Abstract
The responses of sensory neurons to repeated presentations of identical stimuli can be highly reproducible. Little is known about the reliability of the motor command signals carried by individual premotor neurons. We measured the variability in the interspike intervals of the high-frequency, saccade-related bursts generated by neurons in the pontine reticular formation. During movements having similar amplitudes and velocity profiles, the interspike intervals of the high-frequency component of the bursts are very similar. The low variability in interspike intervals cannot be attributed to a burst mode characterized by fixed interspike times. Different, but repeatable, burst patterns are observed when movements having approximately the same amplitude but different velocity profiles occur. These findings suggest that the discharge of a single pontine cell is strongly correlated with the activity of other pontine burst cells. Both the high temporal precision of the saccade-related bursts and the correlated activity of pontine burst cells reduce variability in the signals sent to the motoneuron pools and, thereby, contribute to the accuracy and precision of saccadic eye movements.
Keywords: monkey, saccade, pons
The responses of visual neurons have been shown to be highly reproducible when the same dynamic stimuli are presented repeatedly (1–4). Few comparable studies of the reliability of the motor command signals carried by individual neurons exist. The goal of the experiment reported in this article was to perform the motor equivalent of these sensory experiments and to measure the reliability of motor commands for saccadic eye movements generated by single premotor neurons.
Saccades are produced by a brief burst of activity in agonist motoneurons and a concomitant brief cessation of activity of antagonist motoneurons. The burst (pulse) of activity gradually declines to a new level (step) of activity. The pulse serves to overcome the viscous resistance of the muscles and other orbital tissue and move the eye at a high speed. The step overcomes the elastic properties of the orbital tissue and holds the eye in the new position (5). Almost all agonist motoneurons participate in generating the pulse of innervation, but a smaller subset of the motoneuron pool contributes to the step.
Neurons in the paramedian zone of the pontine reticular formation (PPRF) generate motor command signals responsible for the changes in the horizontal positions of the eyes during saccades (for recent reviews, see refs. 6–8). These neurons display extremely low rates of spontaneous activity and produce a vigorous burst of activity shortly before the onset of ipsilateral saccades. A subset of the burst neurons, excitatory burst neurons, have monosynaptic connections with motoneurons (9). The number of spikes in the saccade-related burst of PPRF neurons is highly correlated with the horizontal amplitude of a saccade and the peak frequency of the burst is highly correlated with the peak velocity of the movement (10–14). We chose the pontine burst cells for a study of the reliability of motor command signals because of these tight linkages between cell activity and movement parameters.
Results
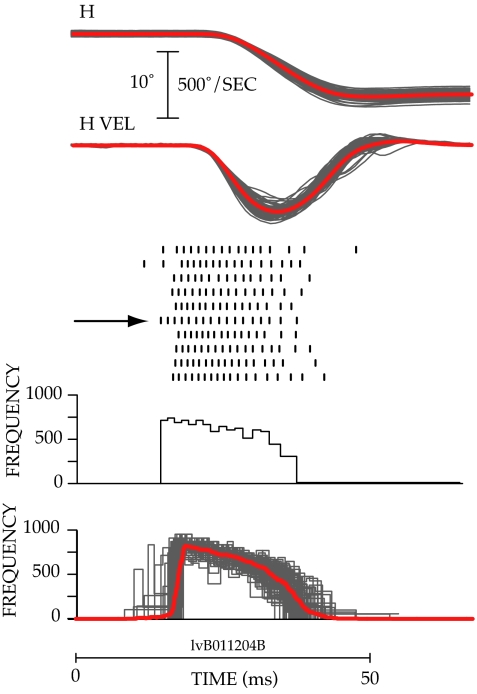
The goal of the experiment was to measure the variability in the burst of pontine neurons when the same motor command was being issued repeatedly. This cannot be accomplished by merely requesting subjects to make saccades from the same initial fixation target to the same eccentric target. As illustrated in Fig. 1, considerable variability in the amplitude, duration, and velocity of movements is observed when this is done. For the 76 movements illustrated, horizontal eye position varied from 0.4° right to 0.6° left (mean 0.1° left) when the animal was looking at the central target. Horizontal position at the end of the primary saccade varied from 8.16° to 10.9° left (mean: 9.3° left). The amplitude of the horizontal component of the movement varied from 8.14° to 10.95° (mean: 9.27), and the duration of the saccades varied from 25 ms to 32 ms (mean: 27.59 ms). Peak horizontal velocity ranged from 407°/sec to 592°/sec (mean: 503.25°/sec).
