Abstract
Two series of peptides that specifically bind to the extracellular domain of the α chain of the human interleukin-5 receptor (IL-5Rα), but share no primary sequence homology to IL-5, were identified from libraries of random recombinant peptides. Affinity maturation procedures generated a 19-aa peptide that binds to the IL-5 receptor α/β heterodimer complex with an affinity equal to that of IL-5 and is a potent and specific antagonist of IL-5 activity in a human eosinophil adhesion assay. The active form of the peptide is a disulfide-crosslinked dimer that forms spontaneously in solution. Gel filtration analysis, receptor-binding studies, and analytical ultracentrifugation reveal that the dimeric peptide binds simultaneously to two receptor α chains in solution. Furthermore, the dimer peptide, but not IL-5, can activate a chimeric receptor consisting of the IL-5Rα extracellular domain fused to the intracellular domain of the epidermal growth factor receptor, thus demonstrating that the peptide also promotes receptor dimerization in a cellular context. The functional antagonism produced by the bivalent interaction of the dimeric peptide with two IL-5R α chains represents a distinctive mechanism for the antagonism of cytokines that use heteromeric receptors.
Interleukin-5 (IL-5) is a T cell-derived hematopoietic cytokine that acts exclusively on cells of the eosinophil and basophil lineage (1, 2). Although IL-5 can regulate many of the functions of mature, terminally differentiated eosinophils such as cell survival (3), adhesion (4), and activation (5), its primary function is to promote the differentiation and expansion of eosinophil precursors in the bone marrow (6).
Transgenic mice that constitutively overexpress IL-5 in their lungs exhibit systemic and airway eosinophilia, bronchial hyperreactivity, and histopathological features characteristic of human asthma (7). Administration of recombinant IL-5 into the airways of either guinea pigs (8) or mildly asthmatic individuals (9) promotes a lung eosinophilia and bronchial hyperreactivity. Moreover, blocking the activity of IL-5 through administration of neutralizing anti-IL-5 antibodies (10–12), or through IL-5 gene deletion (13), prevents allergen-induced airway eosinophilia and bronchial hyperreactivity. Together, these data demonstrate that IL-5 plays a central role in the development of allergen-induced eosinophilia in animals and suggest that an anti-IL-5 therapeutic could provide an alternative approach to the treatment of human asthma perhaps more specific than current anti-inflammatory therapies such as inhaled corticosteroids.
The IL-5 receptor is a heterodimer consisting of an α chain that specifically binds IL-5 and a signal-transducing βc chain shared with the receptors for two structurally related cytokines: granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-3 (14). A number of protein-based IL-5 antagonists have been reported, including soluble IL-5 receptor α chains (IL-5Rαs) that sequester the ligand in solution (15), single point mutants of IL-5 that occupy the receptor but fail to initiate receptor activation (16, 17), and neutralizing antibodies directed against IL-5 (18). Detailed structure–function analysis of the IL-5/IL-5 receptor interaction, by scanning alanine mutagenesis of IL-5, has revealed that the key charged residues important for the formation of the ligand–receptor complex are spatially distinct and are separated across a large surface area (17, 19). This topography may provide a molecular explanation for the lack of small molecule IL-5 receptor antagonists that have been reported.
The screening of libraries of random recombinant peptides has previously led to the discovery of both agonistic and antagonistic peptides to several other cytokine receptors (20–22). In this study, we report the identification and characterization of two distinct series of specific antagonists of IL-5 that bind to the ligand-binding α subunit of the receptor. Furthermore, we demonstrate that one of these series of antagonist peptides exhibits a distinctive mechanism of antagonist action.
Methods
Peptide Library Screening.
The extracellular domain (ECD) of the human IL-5Rα was expressed in CHO cells as a phosphatidylinositol glycan-linked, epitope-tagged fusion protein. Receptor was harvested from the cell surface by cleavage with phosphatidylinositol-specific phospholipase C and immobilized by using a capturing antibody (mAb 179) directed toward the tag (23).
