Abstract
The natural communication of apes may hold clues about language origins, especially because apes frequently gesture with limbs and hands, a mode of communication thought to have been the starting point of human language evolution. The present study aimed to contrast brachiomanual gestures with orofacial movements and vocalizations in the natural communication of our closest primate relatives, bonobos (Pan paniscus) and chimpanzees (Pan troglodytes). We tested whether gesture is the more flexible form of communication by measuring the strength of association between signals and specific behavioral contexts, comparing groups of both the same and different ape species. Subjects were two captive bonobo groups, a total of 13 individuals, and two captive chimpanzee groups, a total of 34 individuals. The study distinguished 31 manual gestures and 18 facial/vocal signals. It was found that homologous facial/vocal displays were used very similarly by both ape species, yet the same did not apply to gestures. Both within and between species gesture usage varied enormously. Moreover, bonobos showed greater flexibility in this regard than chimpanzees and were also the only species in which multimodal communication (i.e., combinations of gestures and facial/vocal signals) added to behavioral impact on the recipient.
Keywords: bonobo, chimpanzee, communication, multimodal
The longstanding debate about the evolution of language typically compares human speech with animal vocalizations (1). The vocal modality offers obvious parallels, yet it has been proposed that our ancestors' first linguistic utterances were not in the vocal but in the gestural domain (2–5). This proposal is all the more intriguing because gestural communication is virtually limited to the Hominoidea (i.e., humans and apes).
Gestures are narrowly defined here as communication by means of hands, feet, or limbs (Table 1). One reason to set gestures apart from other bodily communication is that the two are neurologically distinct in both production and perception by others (6, 7). Whereas all primates regularly communicate by means of vocalizations, orofacial movements, body postures, and locomotion patterns, free brachiomanual gestures (i.e., manual communication without touching another individual or a substrate) are typical of humans and apes (8). Gestures were first described for chimpanzees (9, 10), followed by other anthropoid apes: bonobos (11, 12), gorillas (Gorilla gorilla; refs. 13 and 14), and orangutans (Pongo pygmaeus; ref. 15).
Table 1.
Operational definition of gesture
|
The discontinuity between the Hominoidea and all other primates regarding gestural modality suggests a relatively recent shift toward a more flexible and intentional communicative strategy in our prehominid ancestors (8). One mark of this shift is contextually defined usage; that is, a single gesture may communicate entirely different needs or intentions depending on the social context in which it is used. For example, a chimpanzee stretching out an open hand toward a third party during a fight signals a need for support, whereas the same gesture toward a possessor of food signals a desire for a share (refs. 16 and 17; Fig. 1). This is not to say that other forms of communication cannot derive extra meaning from the context in which they occur (18), but gestures seem less closely tied to specific emotions, hence they permit greater cortical control than other forms of communication (19, 20).
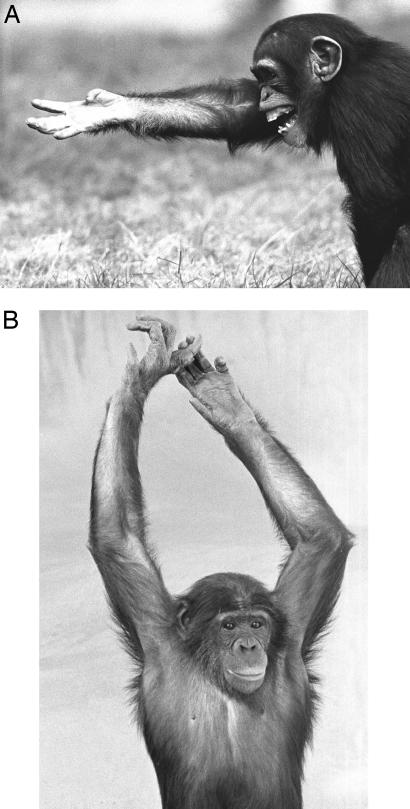
Fig. 1.
Meaning often needs to be extracted from the specific context in which a gesture is being used. (A) A juvenile chimpanzee tries to reclaim food that a dominant has taken away by combining the reach out up begging gesture with a scream vocalization. (B) An adolescent bonobo male making sexual advances to a female adds the arm raise gesture. Photographs by Frans de Waal.
This difference in control was dramatically illustrated by the failure of efforts to teach chimpanzees vocal modifications to “speak” (21, 22), whereas this species had no trouble learning to use the gestures of American sign language in a referential manner (23). Greater control over natural gestures than facial expressions is also suggested by observations of (i) deception, in which apes may use a hand to modify a facial expression (24, 25), and (ii) cultural transmission, with gestures being far more population-specific in both humans and apes than facial expressions, which tend to be relatively invariant (8, 26–28).