Fig. 1.
Variability in eye position, eye velocity, and spike frequency during 76 saccades to the same target. (Top) Changes in horizontal position (H) and horizontal velocity (HVEL) observed during 76 saccades made to a target presented 10° to the left of a central fixation target are plotted by thin gray lines. The averages of the position and velocity traces are shown by bold red lines. (Middle) Raster plots of spike occurrence are shown for 10 of the 76 movements below the velocity traces. Each tick mark indicates the time of occurrence of an action potential. Each row shows the action potentials recorded during a single movement, and the horizontal arrow marks the burst for which the instantaneous frequency plot was constructed. (Bottom) A plot of the instantaneous frequency firing rate (reciprocal of ISI × 1,000) of the cell during the burst indicated by the horizontal arrow is shown below the rasters. Instantaneous frequency plots for all 76 trials are superimposed at the bottom, and the average is shown by the bold red line.
Variation in the concomitant neural activity also was observed. The number of spikes in the bursts associated with the 76 saccades ranged from 13 to 19 (mean: 16.1). Burst duration varied from 20 ms to 37 ms (mean: 26.3), and the peak of the instantaneous frequency records ranged from 702 spikes/sec to 930 spikes/sec (mean: 841.4 spikes/sec).
Methods used to assess the reliability of the signals carried by pontine neurons are illustrated in Fig. 2. The variability in the saccade-related activity of pontine neurons was measured during selected subsets of movements that had very similar amplitudes and velocity profiles. The rationale for the methods is as follows. Saccades of the same amplitude with very similar velocity profiles occur when, at the level of the motoneuron pools, the same or equivalent motor commands are issued. Measures of the activity of premotor neurons when the same or equivalent motor commands are being issued by motoneurons can be used to measure the reliability of motor commands generated more centrally. Note that this method may not be valid if the head is unrestrained or the saccade targets are located at different depth planes, conditions in which other oculomotor subsystems are contributing to the excitability of the motoneuron pools (15).
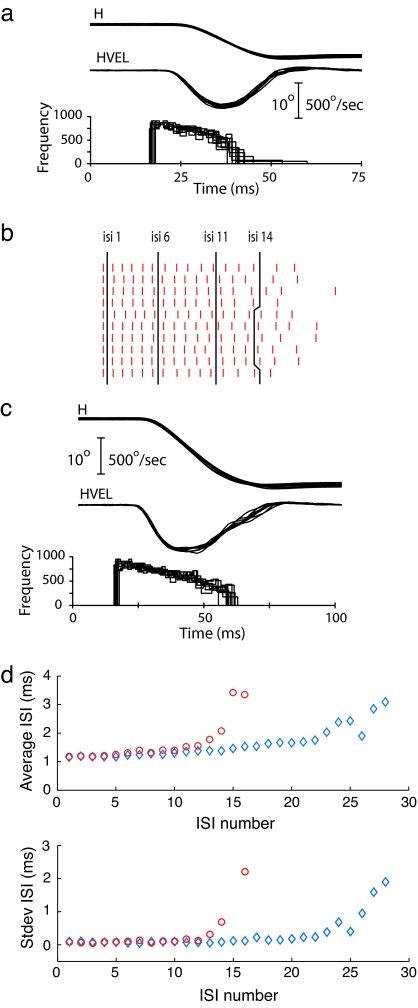
Fig. 2.
Illustration of the general methods used to assess the reliability of signals carried by pontine neurons. (a) Plots of horizontal position (H), horizontal velocity (HVEL), and instantaneous frequency for a subset of 10 of the 76 movements shown in Fig. 1, selected for similarity of velocity profile. The same cell is illustrated in Fig. 1. (b) Raster plots of spike occurrence for the 10 bursts associated with the 10° movements. Spikes are aligned on the first spike of the high-frequency burst. Black lines are through ISIs 1, 6, 11, and 14. (c) Horizontal position and velocity traces for 10 saccades to a target 20° to the left of a central fixation stimulus and the instantaneous frequency profile of the associated bursts. (d) Plots of the average ISI and standard deviation for the 16 ISIs common to the 10 bursts preceding the 10° movements (red) and the 28 ISIs common to the 10 bursts associated with the movements to the 20° target (blue).