A recombinant library was constructed encoding peptides of the form X3CX5–10CX3 (where X is any amino acid and C is a fixed cysteine residue) and expressed as a C-terminal fusion to the lac repressor (24). In this method, peptides are linked to the corresponding random coding sequence through binding of the lac repressor to lac operator sequences also present on the pJS142 library plasmid (25). The library was screened by incubating peptide–repressor–plasmid complexes with antibody-immobilized IL-5Rα ECD in microtiter wells. After washing away unbound plasmid complexes, receptor-bound plasmids were eluted from the wells with 1 mM isopropyl β-d-thiogalactoside/0.2 M KCl/35 mM Hepes (pH 7.5)/0.1 mM EDTA. The recovered plasmids were used to transform Escherichia coli; plasmid complexes were prepared from the transformed cells, and the process was repeated through a total of four rounds of affinity selection.
Secondary libraries were constructed, based on peptides isolated from the primary library, as fusions to the lac repressor headpiece dimer, an affinity selective variant of the lac repressor library method (30). To facilitate further analysis, the DNA encoding the enriched pools of receptor-binding peptides were transferred to a plasmid that allowed expression of the peptides as C-terminal fusions to the E. coli maltose-binding protein. Clones for analysis were identified by using colony lifts probed with 33P-labeled IL-5Rα ECD. Relative binding affinities of the resulting clones were then determined by ELISA (25).
Peptide Synthesis and Dimer Peptide Structure Determination.
Peptide synthesis of was performed on p-hydroxymethylphenoxymethyl polystyrene resin using an Applied Biosystems peptide synthesizer 431A or 433A and fluorenylmethoxycarbonyl (Fmoc) chemistry. Each step was double coupled by using O-benzotriazol-1-yl-N,N,N′,N′-tetramethyluronium hexafluorophosphate/N-hydroxybenzotriazole (HBTU/HOBt) reagent. The products were purified by C18 RP-HPLC and confirmed by MS.
For peptide AF18748, the acetamidomethyl protecting group was used for the cysteine in position 9 and trityl (Trt-) for the cysteines in positions 15 and 18. The peptide was cleaved from the resin and deprotected with trifluoroacetic acid, dichloromethane, anisole, and 2-mercaptoethanol (89.7%/10%/0.1%/0.2%). The crude peptide was further purified by C18 RP-HPLC. The peptide was oxidized overnight in 30% dimethyl sulfoxide/water at 1 mg/ml to form the intrachain disulfide bond (26). The interchain disulfide bond was formed by using silver trifluoromethanesulfonate/trifluoroacetic acid followed by dimethyl sulfoxide/aqueous HCl (27).
To determine the disulfide pattern of the dimeric form of AF17362, a 1 mg/ml solution of the peptide in 200 mM ammonium bicarbonate was digested with 20 μg/ml trypsin (Boehringer Mannheim) for 4 h. Aliquots were removed every hour and analyzed by liquid chromatography–electrospray MS and matrix-assisted laser desorption ionization MS.
Pharmacological Assays.
Binding of peptides to the IL-5Rα ECD was measured by using a 125I-IL-5 competition-binding assay in a scintillation proximity assay format (28). TF-1 cell-based radioligand-binding studies were carried out as described previously (19). In brief, TF-1 cells (1 × 106 per point) were incubated with 200 pM 125I-IL-5 (1,130 Ci/mmol; 1 Ci = 37 GBq) for 2 h at room temperature in the presence of increasing concentration of unlabeled cytokine or peptide. After separation of bound and free ligand by centrifugation through oil (16% paraffin oil/84% silicone oil) the cell-associated radioligand was quantified in a γ counter. Nonspecific binding was defined in the presence of a 300-fold molar excess of unlabeled IL-5.
Human peripheral blood eosinophils were purified from healthy donors with mildly elevated eosinophil levels by a CD16-negative selection procedure, as previously described (29). Eosinophil purity was consistently >98%. The cytokine-induced adhesion of eosinophils to IgG-coated plates was measured as previously described (29). In brief, purified eosinophils (5 × 103 per point) were incubated with peptides for 5 min before stimulation with the indicated cytokines for 30 min at 37°C in a 96-well microtiter plate precoated with human IgG. After a washing step, the adherent cells were lysed and the endogenous peroxidase activity was measured in a colorimetric assay using o-phenylenediamine as a substrate.