All of these observations are in line with the gestural hypothesis of human language origins, which is further supported by differential growth of the brain and vocal apparatuses, as seen in paleoarchaeological remains (29), the appearance of gestural communication in human infants before speech (30), and the right-hand (hence left-brain) bias of both ape and human gestures (31–33). The ape homologue of Broca's area (i.e., Brodmann's area 44) is enlarged in the left hemisphere (34). In monkeys, this area is activated during both the production and perception of gestures but not vocalizations (35). It has been speculated, therefore, that the neural structures underlying manual movements in the great apes, perhaps also including tool use (36), are homologous with the lateralized language areas in the human brain (37, 38).
Gesture remains very much alive in human communication. Recent research demonstrates the universal importance of gesture to human cognitive functioning, such as enhanced information transfer (39), lexical retrieval (40), and even the provision of a supplemental cognitive arena for thought (41). Gesture production in humans is so automatic that it is relatively immune to audience effects: blind subjects gesture at equal rates as sighted subjects to a known blind audience (42).
Gestures are rarely produced in the absence of other communicative signals, such as facial expressions and vocalizations. Multimodal communication has been appreciated in humans for several decades (43) and is becoming increasingly important in the study of animal communication (44). Multimodal signaling occurs across taxa, from snapping shrimps to spiders and birds, and in all contexts, although those related to courtship and mating are best documented (A.S.P., unpublished work). This communication strategy can have a variety of functions, including amplification and modulation of signal meaning. Combined with the graded facial/vocal signals typical of the apes (46), gestural flexibility has the advantage over the more stereotyped signaling by monkeys that it permits greater communicative complexity.
Despite prior work on ape gesture, no research has investigated how these signals combine with other forms of natural communication and how variations in usage affect behavior. Here, we set out to test how the natural gestural modality combines with and differs from the facial/vocal modality in the communication of the two ape species genetically closest to humans: bonobos and chimpanzees. Given the above background, we formulated a gestural flexibility hypothesis according to which the usage of gestures will be more flexible than that of facial/vocal displays, both within and between species. In testing this hypothesis, we will use context as a proxy for usage, meaning, and function; that is, we predict that gestures will be less context-bound than other types of signals.
The Pan line, which includes both chimpanzees and bonobos, split off from the line that produced our species ≈6 million years ago (47), whereas the two ape species themselves split apart only ≈2.5 million years ago (48). Chimpanzees and bonobos are, therefore, genetically equidistant to us. Studying similar types of communicative signals in closely related species allows one to determine homologies, i.e., shared evolutionary ancestry (49). A signal that occurs in both of these apes as well as humans likely was present in the last common ancestor. An additional impetus for comparing these two ape species is the suggestion that bonobos have greater language-like ability in the vocal domain than chimpanzees (11, 50, 51), which may extend to their gestural communication.
Results
Species-Typical Gesture Profiles.
To establish possible species differences in the production of gestures and facial/vocal signals, an analysis was conducted on focal observations concerning individuals of both species matched with regards to sex, age, rearing history, and social rank (see Methods).
In these data, bonobos produced a total of 375 signals: 78.4% gestures, 13.8% facial/vocal signals, and 7.8% combinations of the two. Chimpanzees produced 383 signals: 55.9% gestures, 22.5% facial/vocal signals, and 21.6% combinations of the two. Looking at only those gestures that both species frequently produced, the species difference in overall frequency distribution across gesture types was significant (χ2 = 16.31, df = 6, P < 0.05). The two species differed considerably in their gesture profiles: chimpanzees performed the gestures arm raise, hard touch, and dab more than bonobos, and bonobos performed reach out down, reach out up, gentle touch, and slap ground more than chimpanzees.
No significant difference was found in the proportion of signals that was gestural versus facial/vocal, but chimpanzees did combine these two signal classes relatively more often than did bonobos (mean proportion of combinations ± SD: bonobos: 6.7 ± 4.0%, chimpanzees: 17.7 ± 3.4%; Wilcoxon, n = 7, P = 0.018, two-tailed).
Context Specificity of Signals.
Because sample size did not permit an analysis distinguishing both the individual performer and the signal, the analysis below is conducted per signal while pooling across individual performers. We recorded how 770 signals in bonobos and 739 in chimpanzees were distributed over seven distinct behavioral contexts (Table 2). The combination matrix between all signals and all contexts deviated from a random distribution calculated on the basis of the matrix's marginal totals in both bonobos (χ2 = 562.58, df = 102, P < 0.001) and chimpanzees (χ2 = 1,213.78, df = 132, P < 0.001). In other words, signals were significantly associated with specific social contexts.
Table 2.
