Abstract
Ovarian estrogen exerts both positive and negative feedback control over luteinizing hormone (LH) secretion during the ovulatory cycle. Estrogen receptor (ER) α but not ERβ knockout mice lack estrogen feedback. Thus, estrogen feedback appears to be primarily mediated by ERα. However, it is now recognized that, in addition to binding to estrogen response elements (EREs) in DNA to alter target gene transcription, ERα signals through ERE-independent or nonclassical pathways, and the relative contributions of these pathways in conveying estrogen feedback remain unknown. Previously we created a knockin mouse model expressing a mutant form of ERα (AA) with ablated ERE-dependent but intact ERE-independent activity. Breeding this allele onto the ERα-null (−/−) background, we examine the ability of ERE-independent ERα signaling pathways to convey estrogen feedback regulation of the female hypothalamic–pituitary axis in vivo. ERα−/AA exhibited 69.9% lower serum LH levels compared with ERα−/− mice. Additionally, like wild type, ERα−/AA mice exhibited elevated LH after ovariectomy (OVX). Furthermore, the post-OVX rise in serum LH was significantly suppressed by estrogen treatment in OVX ERα−/AA mice. However, unlike wild type, both ERα−/AA and ERα−/− mice failed to exhibit estrous cyclicity, spontaneous ovulation, or an afternoon LH surge response to estrogen. These results indicate that ERE-independent ERα signaling is sufficient to convey a major portion of estrogen's negative feedback actions, whereas positive feedback and spontaneous ovulatory cyclicity require ERE-dependent ERα signaling.
Keywords: neuroendocrine, reproduction
Neuroendocrine control of the ovulatory cycle is mediated by the pulsatile neurosecretion of gonadotropin-releasing hormone (GnRH) (1). The decapeptide is conveyed through the hypophysial portal vessels to the extracellular spaces of the anterior pituitary gland where it binds GnRH receptors on the plasma membranes of gonadotropes. GnRH receptor activation directs the synthesis and secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone into the peripheral circulation to govern ovarian steroidogenesis and folliculogenesis. Ovarian steroid secretions, in turn, exert homeostatic feedback actions that alter GnRH and LH secretions. Throughout most of the rodent estrous cycle, estrogen exerts negative feedback actions on GnRH and LH secretion until, on the afternoon of proestrous, elevated follicular estrogen secretions evoke an abrupt release of a preovulatory GnRH surge, and hence LH surge, which triggers ovulation (1, 2). Thus, both negative and positive feedback actions of estrogen are critically important in the physiological control of cyclic hormone secretions and ovulatory cyclicity.
In ovariectomized (OVX) animals, virtually all physiological or supraphysiological regimens of estrogen replacement can manifest a pronounced suppression of LH secretion, which is at least in part mediated by retardation of the hypothalamic “GnRH pulse generator” (3, 4). The ability of estrogen to elicit positive feedback effects, however, depends on a sustained elevation of circulating estrogen levels to evoke GnRH and LH surges (1, 3, 5). It remains unclear whether the signaling pathways that mediate estrogen's positive and negative feedback actions are completely separable, partially dissociable, or largely overlapping. The complexity of estrogen feedback actions has largely confined experiments to whole-animal in vivo models and limited detailed mechanistic studies. Recently, estrogen receptor (ER) α and ERβ knockout (KO) mouse models have been used to identify ERα as the predominant receptor isoform that conveys estrogen negative feedback regulation of LH (6). Moreover, ERα expression in a neuronal cell population was shown to be required for estrogen positive feedback (7). However, the precise cellular and molecular mechanisms of estrogen feedback remain elusive because ERα has been shown to signal through multiple pathways, even within a single-cell context (8). In the classical genomic pathway, ERα binds to estrogen response elements (EREs) in DNA to alter the transcription of genes (9). In contrast, in the ERE-independent, or nonclassical, genomic pathway, ERα participates in protein–protein interactions to alter transcription at non-ERE sites by tethering to other transcription factors such as AP1 and NF-κB (10, 11). ERα has also been shown to participate in rapid membrane-initiated signaling (12).