In Vitro Receptor Dimerization Assays.
The microvolume fluorimetry assay, measuring ligand-dependent association of fluorescently labeled IL-5Rα ECD with unlabeled bead-bound receptor, was performed using an fluorometric microvolume assay technology (FMAT) instrument (Perkin-Elmer) as described previously (31). Gel filtration chromatography was performed in 50 mM Tris (pH 7.5)/0.5 M NaCl on a Pharmacia Smart system using a Precision Superdex 200 3.2/30 column at 25°C with a flow rate of 10 μl/min. The column was calibrated by using Bio-Rad gel filtration molecular weight standards. Velocity sedimentation experiments were conducted on a Beckman XLI ultracentrifuge equipped with absorption and interference optics and an An60Ti rotor. The experiments were run at 40,000 rpm in charcoal-filled Epon double sector centerpieces at 25°C in 50 mM Tris (pH 7.5)/0.5 M NaCl. Data were collected at 280 nm as single scans at a spacing of 0.01 cm in continuous-scan mode and analyzed by using both ultrascan (B. Demeler, University of Texas, San Antonio) and dcdt (W. F. Stafford, Boston Biomedical Research Institute, Boston).
Chimeric IL-5Rα/Epidermal Growth Factor Receptor (EGFR) Reporter Cell Assay.
A reporter cell line was established by transfecting the gene encoding a chimeric receptor consisting of the IL-5Rα ECD fused to the transmembrane and intracellular domains of the EGFR into Ba/F3 cells containing a luciferase reporter gene under the transcriptional control of the c-fos enhancer and minimal thymidine kinase promoter. Before assay, the cells were starved overnight of the WEHI-conditioned medium in which they were maintained and then seeded into 96-well plates at a density of 105 cells per well. After addition of IL-5 or antagonist peptide, the cells were incubated for 4 h and then LucLite reagent (Packard) was added to the wells, and the plates were assayed for luciferase activity by counting in a Topcount instrument (Packard).
Results
Identification of IL-5 Antagonist Peptides by Peptide Library Screening.
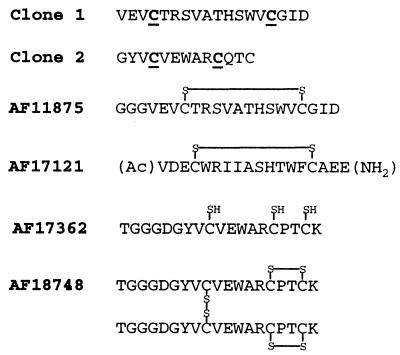
A recombinant peptide library was generated that expressed random peptides 13–18 amino acids in length as C-terminal fusions to the lac repressor protein (24). The library peptides contained two fixed cysteine residues to ensure the expression of a high percentage of conformationally constrained, cyclic peptides. After four rounds of affinity selection against IL-5Rα ECD, two individual clones were isolated that expressed peptides that bound specifically to IL-5Rα ECD when assayed by ELISA. These peptides also blocked the binding of 125I-IL-5 to IL-5Rα ECD in a scintillation proximity assay (data not shown). The peptide sequences of both clones were determined by DNA sequencing [clones 1 and 2 (Fig. 1)], and neither showed any sequence similarity to IL-5.
Figure 1.
Peptide sequences and structures. Amino acid residues are indicated with single letter code. Sequences of clones 1 and 2 determined by DNA sequencing. AF-numbered compounds were prepared synthetically and their structures were confirmed by MS. In the AF17121 sequence, (Ac) and (NH2) indicate acetylation and amidation, respectively. Boldface and underlined letters C in the sequences of clone 1 and clone 2 indicate the invariant cysteine residues specified in the library design. Letter S in AF11875, AF17121, and AF18748 structures indicates cysteine sulfur involved in the disulfide bonds shown. Letters SH in AF17362 structure indicate free cysteine sulfhydryls.