Behavioral contexts
| Behavioral category | Behavioral description |
|---|---|
| Affiliative | Nonagonistic body contact, or invitation for body contact by staring, approaching, and/or gesturing to another; greeting behaviors such as between individuals who were not previously in contact and can include pant grunt, embrace, head bob, and/or gentle touch |
| Agonistic | Individual performs or receives aggressive behaviors such as bark, grunt, chase, hit/punch, bite, flee, or scream; situations where no clear agonistic behaviors are present but there is clear conflict; reconciliation and support behaviors such as two individuals engaging in friendly body contact while at least one of them seems distressed, frightened, or hurt can be between either aggressor and victim, or between victim and third individual, as well as individual supporting another who is involved in agonism with opponent |
| Food | Gathering and/or eating food; anticipating food when provision is imminent, as evident from arrival of caretakers with food; competing for food by way of grabbing, pulling, picking up parts, and or begging; simply a keen interest in other's food by way of staring or peering; nursing |
| Groom | Using one or both hands individual pushes another's hair back with the thumb or index finger and picks at the exposed skin; can give or receive or perform on self; invitation to groom by staring, approaching, and/or signaling |
| Play | Individuals wrestle, chase, and/or tickle each other in nonagonistic, relaxed manner; invitation to play by running toward and away from another and/or communicative signals |
| Sex | One individual mounts another, stimulates another's genitals with hand or mouth, or rubs own genitals against another's genitals, or invitation to engage in sex by signaling to partner through exhibition of genitals by spreading legs or presenting hindquarters |
| Locomotion | Individual walks with, runs with, or follows another, or walks or runs away from another; also includes signaling to another to induce movement with or away from signaler |
Behavioral categories were comprised of 29 collapsed contexts; unclear contexts were eliminated from analysis.
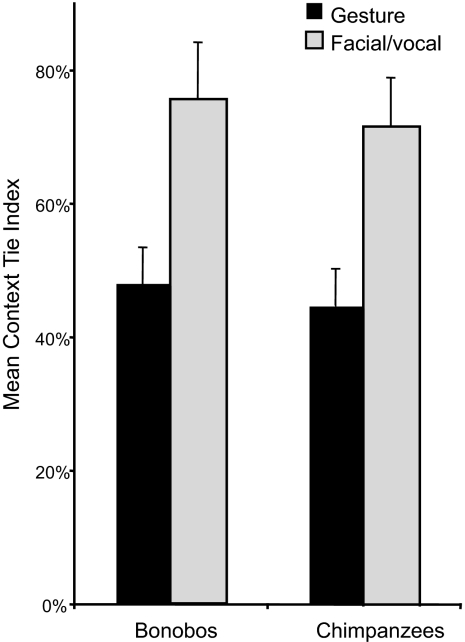
The gestural flexibility hypothesis predicts that facial/vocal signals will be more closely tied to specific contexts than gestures. For each communicative behavior we calculated a context-tie index (CTI), i.e., the percentage of occasions on which the signal occurred in its most typical context. We then compared the CTI for gestures with that for facial/vocal signals for each species, limiting the analysis to signals that occurred minimally five times (Fig. 2). In the first analysis we compared only those signals that both species regularly produced, and as predicted, the average gesture had a significantly lower CTI than the average facial/vocal signal in both bonobos (Mann–Whitney U test: U = 7.5, N1 = 13, N2 = 5, P < 0.005, one-tailed) and chimpanzees (U = 16.5, N1 = 14, N2 = 9, P = 0.001, one-tailed), meaning that facial/vocal displays were more narrowly context-bound than gestures.
Fig. 2.
For each communicative signal, the context in which it occurred most often was expressed as a percentage of all occurrences: the context tie index. Both species displayed a higher mean (+SEM) index for facial/vocal signals than for gestures.
Stability of Signal Context.
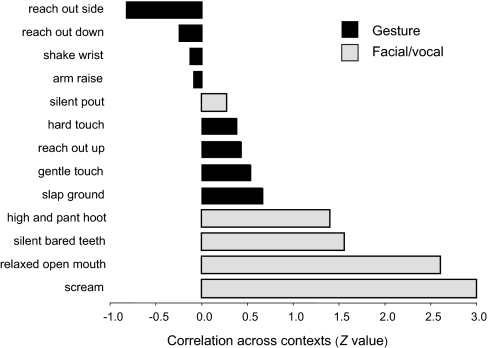
According to the gestural flexibility hypothesis, the way signals are used should vary more for gestures than facial/vocal displays. As above, the analysis focused on variation in behavior rather than individual performers. Using behavioral context as a proxy for usage and function, the comparison was limited to signals present in both species and performed more than five times by each (i.e., eight gestures and five facial/vocal displays). For each signal, we calculated the Pearson correlation between both species with regard to the number of times the signal occurred across seven behavioral contexts (Table 2). For statistical comparison and graphing, the r values were converted to Z values (Fisher's r-to-z conversion). Given Z's approximately normal distribution, Z values were compared by means of t tests. Facial/vocal signals showed significantly positive Z values (one-sample t = 3.22, df = 4, P = 0.016, one-tailed), but not gestures (t = 0.65, df = 7, not significant). A comparison between both signal categories yielded a significant difference in the predicted direction (two-sample t = 3.55, df = 11, P = 0.0025, one-tailed). Fig. 3 illustrates the higher Z values for most facial/vocal signals compared with gestures.