One approach to dissecting multiple signaling activities in vivo is the use of gene “knockin” models. A mouse expressing a dimerization-deficient glucocorticoid receptor (GR) unable to bind DNA was previously used to elucidate the roles of DNA binding-dependent and binding-independent GR signaling pathways in the hypothalamic–pituitary–adrenal axis (13). Taking a similar approach with the ERα, we previously generated a (E207A/G208A; “AA”) mutant receptor with disrupted DNA binding and intact ERE-independent activity (14). Using this AA mutant ERα we created a nonclassical ERα knockin mouse model. Infertility in the ERα+/AA due to uterine defects and anovulation (15) precluded breeding of the AA mutation to homozygosity. Therefore, we used an alternate approach of breeding the AA mutant ERα onto the ERαKO (ERα−/−) background to create an in vivo model of isolated ERE-independent signaling. In this system, a rescue of ERα−/− phenotypes by AA in ERα−/AA mice reveals a physiologic role for the ERE-independent ERα pathway. Conversely, a lack of effect implies a role for ERE-mediated pathways. To date, this model has been used to characterize the in vivo roles of the ERE-independent pathway in the uterus (16) and bone (17). This report investigates the relative contributions of the ERE-dependent (classical) and -independent (nonclassical) ERα signaling pathways in providing estrogen feedback in the female hypothalamic–pituitary–gonadal axis. We find that ERE-independent estrogen signaling is sufficient to convey partial negative feedback, whereas estrogen induction of the LH surge (positive feedback) and spontaneous ovulation require ERE-dependent signaling.
Results
Ovarian Hemorrhagia and Cyclicity in ERα−/AA Versus ERα−/−.
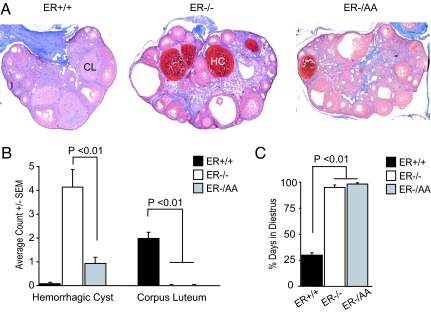
In previous reports, ERαKO females were shown to have elevated serum LH and estradiol, as well as ovarian hemorrhagia (18). Consistent with these data, here ERα−/− females exhibited an average of four hemorrhagic cysts per ovary (range 0–16), as compared with wild type (ERα+/+), which were essentially devoid of hemorrhagic cysts (one cyst in 14 ovaries examined) (Fig. 1 A and B). Upon selectively restoring ERE-independent ERα signaling, ERα−/AA mice exhibit an average of 0.93 (range 0–4) hemorrhagic cysts, a 77.5% decrease compared with ERα−/− animals (Fig. 1 A and B).
Fig. 1.
Ovarian morphology and estrous cyclicity. Selectively restoring ERE-independent ERα signaling decreases ovarian hemorrhagia but does not mediate spontaneous ovulation or estrous cyclicity. (A) Representative ovarian sections from ERα+/+, ERα−/−, and ERα−/AA mice. CL, corpus luteum; HC, hemorrhagic cyst. (B) Average incidence of hemorrhagic cyst and corpus luteum (n = 25–30). Note the excess of hemorrhagic cysts in ERα−/− mice and the presence of corpus luteum only in ERα+/+ mice. (C) Estrous cyclicity (n = 7–13). ERα+/+ mice exhibit estrous cycles, whereas ERα−/− and ERα−/AA mice are in constant diestrus.
ERαKO mice appear anovulatory based on the absence of ovarian corpus lutea (18). We observed a similar absence of corpora lutea in ERα−/− and also ERα−/AA ovaries (Fig. 1 A and B). Estrous cycles were examined in each genotype to determine whether the lack of spontaneous ovulation was due to a lack of cyclicity. In contrast to ERα+/+ females, neither ERα−/− nor ERα−/AA mice exhibited evidence of estrous cyclicity. Rather, both appeared to be in constant diestrus (Fig. 1C). These results indicate that ERE-independent (nonclassical) ERα signaling is not sufficient to mediate estrous cyclicity and spontaneous ovulation in vivo.
Serum LH and Estradiol Levels in Ovary-Intact Mice.