Peptides Derived from Clone 1 Are Cyclic Monomers.
A synthetic linear peptide corresponding to clone 1 did not measurably compete with 125I-IL-5 for binding to IL-5Rα ECD when analyzed by scintillation proximity assay, whereas the disulfide-cyclized form, AF11875 (Fig. 1), blocked 125I-IL-5 binding with an IC50 of 18 μM (data not shown). Additional peptide libraries were designed to contain variants of the clone 1 sequence in the affinity-selective lac repressor headpiece dimer system (30). Screening of these libraries against the IL-5Rα ECD resulted in a number of clones that appeared by ELISA to bind the IL-5Rα ECD with considerably higher affinity than AF11875. A disulfide-cyclized peptide was then synthesized corresponding to the sequence of the clone that appeared to bind with highest affinity. This peptide, AF17121 (Fig. 1) competed with 125I-IL-5 for binding to IL-5Rα ECD with an IC50 of 69 nM (data not shown).
Peptides Derived from Clone 2 Are Disulfide-Linked Dimers.
The peptide sequence of clone 2 contained a third cysteine residue in addition to the fixed cysteines defined in the original library construction (Fig. 1), giving rise to a large number of potential disulfide bond configurations. To determine the importance of each cysteine residue, the cysteines were allowed to vary during subsequent rounds of library mutagenesis and affinity maturation (30). Analysis of the DNA sequences of 31 distinct clones expressing peptides with high affinity for the IL-5Rα ECD revealed complete conservation of all three of the cysteine residues from the clone 2 sequence, indicating their absolute requirement for receptor-binding activity in this series. There was also a glutamine-to-proline substitution at position 11 in 30 of the 31 clones, suggesting that this change strongly favors high affinity binding.
A peptide corresponding to the clone with the highest apparent receptor-binding affinity as measured by ELISA was synthesized with all three cysteine residues in the free sulfhydryl form. When this peptide, AF17362 (Fig. 1), was dissolved in 50 mM Tris buffer (pH 7.8) and assayed immediately, it competed with 125I-IL-5 for binding to the IL-5Rα ECD with an IC50 of 120 nM. However, when the peptide solution was left at room temperature for 10 days the IC50 in the binding assay dropped to 550 pM. Concomitant with this change in binding affinity, the peptide's retention time by C18 RP-HPLC shifted (but remained a single peak), and MS revealed that the peptide had spontaneously formed a disulfide-linked dimer (data not shown).
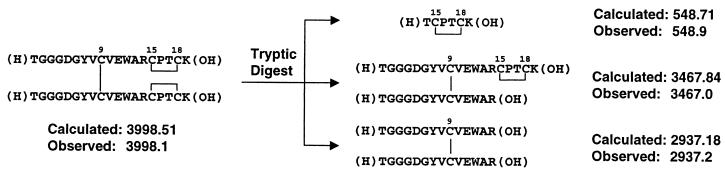
To determine the disulfide pattern of the active peptide, the dimeric form of AF17362 was digested with trypsin and analyzed by MS. The MS analysis of the tryptic digest revealed fragments consistent with the presence of a single symmetrical dimer structure containing an interchain disulfide bond between the cysteines in position 9 and intrachain disulfide bonds between the cysteines in positions 15 and 18 on each chain (Fig. 2). Kinetic analysis of the dimerization process by NMR spectroscopy demonstrated that the formation of the intramolecular bond preceded intermolecular dimerization (data not shown).
Figure 2.
Structure determination of dimeric AF17362 by tryptic digestion and MS. Aliquots from a tryptic digest of the dimer form of AF17362 were removed every hour and analyzed by MS. Shown are the molecular masses (in daltons) of all of the species observed (not all were observed in a single time point). The structures shown, along with their calculated molecular masses, are the only ones consistent with the observed molecular masses, the sequence of AF17362, and the cleavage specificity of trypsin.