Fig. 3.
Comparing the frequency distribution of particular communication signals across behavioral contexts in both species, gestures showed lower correlations (presented as Z values) than did facial/vocal signals, thus indicating greater flexibility in the gestural modality.
The facial expressions silent bared teeth and relaxed open mouth showed extremely high correlations across contexts as did the vocalizations scream and pant hoot. The only exception in this category was silent pout face, which showed a relatively low, but still positive, correlation. The few silent pout faces in bonobos were mostly food-related, whereas the chimpanzees' silent pout faces were used in food, grooming, and affiliative contexts. None of the gestures, in contrast, reached high contextual correlations. Half of the gestures even correlated negatively across contexts, suggesting dramatically different functions in each species.
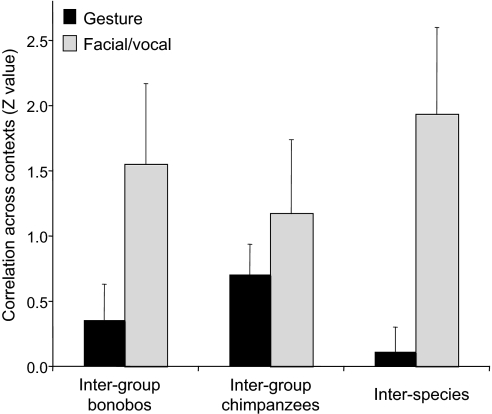
The same analysis was applied to compare groups within each species to test the “cultural” variation aspect of the gestural flexibility hypothesis according to which the usage of gestures should vary from group to group, whereas the usage of facial/vocal displays should be culturally invariant. A larger sample of communication signals was available for this analysis than the previous one as it added signals unique to each species. Comparing the two separate bonobo groups, facial/vocal signals showed significantly positive Z values (one-sample t = 2.88, df = 3, P = 0.032, one-tailed), but not gestures (t = 1.34, df = 12, not significant). Comparing the two chimpanzee groups, both facial/vocal signals and gestures showed significantly positive Z values (facial/vocal signals: t = 2.22, df = 6, P = 0.034; gestures: t = 3.17, df = 13, P = 0.0035). The analysis (Fig. 4) confirmed stronger contextual correlations for facial/vocal signals than gestures in bonobos (t = 2.14, df = 15, P = 0.025, one-tailed), but not chimpanzees (t = 0.96, df = 19, not significant).
Fig. 4.
Comparing the frequency distribution of communication signals across behavioral contexts in both bonobo groups, facial/vocal displays showed much higher correlations (presented as mean + SEM Z values) than gestures. The two chimpanzee groups showed the same, but nonsignificant, trend. Comparing both species (based on data in Fig. 3), the contextual usage of facial/vocal signals correlated much better than that of gestures.
Responsiveness to Multimodal Signals.
Gestures are often produced concurrently or in rapid alternation with facial expressions and/or vocalizations. Here, we define multimodal signaling as the occurrence of two signals within 10 s of each other, and in the majority of such combinations, the two signals overlapped in time.
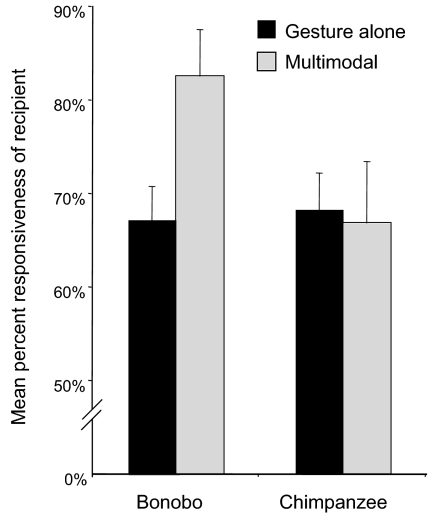
We investigated whether such multimodal communication was more effective in eliciting a response. “Responsiveness” was defined as the proportion of total signals in a given category that elicited a response of any kind from the recipient to whom the signals were directed (see Methods). A Wilcoxon signed ranks test per individual signal performer compared recipient responsiveness with gestures alone versus multimodal communication, which comprised of gestures combined with facial/vocal signals (Fig. 5). In bonobos, multimodal communication elicited more responses than did gestures alone (n = 13, Wilcoxon T = 9, P < 0.01, two-tailed), whereas no such difference was found in chimpanzees (n = 29, T = 149.50, not significant).
Fig. 5.
The mean (+SEM) responsiveness to combinations of facial/vocal signals and gestures was higher than to gestures alone in bonobos. No such difference was found in chimpanzees.
Discussion
Our data strongly support the gestural flexibility hypothesis according to which our closest primate relatives use brachiomanual gestures more flexibly across contexts than they do facial expressions and vocalizations. Gestures seem less closely tied to particular emotions, such as aggression or affiliation, hence possess a more adaptable function. Gestures are also evolutionarily younger, as shown by their presence in apes but not monkeys, and likely under greater cortical control than facial/vocal signals (see Introduction). This observation makes gesture a serious candidate modality to have acquired symbolic meaning in early hominins. As such, the present study supports the gestural origin hypothesis of language (4).