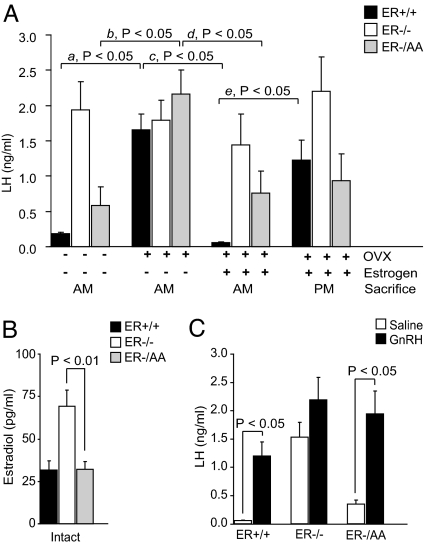
ERαKO female mice have elevated LH, excess steroidogenesis, and elevated serum estrogen compared with wild-type counterparts (18, 19). In our study, ERα−/− females exhibited 10.6-fold greater serum LH (Fig. 2A) (P < 0.05) and 2.2-fold greater serum estradiol (Fig. 2B) (P < 0.01) compared with ERα+/+ mice. Serum LH levels in ERα−/AA females, however, were 69.9% lower than LH levels in ERα−/− mice (Fig. 2A) (P < 0.05) but remained higher than in the ERα+/+ (Fig. 2A) (P < 0.05). ERα−/AA females exhibited serum estradiol levels that were indistinguishable from ERα+/+ and 46% lower than those in ERα−/− mice (Fig. 2B).
Fig. 2.
Estrogen feedback. The ERE-independent ERα signaling pathway is sufficient to convey estrogen negative but not positive feedback. (A) Serum LH from intact, OVX, OVX/estrogen-replaced females killed in the morning for negative feedback and afternoon for positive feedback (n = 5–16). (B) Serum estradiol from intact animals (n = 5–6). (C) Pituitary response to exogenous GnRH in OVX/estrogen-replaced females (n = 6–10).
Serum LH Response to OVX.
Animals of each genotype were OVX to investigate the serum LH response to a loss of negative feedback. OVX of ERα+/+ females increased serum LH 9-fold when compared with intact mice (Fig. 2A, a). In contrast, LH in ERα−/− mice remained high and did not further increase after OVX. OVX in ERα−/AA females resulted in 3.7-fold elevated serum LH compared with ovary-intact ERα−/AA (Fig. 2A), indicating that one or more ovarian factors provide negative feedback through an ERE-independent ERα pathway to suppress serum LH.
Estrogen Negative Feedback.
To examine whether estrogen is responsible for negative feedback provided by the presence of ovaries in the three genotypes, estrogen was replaced in OVX females using a 17-β-estradiol silastic capsule implanted at OVX followed by a s.c. injection of estradiol benzoate administered in the morning on day 6 after OVX. In this model, the mice are under estrogen negative feedback in the morning and positive feedback by late afternoon of day 7. In ERα+/+ females killed in the morning on day 7, the post-OVX rise in LH was completely abrogated (Fig. 2A, c). In ERα−/AA mice, estrogen replacement also returned serum LH to intact levels (Fig. 2A, d). As previously reported (20), estrogen failed to decrease LH in OVX ERα−/− mice. These data suggest that estrogen acting via the ERE-independent (nonclassical) ERα signaling pathway mediates a functionally significant, albeit incomplete, level of estrogen negative feedback.
Estrogen Positive Feedback.
When killed in the afternoon, immediately preceding lights-out, positive feedback or the endogenous LH surge response to estrogen can be examined (21). ERα+/+ mice exhibited a 21-fold increase in serum LH when compared with animals killed in the morning on day 7 (Fig. 2A, e). In contrast, neither ERα−/− nor ERα−/AA mice showed a significant response. These data indicate that the ERE-dependent (classical) ERα pathway is required for positive feedback and estrogen induction of an LH surge.
Pituitary Response to Exogenous GnRH.
