same periods with a three-way ANOVA [genotype, treatment (repeated measures), and day (repeated measures)]. Similar analyses were used to analyze distance traveled after amphetamine injection relative to saline injection (n = 11–13 per genotype per treatment).
Autoradiography.
Quantitative ligand binding to D1-like receptors was done with [3H]SCH23390 (65.9 Ci/mmol; Amersham, Piscataway, NJ) as in ref. 50. D2-like binding with [3H]spiperone and DAT with [3H]mazindol were done as in ref. 51 with minor changes. Binding in drug-näive adult (P112) WT and Tg mice was compared by using a Student t test (n = 10 per genotype). For details see SI Methods.
Amperometry.
Voltammetric recordings in the core region of the nucleus accumbens were performed in brain slices as described in ref. 52 while blind to genotype. Amplitude of evoked dopamine release was compared between genotypes by using a Student t test (n = 10 per genotype).
C-fos Induction.
Animals were injected with amphetamine (4 mg/kg), DHX (10 mg/kg), or vehicle (0.9% saline or 0.1% ascorbic acid, respectively) and perfused with 4% paraformaldehyde 2 h later. Preparation of tissue, immunostaining, and stereological estimation of c-fos+ cells after DHX injections were performed blind as described in ref. 53. For the amphetamine studies the same regions were analyzed by using the fractionator technique. Tissue shrinkage and size of c-fos+ cells did not differ between genotypes. The number of c-fos+ cells per cubic millimeter (DHX or ascorbic acid) or profiles per cubic millimeter (amphetamine or saline) was compared using nonparametric Mann–Whitney U tests (n = 6 per genotype, amphetamine/saline; n = 10 per genotype, DHX/ascorbic acid).
Acknowledgments
This research was supported in part by National Institute of Neurological Disorders and Stroke Grants R01 NS35884 (to G.C.) and F31 NS048630 (to B.M.H.); National Multiple Sclerosis Society Grant RG 3786 (to G.C.); National Institute of Mental Health Grants R43 MH070264 (to D.B. and G.C.), R01 MH60131 (to P.O.), and P50 MH66171 (to H.M.); a National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (to G.C.); Development Disability Research Center Grant NIH P30-HD 18655 (to G.C.); National Institute of Neurological Disorders and Stroke Training Grant NS7473 (to K.R.); a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to K.R.); a National Multiple Sclerosis Society Postdoctoral Fellowship (to J.C.M.); a Lefler Postdoctoral Fellowship (to S.P.S.); the Lieber Center for Schizophrenia Research at Columbia University (H.M.), and a Pew Scholar Award (to C.C.).
Abbreviations
- Tg
transgenic
- OL
oligodendrocyte
- NRG1
neuregulin 1
- DHX
dihydrexidine
- DAT
dopamine transporter
- CNP
2′,3′-cyclic nucleotide 3′-phosphodiesterase
- PLP
proteolipid protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702157104/DC1
References
- 1.Corfas G, Roy K, Buxbaum JD. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 2.Georgieva L, Moskvina V, Peirce T, Norton N, Bray NJ, Jones L, Holmans P, Macgregor S, Zammit S, Wilkinson J, et al. Proc Natl Acad Sci USA. 2006;103:12469–12474. doi: 10.1073/pnas.0603029103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, Williams HJ, Preece AC, Dwyer S, Wilkinson JC, et al. Am J Med Genet B. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 4.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. Am J Med Genet B. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 5.Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Gerlai R, Pisacane P, Erickson S. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- 7.Golub MS, Germann SL, Lloyd KC. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. NeuroReport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- 9.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, et al. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL. Depress Anxiety. 2006 Nov 9; doi: 10.1002/da.20246. [DOI] [PubMed] [Google Scholar]
- 11.Hajek T, Carrey N, Alda M. Bipolar Disord. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan EV, Pfefferbaum A. Eur J Radiol. 2003;45:244–255. doi: 10.1016/s0720-048x(02)00313-3. [DOI] [PubMed] [Google Scholar]