Facial and vocal communication are not only more closely tied to specific contexts in the two ape species studied, the contexts in which they typically occur also correlate better across different groups of the same species and even between species. From knowing one species, one can reliably predict in what kind of situation a certain facial/vocal display will occur in the other species. For example, the scream is typical for victims of intimidation, threat, or attack. This behavior is so for all groups of both ape species, hence the function of this signal is relatively invariant and probably evolutionarily ancient as supported by the fact that, in both form and typical usage, the scream of chimpanzees and bonobos resembles that of other primates (10, 52).
The same cannot be said for gestures, which, first, are virtually absent outside the Hominoidea, and second, show far weaker correlations across contexts between both ape species in this study, and even between different groups of the same species. This finding suggests that the function of, for example, gentle touch is informed by other signals and behavior (e.g., a touch by a male to a genitally swollen female has a different meaning than one by a milk-dependent infant approaching her mother), hence recipients need to interpret gestures within a larger combinatorial context.
The cross-group differences in the context of gestures, especially in bonobos, may be caused by both individual learning (53) and a particular sensitivity of gestures to “cultural” variation (8). The latter sensitivity is supported by the capacity of apes to imitate gestures (54, 55) as well as the population specificity of many gestures, such as the so-called grooming hand clasp (56–58), hand clapping (11), and the leaf-clip display and social scratching (59, 60). Far more than facial expressions and vocalizations, gestures seem subject to modification, conventionalization, and social transmission.
This contrast in adaptability between gestures and other modes of communication emerges only if gesture is strictly defined and kept separate from general body postures and locomotion patterns. But also within the category of facial/vocal communication, distinctions may need to be made. Signals involving orofacial movements (“facial gestures”) may be more open to action-understanding, a likely component of language evolution, than purely auditory signals (61). It is interesting, therefore, that bonobos show a degree of voluntary control over their facial musculature as reflected in games that consist of “pulling” unusual faces in situations that appear to lack emotional engagement (62, 63).
There are several other differences between bonobo and chimpanzee communication that may make bonobos the better model regarding the prerequisites of language evolution. This has already been noted for vocal communication (50, 51), which appears more dialogue-like in bonobos, and includes soft peeps to draw attention to and “comment” on novel items or environmental events (11), a characteristic shared with early human language development (64). But the present study adds further differences. First, observation of gestures in one chimpanzee group allows one to predict the usage of these gestures in another. This is not true for bonobos, which seem culturally more diverse in their gesture usage. Second, when bonobos combine gestures with facial/vocal signals, they are more effective at eliciting a response than when they use gestures alone. Responsiveness is rather invariable ≈67% for all signal categories except multimodal signals in bonobos, for which it jumps to 83% (Fig. 5). This jump applies to multimodal signals in every single context (unpublished data). That this contrast between multimodal and single modality utterances held for bonobos only is interesting given that multimodal combinations are less common in bonobos. Could the relative scarcity of multimodal signaling in bonobos relate to a more deliberate combination of gestures with other forms of communication, perhaps in an attempt to add critical information to the message instead of merely amplifying it?
Although they are genetically equidistant to us, the question which of our two closest relatives most resembles the last common ancestor of humans and apes remains as yet unanswered. But we speculate that the bonobos' variable gestural repertoire and high responsiveness to combinatorial signaling may have characterized our early ancestors, which in turn may have served as a stepping stone for the evolution of symbolic communication.
Methods
All data derive from videotaped spontaneous behavior of captive apes. A gesture was included in the analysis only if it initiated a social interaction, in which one individual approached another and attempted to engage the recipient with a communication signal (Table 1). The two individuals may have been in proximity before, but without observable interaction. Signals were not included in the analysis, therefore, if they occurred in the middle or toward the end of an ongoing interaction. This omission greatly restricted the amount of data presented in this study, but was necessary as it increased the reliability of the functional analysis, i.e., behavioral effects of the various signal classes were easier to detect whether a signal started the interaction.
Subjects and Sites of Study.
Two chimpanzee groups of 17 individuals each were housed at the Yerkes National Primate Research Center's Field Station, in Lawrenceville, GA. Both groups were well established: FS1 in 1978 and FS2 in 1993. Each group had an outdoor enclosure (FS1: 697 m2; FS2: 520 m2) with climbing structures, as well as a heated indoor building composed of interconnected rooms with nesting sites and swings. The two groups have never been in nonauditory contact with each other. For further details see supporting information (SI) Table 3.
One bonobo group was observed at the San Diego Zoo in San Diego, CA. This group contained six individuals in an outdoor exhibit of 560 m2. The second bonobo group was observed at the San Diego Wild Animal Park in Escondido, CA. This group contained seven individuals in an outdoor exhibit of >3,000 m2. For further details see SI Table 3.