The inability to produce an LH surge may be caused either by a lack of estrogen positive feedback or by decreased pituitary response to GnRH. To examine the latter possibility, OVX, estrogen-treated animals of each genotype were given a submaximal dose of exogenous GnRH (Fig. 2C). Both ERα+/+ and ERα−/AA mice responded with significant elevations of serum LH to >1 ng/ml. Therefore, a lack of positive feedback in the ERα−/AA is not likely due to diminished GnRH responsiveness. Of note, ERα−/− mice did not exhibit a significant LH response to exogenous GnRH. This is likely because the saline-treated group is already under stimulation by elevated endogenous GnRH in the absence of estrogen negative feedback.
Discussion
Physiological studies in ER KO models suggest that estrogen negative feedback occurs primarily through ERα (6, 20, 22). In general, analyses of ERα signaling in vivo and in vitro have focused primarily on ERE-mediated gene transcription (9), and these studies have been invaluable for defining many of the cofactors and alterations in chromatin that accompany E2-induced changes in transcription. However, ER also acts through ERE-independent genomic pathways as well as nongenomic signaling pathways initiated at the cell membrane (8, 12). Currently, the roles of these distinct ERα signaling pathways in mediating estrogen feedback effects in the hypothalamic–pituitary–gonadal axis are unclear.
This report finds that the ERE-independent ERα pathway partially restores estrogen feedback inhibition of LH. This was evidenced by lower serum LH in ovary-intact animals in mice expressing the ERα−/AA allele, compared with their ERα−/− counterparts, resulting in less ovarian hyperstimulation and, hence, decreased hemorrhagia and steroidogenesis. Also, estrogen replacement in OVX ERα−/AA mice was sufficient to convey negative feedback and reduce serum LH to levels observed in ovary-intact animals. However, the ERE-independent ERα signaling pathway was not sufficient to mediate estrogen positive feedback or spontaneous ovulation despite an unaltered pituitary response to GnRH. Therefore, we conclude that the ERE-independent ERα signaling pathway is sufficient for estrogen negative feedback whereas the ERE-dependent ERα signaling pathway is required for estrogen positive feedback. In comparison to 100% of wild type, only 50% of ERα−/AA mice responded to gonadotropin-induced superovulation, and with fewer oocytes recovered (data not shown). Consistent with ERαKO superovulation (18) this result suggests that, in addition to hypothalamic and pituitary components, full ovulatory capacity may require ERE-mediated ERα signaling within the ovary.
Recently, a neuron-specific ERα mutant female mouse was shown to lack estrogen positive feedback (7). Therefore, our results might further specify that it is ERE-dependent ERα signaling that is required in this neuronal population for estrogen positive feedback. Estrogen-induced progesterone receptor (PR) transcription has been shown to depend on EREs in vitro (23) and is known to occur in brain regions involved in estrogen positive feedback (24, 25). Consistent with a requirement for the estrogen induction of PR, and like the ERα−/− and ERα−/AA, the PRKO female mice lack estrogen positive feedback and spontaneous ovulation (26). Of note, PR expression was shown to be reduced in the ERα−/AA compared with ERα+/+ uterus (16). Thus, the ERE-mediated induction of PR provides a possible explanation for the lack of positive feedback regulation in the ERα−/AA. Of interest, we have previously shown that ERα+/AA females are anovulatory (15) whereas ERα+/− are fertile (19). Therefore, it seems possible that the ERE-independent pathway may in some manner inhibit responsiveness to the positive feedback actions of estrogen. The mechanisms mediating this putative antagonism and their physiological significance remain to be clarified.
Treatment of OVX, estrogen-replaced, ERα−/AA and ERα+/+ mice with GnRH produced equivalent LH responses, indicating that normal pituitary responsiveness to GnRH is maintained by the presence of the ERα−/AA allele. Moreover, the saline-treated animals in this experiment again demonstrated that the expression of the ERα−/AA allele rescues the majority of estrogen's negative feedback actions that are evident in the ERα+/+ mice. Negative feedback, in contrast to positive feedback, appears to be intact in the neuron-specific ERα mutant, suggesting that estrogen's actions in hypothalamic glial cells, or pituitary gonadotropes, may be sufficient to convey estrogen negative feedback (7). Furthermore, nongenomic estrogen signaling has been shown to be capable of suppressing LH secretion but not inducing the preovulatory LH surge in the ewe (27). Therefore, it is possible that some of the observed estrogen negative feedback actions in ERα−/AA animals reflect a rescue of estrogen's nongenomic actions.