Fig. 2a presents traces of horizontal position, horizontal velocity, and instantaneous firing rate for 10 movements to a 10° target, selected from the 76 movements illustrated in Fig. 1. Ten movements to a 20° target are shown in Fig. 2c. The two sets of movements were selected based on the similarity of saccade amplitude and velocity profiles. For the 10 movements to the 10° target, horizontal amplitude varied from 9.1° to 9.7°; movement duration ranged from 25 ms to 27 ms; and peak velocity from 544°/sec to 565°/sec.
The raster plot in Fig. 2b illustrates the time distribution of the spikes in the saccade-related bursts recorded during the movements to the 10° target. The means and standard deviations of the duration of the interspike intervals (ISIs) common to the bursts associated with the movements to the 10 and 20° targets are plotted in Fig. 2d.
For the bursts associated with the 10° movements (red symbols), average ISI gradually increased from 1.18 ms at interval 1 to 1.55 ms at interval 12. The increase in average ISI was steeper for the last few intervals. Standard deviations of the average ISI were <0.1 ms for the first 5 intervals and <0.2 ms for the first 12 intervals. For the bursts associated with the 20° movements, average ISI gradually increased from 1.15 at interval 1 to 1.75 at interval 22 and then increased more rapidly. Standard deviations of the average ISIs were <0.2 ms for the first 16 intervals and gradually increased to 0.23 at interval 22.
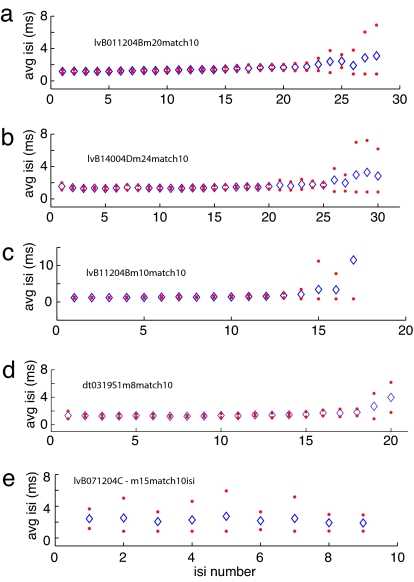
Fig. 3 plots the mean and 95% confidence intervals (mean ± 2 standard deviations) for the ISIs of the bursts of four pontine neurons generating a high-frequency presaccadic burst (Fig. 3 a–d) and one pontine cell with a lower burst rate (Fig. 3e). Data in each plot were obtained from the bursts associated with 10 movements having similar amplitudes and velocity profiles. The first ISI often is more variable than successive intervals and a gradual increase in average ISI is sometimes observed, but variability is low until the intervals at the end of the burst. For example, the average ISI for the data in Fig. 3d gradually increased from 1.3 ms for the first interval to 1.8 ms at interval 18. The standard deviation for the first interval was 0.32 ms but <0.2 ms for intervals 2–15 and <1 ms for all but the last two intervals. In contrast, pontine burst cells that do not generate a high-frequency burst of activity have higher average ISIs, but there is also more variability in the ISIs (Fig. 3e). Means, standard deviations, and the standard deviation expressed as a percent of the mean (coefficient of variation) for the ISIs of the 34 data sets that form the basis of this report are presented in Table 1. The data were obtained from 21 high-frequency burst neurons and 4 cells with lower burst rates (bold italics). For the high-frequency burst neurons, the means, standard deviations, and coefficients of variation are given for the high-frequency component of the burst, defined as the ISIs between the first of three consecutive ISIs <2 ms in duration and the next interval >2 ms in duration.
Fig. 3.
Plots of the average ISI ± 2 standard deviations for 4 PPRF neurons characterized by a high-frequency saccade-related burst (a–d) and one pontine burst cell with a low frequency burst (e).
Table 1.