Data Collection.
Observations started at 8 a.m. and continued through midafternoon. Feeding (both routine feedings and occasional treats) by zookeepers or care staff occurred on a regular basis and were included in the observations.
Video sequences.
Data were collected on all communicative events, defined as an interaction between two individuals in which one used either a gestural (Table 1), facial, or vocal signal to initiate the interaction. The signaler needed to be at least halfway oriented toward the recipient, and the signal (if visual) also needed to be directed toward a 180° field of space around the front of the recipient. From February 2004 through June 2005, we collected behavioral data on a GL2 digital video camera (Canon, Lake Success, NY) equipped with a directional microphone (Sennheiser, Old Lyme, CT). All-occurrence data (65) were collected for 300 h on the bonobos and 219 h on the chimpanzees. The video tapes were reviewed, and all social interactions that were initiated by a gesture or a facial/vocal signal were extracted into a QuickTime movie file. A total of 963 video sequences was coded. The sequences were typically ≈20 s in length, with 10 s preceding and 10 s succeeding the opening signal. If the same signal was repeated within 10 s, the captured sequence was extended until 10 s after the last signal was produced.
Baseline focal data.
Focal-animal data (65) were collected for an additional 73 h in each species, for a total of 146 focal observation hours. These data were used to directly compare rates of initiatory signaling in both species. Seven individuals of each species served as focal subjects. Focal subjects were matched as closely as possible between both species in terms of sex, age, rearing history, and social rank. Focal data were collected in real time by using a stopwatch, binoculars, and a sheet with a clipboard onto which all signals that initiated a social interaction were recorded.
Unless stated otherwise, the present analysis combines video sequences and baseline focal data.
Coding Protocol.
Many facets of communication were coded from the video sequences ranging from the performed behavior and its intensity to the response and its latency. A condensed list of defined communicative behaviors is provided in SI Table 4, based on previous ethograms for both apes species (11, 17, 66, 67). We distinguished 31 different gestures, 18 facial/vocal signals, and 7 context types (Table 2). If the communicative event contained more than two contexts simultaneously, we prioritized the one that occurred less frequently at that point in data collection. The baseline focal data used a simpler coding scheme as these data were collected in real time: the signaler, recipient, initiatory signal(s), behavioral context, recipient's behaviors before and after the signals(s), and the state change, a single code that captured the difference between the presignal and postsignal states of behavior in the recipient. The state change data were used to define responsiveness to the signal(s). All responses were pooled into a dichotomous “response/no response” category, that is, for every social interaction that involved initiatory signals, any observable behavioral response of the part of the signal recipient was labeled response, and if the recipient either ignored the signaler or did not respond in any observable way, this was labeled no response. Thus, the response category included all possible responses, from touching and sex to fleeing and avoiding. Whether they were contextually appropriate responses was not examined. This was done because there were few multimodal interactions and the data needed to be pooled to enable statistical analysis.
Interobserver Reliability.
The data were collected solely by A.S.P., but coding of video sequences was equally split between A.S.P. and a well trained assistant. Interobserver reliability of coding was assessed for the following variables: initial signal, behavioral context, receiver response, and receiver behavioral state change. This comparison was made for ≈10% of the video sequences. Cohen's Kappa coefficient (κ) was used to assess interobserver reliability, which was in the “excellent” range for signal (κ = 0.78), behavioral context (κ = 0.83), receiver response (κ = 0.77), and behavioral state change or responsiveness in the receiver (κ = 0.83) (45).
Acknowledgments
We thank Annette Jeneson for technical assistance with the project; Richard Byrne, Michael Corballis, Harold Gouzoules, and William Hopkins for feedback on the manuscript; Laura Namy, Lisa Parr, and Larry Barsalou for helpful discussion; and the animal care staff at the San Diego Zoo, the San Diego Wild Animal Park, and the Yerkes Primate Center for their support and assistance. Research was supported by a National Institutes of Health Grant RR-00165 (to the Yerkes National Primate Research Center), the Wenner-Gren Foundation, the Leakey Foundation, Emory University's Research Committee, and a National Science Foundation Graduate Research Fellowship (to A.S.P.). The Yerkes Center is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702624104/DC1.