Ovary-intact and OVX estrogen-treated ERα−/AA mice consistently exhibit elevated serum LH levels compared with ERα+/+ mice. The basis for the incomplete suppression of LH in ERα−/AA is unclear. It is possible that classical ERE signaling confers aspects or components important for negative feedback. Alternatively, the AA allele may alter ERβ-mediated estrogen signaling. Data obtained from the use of ERα- and ERβ-selective ligands in rats (28) continue to support the conclusion that ERα is the predominant receptor isoform mediating negative feedback. However, ERα is known to heterodimerize with ERβ, and there is evidence that ERβ may also have a role in conveying estrogen feedback effects. Higher LH in the ERαβ double KO compared with ERαKO has been observed (6), and ERβ can modify ERα effects in pituitary gonadotropes (29). Also, we cannot rule out the possibility of an allelic dosage effect where the single AA allele provided in the ERα−/AA is insufficient to mediate complete negative feedback. Of note, haploinsufficiency has been shown for the repressor of ER activity, a cofactor capable of altering both ERE-dependent and -independent genomic signaling (30).
Although GnRH neuronal activity is altered under estrogen feedback (31, 32), ERα mRNA and protein are absent or undetectable in adult GnRH neurons (33). Therefore, estrogen feedback is likely conveyed indirectly to GnRH neurons by ERα-expressing interneurons (2, 34–36). Moreover, lesion studies performed in the rat and hamster (37, 38) implicated anatomically distinct brain regions for the conveyance of positive and negative feedback. These studies localized negative feedback to the arcuate and median eminence regions of the medial basal hypothalamus and positive feedback to the preoptic area and suprachiasmatic nucleus. Kiss-1 neurons are ERα-positive and present in both the anteroventral periventricular nucleus (AVPV) and the arcuate regions. Recent evidence has shown an ERα-dependent estrogen up-regulation of Kiss-1 mRNA in the AVPV and down-regulation in the arcuate region (39, 40). Although circumstantial, these data support the notion of anatomically distinct responses to estrogen mediated by ERα during feedback. Results provided here further suggest that estrogen positive and negative feedback may be separable by their molecular mechanisms of ERα signaling. Thus, it remains to be determined whether ERE-dependent and ERE-independent ERα signaling mechanisms predominate in AVPV and arcuate neurons, respectively.
In summary, the ERα−/AA mouse provides a novel model for dissecting ERE-dependent from -independent estrogen effects in the female hypothalamic–pituitary–gonadal axis. Our studies of this animal clearly document that nonclassical ER signaling can mediate at least some of estrogen's negative feedback actions in the hypothalamus and/or pituitary gland. Additional studies will further distinguish the relative importance of nonclassical genomic, nongenomic, and/or membrane-initiated ER signaling in these key physiological actions of estrogen.
Materials and Methods
Animals.
Animals were housed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (41). Animal use procedures were approved by the Northwestern University Animal Care and Use Committee. Nonclassical ERα knockin mice were created on a 129SvJ background (15), and ERαKO mice (obtained from Pierre Chambon, Collège de France, Illkirch, France) were on a C57BL/6 background (42). ERα−/AA used in these experiments are the result of the AA mutant allele crossed 7–11 generations onto the C57BL/6 background. Mice were maintained on a 14-h light:10-h dark cycle with standard chow (7912; Harlan Teklad, Madison, WI) and water available ad libitum.
Ovarian Morphology.
Ovaries were dissected, fixed in 10% neutral buffered formalin for 24 h, transferred to 70% ethanol, processed, and embedded. Slides were prepared by using 5-μm sections, deparaffinized, and then stained by rapid Giemsa or hematoxylin and eosin (43). The number of corpora lutea and hemorrhagic follicles were scored in a blinded fashion by using one section per ovary and one ovary per animal, stained with hematoxylin and eosin.
Estrous Cycles.
Female mice were individually housed 2 weeks before experiments. Vaginal smears were taken at 1000 hours each morning, and cytology was used to identify the phase of the estrous cycle (44) for 10–20 consecutive days.