Means, standard deviations, and coefficient of variation for the ISIs for all the data sets and cells
| Cell | Saccade amplitude | Total spikes | High-frequency spikes | Avg ISI | Avg SD | Avg CV |
|---|---|---|---|---|---|---|
| 071204C | 15 | 10 | 0 | 2.30 | 0.90 | 0.40 |
| 181104B | 20 | 18 | 0 | 1.90 | 0.61 | 0.30 |
| 191004B | 24 | 12 | 0 | 2.60 | 2.15 | 0.57 |
| 151004A | 15 | 17 | 0 | 1.78 | 1.08 | 0.29 |
| 011204B | 20 | 28 | 22 | 1.40 | 0.10 | 0.08 |
| v | v | 24 | 1.39 | 0.12 | 0.08 | |
| 121104B | 10 | 13 | 9 | 1.82 | 0.32 | 0.17 |
| 141004D | 24 | 31 | 25 | 1.45 | 0.13 | 0.09 |
| 15 | 19 | 14 | 1.48 | 0.24 | 0.16 | |
| 161104A | 10 | 13 | 10 | 1.40 | 0.25 | 0.17 |
| v | v | 24 | 1.39 | 0.15 | 0.10 | |
| 181004A | 10 | 21 | 18 | 1.37 | 0.16 | 0.11 |
| v | v | 18 | 1.39 | 0.17 | 0.12 | |
| 181004C | 10 | 18 | 15 | 1.44 | 0.22 | 0.14 |
| 20 | 31 | 27 | 1.41 | 0.14 | 0.09 | |
| 201104A | 20 | 23 | 16 | 1.68 | 0.29 | 0.16 |
| 072105Cc1 | 20 | 39 | 36 | 1.05 | 0.14 | 0.12 |
| 080305Ac1 | 10 | 26 | 23 | 1.27 | 0.28 | 0.15 |
| 080305Ac2 | 10 | 26 | 23 | 1.29 | 0.21 | 0.12 |
| 091305Bc2 | 12 | 38 | 33 | 1.08 | 0.09 | 0.08 |
| 7 | 34 | 31 | 1.10 | 0.11 | 0.09 | |
| 102205Ac1 | 20 | 19 | 11 | 1.53 | 0.17 | 0.11 |
| DT0319S1 | 18 | 28 | 24 | 1.44 | 0.22 | 0.15 |
| 8 | 21 | 18 | 1.39 | 0.15 | 0.10 | |
| DT0320S1 | 10 | 21 | 19 | 1.30 | 0.14 | 0.09 |
| DT0404S1 | 14 | 20 | 15 | 1.59 | 0.25 | 0.16 |
| DT10291P | v | v | 32 | 1.56 | 0.13 | 0.08 |
| v | v | 24 | 1.42 | 0.12 | 0.08 | |
| DT11081P | 20 | 31 | 27 | 1.54 | 0.20 | 0.12 |
| DT11271P | v | v | 30 | 1.40 | 0.23 | 0.14 |
| DT11291P | 18 | 40 | 34 | 1.39 | 0.20 | 0.13 |
| da0911 | 12 | 19 | 15 | 1.29 | 0.16 | 0.12 |
| da0618 | 16 | 20 | 16 | 1.23 | 0.18 | 0.13 |
| 16 | 20 | 13 | 1.45 | 0.27 | 0.17 |
The correlation between average ISI and average SD is 0.56. Data from pontine cells generating low-frequency bursts are shown in bold italic. v, velocity match using saccades of different amplitudes; CV, coefficient of variation.
The data shown in Figs. 2 and 3 indicate that when the same or similar saccadic commands are being issued at the level of the motoneurons, there is very little variability in the initial portion of the bursts of pontine neurons generating high-frequency bursts. Indeed, except for jitter in the duration of the first interval and variability in the last few intervals of the burst, most of the ISIs are similar, at a submillisecond time scale, from movement to movement. This is true despite the facts that the movements selected for having similar amplitudes and velocity profiles occurred over a relatively long time period and that movements of other directions and amplitudes and velocities were interspersed. For example, the saccades to the 10° and 20° targets illustrated in Fig. 2 were distributed throughout a span of 100 trials in which saccades to 10° and 20° targets were randomly interleaved. Movements having a range of different amplitudes and a variety of velocity profiles occurred during these trials.