References
- 1.Hockett CF. In: Universals of Language. Greenberg J, editor. Cambridge, MA: MIT Press; 1963. pp. 1–22. [Google Scholar]
- 2.Hewes GW. Curr Anthropol. 1973;14:5–24. [Google Scholar]
- 3.Corballis MC. Am Sci. 1999;87:38–145. [Google Scholar]
- 4.Corballis MC. From Hand to Mouth: The Origins of Language. Princeton: Princeton Univ Press; 2002. [Google Scholar]
- 5.Byrne RW. In: The Roots of Human Sociality: Culture, Cognition and Interaction. Levinson SC, Enfield NJ, editors. Oxford: Berg; 2006. pp. 478–505. [Google Scholar]
- 6.Perrett DI, Smith PAJ, Mistlin AJ, Chitty AJ, Head AS, Potter DD, Broenniman R, Milner AP, Jeeves MA. Behav Brain Res. 1985;16:153–170. doi: 10.1016/0166-4328(85)90089-0. [DOI] [PubMed] [Google Scholar]
- 7.Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Perani D, Fazio F. Exp Brain Res. 1996;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 8.de Waal FBM. In: Emotions Inside Out: 130 Years After Darwin's The Expression of the Emotions in Man and Animals. Ekman P, Campos JJ, Davidson RJ, de Waal FBM, editors. New York: New York Acad Sci; 2003. pp. 7–31. [DOI] [PubMed] [Google Scholar]
- 9.van Lawick-Goodall J. Anim Behav Monogr. 1968;1:161–311. [Google Scholar]
- 10.van Hooff JARAM. Symp Zool Soc London. 1967;8:97–125. [Google Scholar]
- 11.de Waal FBM. Behaviour. 1988;106:183–251. [Google Scholar]
- 12.Pika S, Liebal K, Tomasello M. Am J Primatol. 2005;65:39–61. doi: 10.1002/ajp.20096. [DOI] [PubMed] [Google Scholar]
- 13.Tanner JE, Byrne RW. In: The Mentalities of Gorillas and Orangutans: Comparative Perspectives. Parker ST, Mitchell RW, Miles HL, editors. New York: Cambridge Univ Press; 1999. pp. 211–239. [Google Scholar]
- 14.Tanner JE, Byrne RW. Curr Anthropol. 1996;37:162–173. [Google Scholar]
- 15.Liebal K, Pika S, Tomasello M. Gesture. 2006;6:1–38. [Google Scholar]
- 16.de Waal FBM, van Hooff JARAM. Behaviour. 1981;77:165–195. [Google Scholar]
- 17.van Hooff JARAM. In: Social Communication and Movement. von Cranach M, Vine I, editors. London: Academic; 1973. pp. 75–162. [Google Scholar]
- 18.Flack JC, de Waal FBM. Proc Natl Acad Sci USA. 2007;104:1581–1586. doi: 10.1073/pnas.0603565104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preuschoft H, Chivers DJ. Hands of Primates. Wien, Slovenia: Springer; 1993. [Google Scholar]
- 20.Wiesendanger M. Exp Brain Res. 1999;128:1–5. doi: 10.1007/s002210050810. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg WN, Kellogg LA. The Ape and the Child: A Comparative Study of the Environmental Influence Upon Early Behavior. New York: Hafner; 1933. [Google Scholar]
- 22.Hayes KJ, Hayes C. Proc Am Philos Soc. 1951;5:105–109. [Google Scholar]
- 23.Gardner RA, Gardner BT, van Cantfort TE. Teaching Sign Language to Chimpanzees. Albany, NY: State Univ of New York Press; 1989. [Google Scholar]
- 24.de Waal F. Chimpanzee Politics: Power and Sex Among Apes. London: Jonathan Cape; 1982. [Google Scholar]
- 25.Tanner JE, Byrne RW. Primates. 1993;34:451–457. [Google Scholar]
- 26.Ekman P. In: Nebraska Symposium On Motivation, 1971. Cole J, editor. Lincoln, NE: Univ of Nebraska Press; 1972. pp. 207–283. [Google Scholar]
- 27.Kendon A. In: Cross-Cultural Perspectives in Nonverbal Communication. Poyatos F, editor. Toronto: Hogrefe; 1988. pp. 131–141. [Google Scholar]
- 28.Whiten A, Goodall J, McGrew WC, Nishida T, Reynolds V, Sugiyama Y, Tutin CEG, Wrangham RW, Boesch C. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman P, Crelin ES, Kaltt DH. Am Anthropol. 1972;74:287–307. [Google Scholar]
- 30.Petitto LA, Marentette PF. Science. 1991;251:1493–1496. doi: 10.1126/science.2006424. [DOI] [PubMed] [Google Scholar]
- 31.Annett M. Left, Right, Hand, and Brain: The Right Shift Theory. London: Erlbaum; 1985. [Google Scholar]
- 32.Hopkins WD, de Waal FBM. Int J Primatol. 1995;16:261–276. [Google Scholar]