OVX and Estrogen Replacement.
Intact adult females 2–6 months (estradiol) or 7–13 weeks (LH) of age were anesthetized with halothane (B4388; Sigma, St. Louis, MO) between 0800 hours and 1000 hours and killed by exsanguination using cardiac puncture. For OVX, females (10–13 weeks of age) were transferred to a low-phytoestrogen diet (2019S; Harlan Teklad) 1 day before surgery. Between 0800 hours and 1000 hours on the day of surgery (day 0), females were anesthetized by i.p. injection of 200 mg/kg 2,2,2-tribromoethanol (T48402; Sigma) in 0.9% sodium chloride (S8776; Sigma) and 2% tert-amyl alcohol (240486; Sigma) vehicle. Females were implanted with either blank silastic capsules (OVX group) or capsules containing 2.5 μg of 17β-estradiol (26) (negative feedback and positive feedback groups). Between 0900 hours and 1000 hours on day 6 after OVX, animals in the negative and positive feedback groups were injected s.c. with 1 μg of estradiol benzoate (E8515; Sigma) in 0.1 ml of sesame oil (S3547; Sigma). OVX group females were injected with 0.1 ml of sesame oil vehicle. OVX and negative feedback animals were killed between 0800 hours and 1000 hours by exsanguination using cardiac puncture on day 7 after OVX. Positive feedback animals were killed 30 min before lights-out.
GnRH Stimulation.
To test the response to GnRH, OVX/E2-treated females received a s.c. injection of 0.1 ml of saline vehicle or 200 ng/kg GnRH (L7134; Sigma) 10 min before they were killed between 0800 hours and 1000 hours on day 7 after OVX. This dose of GnRH is submaximal as determined in a preliminary titration experiment (data not shown).
Serum Collection and Assays.
Blood was allowed to coagulate for 90 min at room temperature and centrifuged at 2,000 × g for 15 min. Serum was transferred to a fresh tube and stored at −20°C. Sera were randomized and assayed by using a mouse LH sandwich immunoradiometric assay and estradiol RIA (Diagnostic Laboratories, Webster, TX) at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. For LH, values below the limit of detection were set to the lowest detectable amount (0.04 ng/ml).
Statistical Analysis.
Incidence of hemorrhagic cysts, corpus luteum, estrous cyclicity, and serum estradiol data were analyzed by using the one-way ANOVA with Bonferroni post hoc test with P < 0.01 as the cutoff for significance. Intact, OVX, negative feedback, positive feedback, and pituitary response to exogenous GnRH serum LH data were log-transformed and analyzed by using two-way ANOVA with a Bonferroni post hoc (all-pairwise) multiple comparisons test for effect of treatment and genotype. P < 0.05 was the cutoff for significance.
Acknowledgments
ERαKO mice were a generous gift from Dr. Pierre Chambon. We thank Donna Emge, Thomas Kotlar, and Dr. Teresa Horton for technical assistance with these studies. This research was supported by National Institutes of Health/National Institute of Child Health and Human Development Grant PO1 HD21921, National Institutes of Health Training Grant T32 GM008061, and National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core).