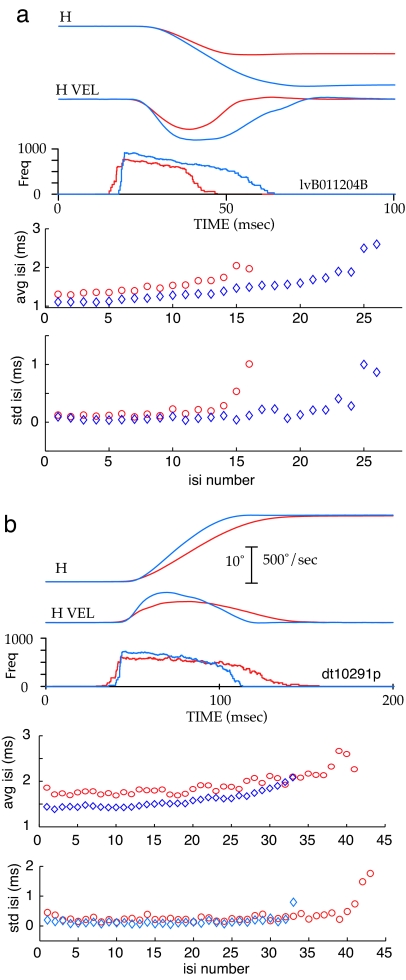
The high repeatability of ISIs is of less interest from the perspective of signal processing if the repeatability is merely the consequence of cells going into a burst mode characterized by relatively constant ISIs. The data in Fig. 4 address this issue. Fig. 4a presents the average horizontal position, horizontal velocity, and instantaneous frequency observed during 10 similar movements to a 10° target (red) and 10 movements to a 20° target (blue). The average ISIs and the standard deviations of the intervals are plotted at the bottom. The peak frequency of the burst of this neuron differed for the two amplitudes, but the standard deviations of the ISIs during the high-frequency component of the bursts were small for both frequencies. Fig. 4b presents data obtained from a more common type of burst cell in which the peak frequency of the burst was similar for all tested movement amplitudes. Data are shown for two subsets of movements to a 20° target, one (red) with slower peak and average velocities and longer durations than the other set (blue). The average instantaneous frequency of the bursts associated with the slower movements (red) reached and sustained a lower frequency than the bursts associated with the faster movements (blue). The plots of average ISIs and standard deviations of the intervals illustrate the regularity of the ISIs during the high-frequency component of the bursts occurring during slow and fast movements. Thus, for this cell, when subsets of movements having approximately the same amplitude, but different velocity profiles occur, the average ISIs in the associated bursts also differ with the slower movements having longer ISIs. Different, but repeatable, burst patterns are associated with movements of the same amplitude but having different velocity profiles. Again, the standard deviations were similar in both patterns. More examples of different, but repeatable, burst patterns for other pontine cells are available as supporting information (SI) Fig. 5.
Fig. 4.
Plots of the horizontal position, horizontal velocity, instantaneous frequency of associated bursts, average ISI, and standard deviation of the ISIs for two different cells. (a) The burst of the cell reaches different peak frequencies for movements of different amplitudes. (b) The burst of this cell reaches similar peak frequencies for movements of different amplitudes, but burst frequency differs for slow and fast movements of the same amplitude.
Discussion
We measured the variability in the presaccadic bursts of pontine neurons when equivalent motor commands were being issued at the level of oculomotor motoneurons. The timing of ISIs in the high-frequency component of the bursts associated with movements having the same amplitude and similar velocity profiles was reproducible with a precision of <1 ms. Moreover, if subsets of movements with distinguishably different velocity profiles were selected, the burst patterns also were distinguishably different. Such a relationship between the activity of a single pontine burst cell and the velocity profile of a subset of movements would not be observed if other members of the large active population of pontine burst cells had quite different temporal profiles of burst activity or if, in general, the burst profiles were heterogeneous and uncorrelated. Thus, these findings indicate that variability in the discharge of a single pontine burst cell is not independent of, but strongly correlated with, the activity of other active pontine cells generating saccade-related bursts.
The ISIs at the end of the burst were longer and more variable. A complete analysis of this observation is beyond the scope of this report, but several factors contribute to the increased variability at the end of the burst. First, the algorithm for matching movements emphasized the peak and rapid components of the velocity profiles, giving less emphasis to the slower, longer tail. Consequently, there often was more variability at the end of the selected movements, the part of the movement with which later intertrial intervals would be associated. Second, the number of spikes in the bursts associated with the selected movements was not constant, so that the last interval for some bursts was averaged with earlier intervals in other bursts. Third, we plotted standard deviations rather than coefficients of variation to emphasize the absolute differences in ISI duration. The relative variability at the end of the burst would be reduced if expressed as a percentage of the average duration of the ISI. Finally, almost all contemporary models of the saccadic system assume that a copy of the motor command is used as a feedback signal for controlling saccade amplitude. If such a feedback signal were introduced before the level of the pontine burst neurons, the ISIs at the end of the burst are the ones most likely to be affected by feedback signals, and this feedback signal would be likely to vary from movement to movement. Note that, on average, the variable part of the burst makes up <20% of the total number of spikes in the burst.