- 33.Hopkins WD, Leavens DA. J Comp Psychol. 1998;112:95–99. doi: 10.1037/0735-7036.112.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantalupo C, Hopkins WD. Nature. 2001;414:505. doi: 10.1038/35107134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jurgens U. Naturwissenschaften. 1998;85:376–388. doi: 10.1007/s001140050519. [DOI] [PubMed] [Google Scholar]
- 36.Wheaton LA, Nolte G, Bohlhalter S, Fridman E, Hallet M. Clin Neurophysiol. 2005;116:1382–1390. doi: 10.1016/j.clinph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Kelly SD, Iverson JM, Terranova J, Niego J, Hopkins M, Goldsmith L. Dev Neuropsychol. 2002;22:323–349. doi: 10.1207/S15326942dn2201_1. [DOI] [PubMed] [Google Scholar]
- 38.Hopkins WD, Russell JL, Cantalupo C. Psychol Sci. 2007 doi: 10.1111/j.1467-9280.2007.02011.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeill D. Hand and Mind: What Gestures Reveal About Thought. Chicago: Univ of Chicago Press; 1992. [Google Scholar]
- 40.Morrel-Samuels P, Krauss R. J Exp Psychol. 1992;18:615–622. [Google Scholar]
- 41.Goldin-Meadow S, Nusbaum H, Kelly SD, Wagner S. Psychol Sci. 2001;12:516–522. doi: 10.1111/1467-9280.00395. [DOI] [PubMed] [Google Scholar]
- 42.Iverson JM, Goldin-Meadow S. Nature. 1998;396:228. doi: 10.1038/24300. [DOI] [PubMed] [Google Scholar]
- 43.Birdwhistell RL. Kinesics and Context: Essays on Body Motion Communication. Philadelphia, PA: Univ of Pennsylvania Press; 1970. [Google Scholar]
- 44.Møller AP, Pomiankowski A. Behav Ecol Sociobiol. 1993;32:167–176. [Google Scholar]
- 45.Bakeman R, Gottman JM. Observing Interaction: An Introduction to Sequential Analysis. Cambridge, UK: Cambridge Univ Press; 1997. [Google Scholar]
- 46.Parr LA, Cohen M, de Waal FBM. Int J Primatol. 2005;26:73–103. [Google Scholar]
- 47.Patterson N, Richter DJ, Gnerre S, Lander ES, Reich D. Nature. 2006;441:1103–1108. doi: 10.1038/nature04789. [DOI] [PubMed] [Google Scholar]
- 48.Sarich VM. In: The Pygmy Chimpanzee: Evolutionary Biology and Behavior. Sussman RL, editor. New York: Plenum; 1984. pp. 43–48. [Google Scholar]
- 49.Preuschoft S, van Hooff JARAM. Folia Primatol. 1995;65:121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- 50.Hopkins WD, Savage-Rumbaugh ES. Int J Primatol. 1991;12:559–583. [Google Scholar]
- 51.Taglialatela JP, Savage-Rumbaugh ES, Baker LA. Int J Primatol. 2003;24:1–17. [Google Scholar]
- 52.Hopkins WD, Carriba SF. In: Comparative Vertebrate Lateralization. Rogers L, Andrews R, editors. Oxford: Oxford Univ Press; 2002. pp. 445–479. [Google Scholar]
- 53.Tomasello M, Call J, Nagell K, Olguin R, Carpenter M. Primates. 1994;35:137–154. [Google Scholar]
- 54.Custance DM, Whiten A, Bard KA. Behaviour. 1995;132:837–859. [Google Scholar]
- 55.Call J. Cybern Syst. 2001;32:97–119. [Google Scholar]
- 56.McGrew WC, Tutin CEG. Man. 1978;13:234–251. [Google Scholar]
- 57.de Waal FBM, Seres M. Am J Primatol. 1997;43:339–346. doi: 10.1002/(SICI)1098-2345(1997)43:4<339::AID-AJP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 58.Bonnie KE, de Waal FBM. Primates. 2006;47:27–34. doi: 10.1007/s10329-005-0141-0. [DOI] [PubMed] [Google Scholar]
- 59.Nishida T. J Hum Evol. 1980;9:117–128. [Google Scholar]
- 60.Nakamura M, McGrew WC, Marchant L, Nishida T. Primates. 2000;41:237–248. doi: 10.1007/BF02557594. [DOI] [PubMed] [Google Scholar]
- 61.Fogassi L, Ferrari PF. Interact Stud. 2004;5:345–363. [Google Scholar]
- 62.de Waal FBM. In: Understanding Chimpanzees. Heltne PG, Marquardt LA, editors. Cambridge, MA: Harvard Univ Press; 1989. pp. 154–175. [Google Scholar]
- 63.Sherwood CC, Holloway RL, Erwin JM, Schleicher A, Zilles K, Hof PR. Brain Behav Evol. 2004;63:61–81. doi: 10.1159/000075672. [DOI] [PubMed] [Google Scholar]
- 64.Tomasello M, Carpenter M. Dev Sci. 2007;10:121–125. doi: 10.1111/j.1467-7687.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 65.Altmann J. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 66.Plooij FX. The Behavioral Development of Free-Living Chimpanzee Babies and Infants. Norwood, NJ: Ablex; 1984. [Google Scholar]
- 67.Nishida T, Kano T, Goodall J, McGew WC, Nakamura M. Anthropol Sci. 1999;107:141–188. [Google Scholar]