To confirm that this structure represented the active conformation, a dimer peptide with an identical disulfide pattern was prepared synthetically by using different protecting groups on the cysteines involved in the intrachain and interchain disulfide bonds. The resulting peptide, AF18748 (Fig. 1), was indistinguishable from the dimeric form of AF17362 by all criteria tested [RP-HPLC, capillary electrophoresis, tryptic digest, and (data not shown)], and competed with 125I-IL-5 for binding to the IL-5Rα ECD with essentially the same potency (IC50 of 780 pM; data not shown).
Because dimerization of <1% of the monomer would produce a sufficient concentration of active dimer to account for the binding activity observed with freshly dissolved AF17362, this activity is probably due to the formation of the dimeric form of the peptide both before and during the binding assay. Moreover, peptides of sequence otherwise identical to AF17362 but containing alanine, serine, or acetamidomethyl-protected cysteine in place of the cysteine in position 9 showed no receptor-binding activity by scintillation proximity assay at concentrations as high as 10 μM (data not shown). Therefore it appears that AF17362 is active only as a disulfide-linked dimer.
Pharmacological Characterization of AF17121 and AF18748.
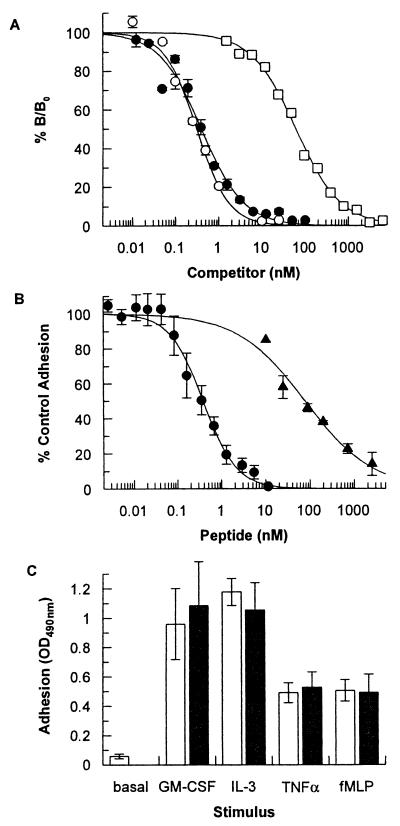
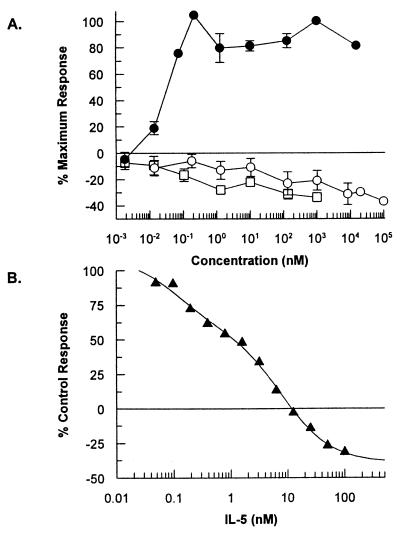
The ability of the peptides AF17121 and AF18748 to bind to the native IL-5R α/β complex on TF-1 human erythroleukemia cells was assessed in a competition-binding assay (28). The binding of 125I-IL-5 to TF-1 cells could be inhibited by either unlabeled IL-5 (IC50 of 310 ± 20 pM), AF17121 (IC50 of 67 nM), or AF18748 (IC50 of 400 ± 40 pM) (Fig. 3A). Thus the dimeric peptide, AF18748, appears to exhibit an affinity for the native heterodimeric form of the receptor similar to the natural ligand, IL-5.
Figure 3.