- 13.Walterfang M, Wood SJ, Velakoulis D, Pantelis C. Neurosci Biobehav Rev. 2006;30:918–948. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Whiteside SP, Port JD, Deacon BJ, Abramowitz JS. Psychiatry Res. 2006;146:137–147. doi: 10.1016/j.pscychresns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Dracheva S, Davis KL, Chin B, Woo DA, Schmeidler J, Haroutunian V. Neurobiol Dis. 2006;21:531–540. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Carter CJ. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Nave KA, Salzer JL. Curr Opin Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Velardez MO, Warot X, Yu ZX, Miller SJ, Cros D, Corfas G. J Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandross KJ, Cohen RI, Paras P, Jr, Gravel M, Braun PE, Hudson LD. J Neurosci. 1999;19:759–774. doi: 10.1523/JNEUROSCI.19-02-00759.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 21.Prevot V, Rio C, Cho GJ, Lomniczi A, Heger S, Neville CM, Rosenthal NA, Ojeda SR, Corfas G. J Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre A, Gallo V. J Neurosci. 2004;24:10530–10541. doi: 10.1523/JNEUROSCI.3572-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. Neuron. 1994;12:1363–1375. doi: 10.1016/0896-6273(94)90451-0. [DOI] [PubMed] [Google Scholar]
- 24.Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallon BS, Shick HE, Kidd GJ, Macklin WB. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murtie JC, Macklin WB, Corfas G. J Neurosci Res. 2007 doi: 10.1002/jnr.21339. in press. [DOI] [PubMed] [Google Scholar]
- 27.Prut L, Belzung C. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 28.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Behav Brain Res. 2004;148:185–198. doi: 10.1016/s0166-4328(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 30.Tan S, Hermann B, Borrelli E. Int Rev Neurobiol. 2003;54:145–197. doi: 10.1016/s0074-7742(03)54005-3. [DOI] [PubMed] [Google Scholar]
- 31.Kienast T, Heinz A. CNS Neurol Disord Drug Targets. 2006;5:109–131. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- 32.Bozzali M, Wrabetz L. Neurochem Res. 2004;29:979–988. doi: 10.1023/b:nere.0000021242.12455.75. [DOI] [PubMed] [Google Scholar]
- 33.Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, et al. Brain Res Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Mol Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 35.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, et al. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 37.Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Proc Natl Acad Sci USA. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker MW, Chen Y, Hallenbeck JM, Ford BD. Neurosci Lett. 2002;334:169–172. doi: 10.1016/s0304-3940(02)01126-6. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Ford BD. Neurosci Lett. 2005;375:181–186. doi: 10.1016/j.neulet.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 40.Blanchard JJ, Cohen AS. Schizophr Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laruelle M. Brain Res Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 42.Braga RJ, Petrides G, Figueira I. Compr Psychiatry. 2004;45:460–468. doi: 10.1016/j.comppsych.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 43.Keller MB. J Clin Psychiatry. 2006;67(Suppl 1):5–7. [PubMed] [Google Scholar]
- 44.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Nat Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 46.Murtie JC, Zhou YX, Le TQ, Armstrong RC. Glia. 2005;49:542–554. doi: 10.1002/glia.20142. [DOI] [PubMed] [Google Scholar]
- 47.Redwine JM, Armstrong RC. J Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. J Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El-Khodor BF, Boksa P. Neuroscience. 1998;87:893–904. doi: 10.1016/s0306-4522(98)00194-8. [DOI] [PubMed] [Google Scholar]
- 50.Wong JY, Clifford JJ, Massalas JS, Finkelstein DI, Horne MK, Waddington JL, Drago J. Eur J Pharmacol. 2003;472:39–47. doi: 10.1016/s0014-2999(03)01862-4. [DOI] [PubMed] [Google Scholar]
- 51.Zeng BY, Heales SJ, Canevari L, Rose S, Jenner P. Exp Neurol. 2004;190:515–524. doi: 10.1016/j.expneurol.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Benoit-Marand M, Jaber M, Gonon F. Eur J Neurosci. 2000;12:2985–2992. doi: 10.1046/j.1460-9568.2000.00155.x. [DOI] [PubMed] [Google Scholar]
- 53.Mura A, Murphy CA, Feldon J, Jongen-Relo AL. Brain Res. 2004;1009:120–128. doi: 10.1016/j.brainres.2004.02.054. [DOI] [PubMed] [Google Scholar]