We know of no previous attempts to measure the reliability of motor command signals generated by individual neurons during a saccadic eye movement. The reliability of the discharge of oculomotor motoneurons in cat and monkey has been examined during the fixation period between saccades (5, 16–20). In monkey, one study (17) reported standard deviations of motoneuron discharge rate ranging from 3.7 to 13.5 with a mean of 6.4 when expressed as a percentage of mean interval, and similar values are reported by others (5, 16). Similar studies in cat (18–20) found that variability increased in proportion to the duration of the fixation and that the firing rate during repeated fixation at the same eye position was affected significantly by the direction of the preceding saccade and by the animal's level of alertness. Variability in the discharge of neurons in motor and parietal areas of the primate cortex was measured during a period that included an arm movement, but no attempt was made to analyze variability in the neuronal discharge associated with the actual movement (21).
The relationships between measures of spike activity and saccade amplitude, duration, and velocity for the cells studied meet criteria for classifying them as excitatory burst neurons (EBNs), but we do not know whether the cells we recorded project monosynaptically to motoneurons as EBNs, by definition, do. However, it would be surprising if the major PPRF input to the motoneuron pools came from the other class of cells with saccade-related activity encountered: burst cells with lower frequencies and higher variability. Therefore, we assume the high-frequency burst neurons are likely to be EBNs.
The low variability in the ISIs of pontine bursts could be due to low variability of input signals, special biophysical properties of the pontine burst cells, or a combination of the two. If special biophysical properties of pontine neuron are the major determinants of signal reliability, predictions can be made about the electrical phenotypes of pontine burst cells. Pontine cells projecting to the motoneurons could be identified by retrograde labeling from injections in the motor nuclei and patch-clamp recordings could be obtained in brainstem slices, as has been done for medial vestibular nucleus neurons projecting to the oculomotor nucleus (22). The cells would have a high threshold for activation, but when activated, they would display high firing rates. The expected response to a step of depolarizing current would be a regularly spaced, steady train of impulses with little or no adaptation over a 200- to 300-ms time period. The ISIs of one subset of cells, corresponding to the functional type with different frequency bursts for saccades of different amplitudes, would vary as a function of the level of current. Another subset of cells may not display a linear relation between current level and discharge frequency, but they would be sensitive to the slope of current ramps. These would correspond to the cells that discharge at the same peak frequency for different saccade amplitudes but would fire at different rates during saccades of different speed.
From the perspective of signal reliability, the high-frequency burst mode of pontine neurons has functional consequences not emphasized in the current literature. When repeated requests for a saccade with a particular amplitude are generated at relatively high levels in the motor control circuitry, the resulting movements will be less variable if the pontine cells contributing to the pulse of innervation produced by motoneurons have properties similar to high-frequency burst cells described in this article. Consider the alternative case in which the dominant pontine input to motoneurons is derived from cells with low burst rates and high variability in ISIs, such as the one illustrated in Fig. 3e. During repeated requests for the same movement, the input received from each pontine cell would be variable, and the population output also would be highly variable across requests. If, however, the pontine input to the motoneurons is produced primarily by neurons generating high-frequency bursts, there will be little variability in the contribution of each cell during repeated requests for the same movement. If, as these data imply, the activity of the high-frequency bursts cells is also correlated, the variability in population output also will be reduced.
The reliability of spike timing in neocortical neurons during repeated presentations of stimulus transients (23) has been interpreted as support for a possible role for precise spike timing, rather than average firing rate, in the processing of information by the neocortex. The precision of spike timing observed in the burst of pontine neurons could be interpreted in a similar manner. But that is not our interpretation. We think it is unlikely that the different, but repeatable, burst patterns observed when movements having similar amplitudes but different velocity profiles occur represents a signal explicitly coding for particular saccadic velocities. Instead, we assume that when the excitability of pontine burst cells is modulated by external and internal factors (e.g., attention, arousal level, target intensity, and/or duration), the properties of the burst are modified in a consistent manner, the changes in the burst profile are faithfully transmitted to motoneurons and muscles, and, therefore, consistent behavioral consequences are observed. Our data emphasize the point that the time distribution of spikes, even at a submillisecond timescale, can have behavioral effects.