Abbreviations
- LH
luteinizing hormone
- GnRH
gonadotropin-releasing hormone
- ER
estrogen receptor
- ERE
estrogen response element
- KO
knockout
- OVX
ovariectomy/ovariectomized
- PR
progesterone receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Levine JE. Biol Reprod. 1997;56:293–302. doi: 10.1095/biolreprod56.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Herbison AE. Endocr Rev. 1998;19:302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 3.Fink G. J Steroid Biochem. 1988;30:169–178. doi: 10.1016/0022-4731(88)90090-8. [DOI] [PubMed] [Google Scholar]
- 4.Kato A, Hiruma H, Kimura F. Neuroendocrinology. 1994;59:426–431. doi: 10.1159/000126688. [DOI] [PubMed] [Google Scholar]
- 5.McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Neuroendocrinology. 1981;33:158–165. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- 6.Couse JF, Yates MM, Walker VR, Korach KS. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 7.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JM, Couse JF, Korach KS. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- 9.O'Malley BW, Tsai MJ. Biol Reprod. 1992;46:163–167. doi: 10.1095/biolreprod46.2.163. [DOI] [PubMed] [Google Scholar]
- 10.Stein B, Yang MX. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray P, Ghosh SK, Zhang DH, Ray A. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 12.Levin ER. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 14.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 15.Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. Mol Endocrinol. 2002;16:2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. J Biol Chem. 2006;281:26683–26692. doi: 10.1074/jbc.M601522200. [DOI] [PubMed] [Google Scholar]
- 17.Syed FA, Modder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S. J Bone Miner Res. 2005;20:1992–2001. doi: 10.1359/JBMR.050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Endocrinology. 1999;140:5855–5865. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- 19.Couse JF, Korach KS. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 20.Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Endocrine. 1999;11:137–143. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- 21.Christian CA, Mobley JL, Moenter SM. Proc Natl Acad Sci USA. 2005;102:15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorling AA, Todman MG, Korach KS, Herbison AE. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 23.Kraus WL, Montano MM, Katzenellenbogen BS. Mol Endocrinol. 1994;8:952–969. doi: 10.1210/mend.8.8.7997237. [DOI] [PubMed] [Google Scholar]
- 24.Brown TJ, Yu J, Gagnon M, Sharma M, MacLusky NJ. Brain Res. 1996;725:37–48. doi: 10.1016/0006-8993(96)00241-7. [DOI] [PubMed] [Google Scholar]
- 25.Shughrue PJ, Lane MV, Merchenthaler I. Endocrinology. 1997;138:5476–5484. doi: 10.1210/endo.138.12.5595. [DOI] [PubMed] [Google Scholar]
- 26.Chappell PE, Schneider JS, Kim P, Xu M, Lydon JP, O'Malley BW, Levine JE. Endocrinology. 1999;140:3653–3658. doi: 10.1210/endo.140.8.6895. [DOI] [PubMed] [Google Scholar]
- 27.Arreguin-Arevalo JA, Nett TM. Biol Reprod. 2006;74:202–208. doi: 10.1095/biolreprod.105.044685. [DOI] [PubMed] [Google Scholar]
- 28.Hillisch A, Peters O, Kosemund D, Muller G, Walter A, Schneider B, Reddersen G, Elger W, Fritzemeier KH. Mol Endocrinol. 2004;18:1599–1609. doi: 10.1210/me.2004-0050. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Criado JE, de Las Mulas JM, Bellido C, Navarro VM, Aguilar R, Garrido-Gracia JC, Malagon MM, Tena-Sempere M, Blanco A. J Endocrinol. 2006;188:167–177. doi: 10.1677/joe.1.06377. [DOI] [PubMed] [Google Scholar]
- 30.Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. Proc Natl Acad Sci USA. 2006;103:16716–16721. doi: 10.1073/pnas.0607768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moenter SM, Defazio RA, Straume M, Nunemaker CS. Ann NY Acad Sci. 2003;1007:143–152. doi: 10.1196/annals.1286.014. [DOI] [PubMed] [Google Scholar]
- 32.DeFazio RA, Moenter SM. Mol Endocrinol. 2002;16:2255–2265. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 33.Herbison AE, Pape JR. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- 34.Kelly MJ, Qiu J, Ronnekleiv OK. Ann NY Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Segura LM, McCarthy MM. Endocrinology. 2004;145:1082–1086. doi: 10.1210/en.2003-1383. [DOI] [PubMed] [Google Scholar]
- 36.Kalra SP. Endocr Rev. 1993;14:507–538. doi: 10.1210/edrv-14-5-507. [DOI] [PubMed] [Google Scholar]
- 37.Shander D, Barraclough CA. Exp Brain Res. 1980;40:123–130. doi: 10.1007/BF00237530. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 39.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dungan HM, Clifton DK, Steiner RA. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 41.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: Natl Acad Press; 1996. pp. 21–55. [Google Scholar]
- 42.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Development (Cambridge, UK) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 43.Preece A. A Manual for Histologic Technicians. Boston: Little; 1972. p. 243. [Google Scholar]
- 44.Marcondes FK, Bianchi FJ, Tanno AP. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]