Additional studies of the pontine burst cells are needed. Relatively little is known about the biophysical properties of pontine burst neurons or the reliability of the motor command signals received by these cells. The degree of correlation between pontine burst cells is unknown. The assumption that the major input to motoneuron pools from PPRF originates from pontine cells generating high-frequency bursts should be tested empirically.
Materials and Methods
Neuronal activity was recorded from the pontine reticular formation of two adolescent rhesus monkeys (Macaca mulatta). In a sterile surgical procedure under isoflurane anesthesia each animal was fitted with a scleral eye coil (24–25) and a fixture for immobilizing the head. After ≈3 months of behavioral training, a cylinder for chronic single-unit recording was implanted in a second sterile surgical procedure. All experimental protocols complied with the National Institutes of Health's Guide for the Care and Use of Animals and were approved by the institutional animal care and use committees.
Using the scleral search coil technique (26), eye position signals were digitized at 500 or 1,000 Hz and stored for later analysis. Commercially available, parylene-coated, tungsten electrodes were used to obtain single-unit, extracellular recordings employing standard chronic-recording techniques (27). For one data set (n = 12), spikes were recorded as time stamps with 1-μs resolution by using an electronic window discriminator to determine the occurrence of action potentials. However, the size of action potentials becomes smaller and irregular in amplitude as the burst of pontine cells progresses (11). Failure to detect one or more of the smaller action potentials in the burst produces spuriously long ISIs expressed as “dropouts” or momentary lower frequencies in the instantaneous frequency records. To avoid this potential error in measurement, we recorded the waveforms of action potentials from a second set of pontine cells and the output of the window discriminator was corrected by adding undetected spikes or removing falsely detected ones. For the second data set (n = 22), ISIs were measured with a 10-μs resolution. In general, the neurons in the data set were well isolated and corrections were applied only occasionally when visual inspection of the data indicated that there were very obvious dropouts or falsely detected spikes. If the situation was ambiguous, the trial was excluded. Corrections were only applied to data sets in which no more than two cells with distinctly different waveforms or large amplitude differences were recorded. Moreover, we did not try to separate the spikes of two cells that both generated high-frequency bursts; corrections were only applied if one of the cells had low-frequency activity not obviously related to eye movements. “Dropped” spikes were manually reinserted, trying to place them at the same location along the spike waveform at which the window discriminator detected spikes.
The waveform recordings verified that most, but not all, apparent dropouts are artifactual. Accordingly, the data presented in this article from the first data set are based on trials in which bursts with two or more dropouts are excluded. The regularity of the high-frequency burst was assessed by computing the mean and standard deviation of ISIs in the burst. Bursts were aligned on the first spike, ignoring the occasional single spike or doublet that precedes burst onset by ≥10 ms. These early spikes were assumed to have little influence on the movement because excitatory postsynaptic potentials recorded from oculomotor motoneurons are usually <10 ms in duration (28, 29). Also excluded from the analysis of ISIs were spikes that (i) preceded movement onset by >8 ms, (ii) preceded saccade offset by <8 ms, or (iii) followed the end of the movement.
Matching of velocity waveforms was accomplished in two stages. First, similarity indices for all pairs of movements to a particular target were computed as (sum of the absolute value of differences between two movements at each sample point) divided by the number of samples. Then, the most similar movements were selected by using a program that superimposed the position and velocity waveforms of a selected movement with each of the movements selected on the basis of the similarity indices.
During each experimental session, the monkey sat in a primate chair facing an array of light emitting diodes (LEDs) in a dimly lit room. Each LED subtended a visual angle of ≈0.2°. Action potentials were recorded from individual pontine burst neurons while monkeys made horizontal saccades to 2 to 5 visual targets, always starting from a few selected initial eye positions varying over a range of ±10°.
Acknowledgments
We thank Kathy Pearson for software development, John Maunsell for helpful comments on earlier versions of the manuscript and useful suggestions about the conduct of the experiments, and Dennis Murray for providing invaluable assistance during surgical procedures and the daily maintenance of the animals. The work was supported by National Institutes of Health Grant EY01189 and by National Science Foundation of China Grants NSFC 30670669 and 30530270, and 973 program Grant 2005CB522803.
Abbreviations
- ISI
interspike interval
- PPRF
pontine reticular formation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702799104/DC1.
References
- 1.Reich DS, Victor JD, Knight BW, Ozaki T, Kaplan E. J Neurophysiol. 1977;77:2836–2841. doi: 10.1152/jn.1997.77.5.2836. [DOI] [PubMed] [Google Scholar]
- 2.de Ruyter van Steveninch RR, Lewen GD, Strong SP, Koberle R, Bialek W. Science. 1997;275:1805–1808. doi: 10.1126/science.275.5307.1805. [DOI] [PubMed] [Google Scholar]
- 3.Reinagel P, Reid RC. J Neurosci. 2000;20:5392–5400. doi: 10.1523/JNEUROSCI.20-14-05392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinagel P, Reid RC. J Neurosci. 2002;22:6837–6841. doi: 10.1523/JNEUROSCI.22-16-06837.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson DA. J Neurophysiol. 1970;33:393–404. doi: 10.1152/jn.1970.33.3.393. [DOI] [PubMed] [Google Scholar]
- 6.Moschovakis AK, Scudder CA, Highstein SM. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 7.Scudder CA, Kaneko CS, Fuchs AF. Exp Brain Res. 2002;142:439–462. doi: 10.1007/s00221-001-0912-9. [DOI] [PubMed] [Google Scholar]
- 8.Sparks DL. Nat Rev Neurosci. 2002;3:952–964. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- 9.Strassman A, Highstein SM, McCrea RA. J Comp Neurol. 1986;249:358–380. doi: 10.1002/cne.902490304. [DOI] [PubMed] [Google Scholar]
- 10.Luschei ES, Fuchs AF. J Neurophysiol. 1972;35:445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- 11.Keller EL. J Neurophysiol. 1974;37:316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- 12.King WM, Fuchs AF. J Neurophysiol. 1979;42:861–876. doi: 10.1152/jn.1979.42.3.861. [DOI] [PubMed] [Google Scholar]
- 13.Van Gisbergen JAM, Robinson DA, Gielen S. J Neurophysiol. 1981;45:417–442. doi: 10.1152/jn.1981.45.3.417. [DOI] [PubMed] [Google Scholar]
- 14.Scudder CA, Fuchs AF, Langer TP. J Neurophysiol. 1988;59:1430–1454. doi: 10.1152/jn.1988.59.5.1430. [DOI] [PubMed] [Google Scholar]
- 15.Sparks DL. Curr Opin Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs AF, Luschei ES. J Neurophysiol. 1970;33:382–392. doi: 10.1152/jn.1970.33.3.382. [DOI] [PubMed] [Google Scholar]
- 17.Keller EL, Robinson DA. Vision Res. 1972;12:369–382. doi: 10.1016/0042-6989(72)90082-x. [DOI] [PubMed] [Google Scholar]
- 18.Delgado-Garcia JM, del Pozo F, Baker R. Neuroscience. 1986;17:929–952. doi: 10.1016/0306-4522(86)90072-2. [DOI] [PubMed] [Google Scholar]
- 19.Gomez C, Canals J, Torres B, Delgado-Garcia JM. Brain Res. 1986;381:401–404. doi: 10.1016/0006-8993(86)90099-5. [DOI] [PubMed] [Google Scholar]
- 20.de la Cruz RR, Escudero M, Delgado-Garcia JM. Eur J Neurosci. 1989;1:288–295. doi: 10.1111/j.1460-9568.1989.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Port NL, Kruse W, Georgopoulos AP. J Neurosci. 1998;18:1161–1170. doi: 10.1523/JNEUROSCI.18-03-01161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekirnjak C, du Lac S. J Neurophysiol. 2006;95:3012–3023. doi: 10.1152/jn.00796.2005. [DOI] [PubMed] [Google Scholar]
- 23.Mainen ZF, Sejnowski TJ. Science. 1995;268:1503–1506. doi: 10.1126/science.7770778. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs AF, Robinson DA. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- 25.Judge SJ, Richmond BJ, Chu FC. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DA. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- 27.Mays LE, Sparks DL. J Neurophysiol. 1980;43:207–232. doi: 10.1152/jn.1980.43.1.207. [DOI] [PubMed] [Google Scholar]
- 28.Precht W, Baker R. Exp Brain Res. 1972;14:158–184. doi: 10.1007/BF00234797. [DOI] [PubMed] [Google Scholar]
- 29.Uchino S, Suzuki S, Watanabe S. Exp Brain Res. 1980;41:45–53. doi: 10.1007/BF00236678. [DOI] [PubMed] [Google Scholar]