Pharmacological characterization of antagonist peptides. (A) Competition-binding assay using TF-1 cells. The binding of 125I-labeled human IL-5 to TF-1 erythroleukemia cells was reduced by competition with various concentrations of human IL-5 (○), AF17121 (□), or AF18748 (●). Results are expressed as percentage of maximum specific binding (% B/B0). Mean total binding was 1,074 ± 188 cpm and nonspecific binding was 115 ± 20 cpm. Data are mean ± SEM for three separate experiments for IL-5 and AF18748, and mean of two separate experiments for AF17121. (B) IL-5-induced eosinophil activation assay. The adherence of purified human eosinophils to IgG-coated plates in response to stimulation with 20 pM IL-5 was assessed in the presence of various concentrations of AF17121 (▴) or AF18748 (●). Data are mean ± SEM for four separate experiments for AF17121 and for six separate experiments for AF18748. The average OD490 reading for IL-5-stimulated cells was 0.90 ± 0.09 compared to unstimulated levels of 0.17 ± 0.03. (C) AF18748 specificity assay. Purified eosinophils were assayed for their ability to adhere to IgG-coated plates after stimulation with 6 pM human GM-CSF, 180 pM human IL-3, 1 nM human tumor necrosis factor-α , or 100 nM fMet-Leu-Phe in the presence (closed bars) or absence (open bars) of 1 μM AF18748. The concentration of each cytokine used is one that elicits 80% of the maximal response achievable with that cytokine. Data are mean ± SEM for three separate experiments.
The functional activity of AF17121 and AF18748 was assessed in a human eosinophil adhesion assay (29). Both peptides alone were devoid of agonist activity but completely inhibited IL-5 induced eosinophil adhesion to immobilized IgG with IC50s of 50 ± 8 nM (AF17121) and 350 ± 70 pM (AF18748) (Fig. 3B). AF17121 had no effect on eosinophil adhesion induced by the related cytokine GM-CSF at concentrations up to 50 μM (data not shown). Furthermore, concentrations of AF18748 up to 1 μM had no effect on eosinophil adhesion induced by the related cytokines GM-CSF and IL-3, the unrelated cytokine tumor necrosis factor-α or the chemotactic peptide fMet-Leu-Phe (Fig. 3C). AF18748 is therefore an extremely potent and selective antagonist of IL-5 in a human eosinophil functional assay.
AF18748 Induces Receptor Dimerization.
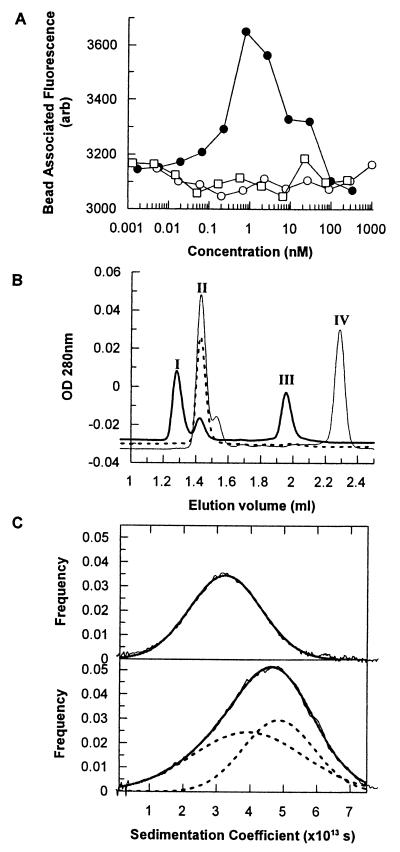
Previous work on peptide ligands for the erythropoietin and thrombopoietin receptors has demonstrated that dimeric peptides can induce receptor homodimerization (21, 22). Several different methods were used to investigate whether AF18748 was similarly able to bind to two IL-5Rα chains. First, a “sandwich-” binding assay was devised using microvolume fluorimetry (31) to measure the ligand-dependent association of two receptor α chains. IL-5Rα ECD was immobilized onto 10-μm beads and incubated with soluble, fluorescently labeled IL-5Rα ECD in the presence or absence of IL-5 or peptide. The addition of increasing amounts of AF18748 over a range from 10 pM to 1 nM caused an increase in the association of the fluorescently labeled receptor to the beads (Fig. 4A), indicating that the peptide is binding both an immobilized receptor and a soluble receptor simultaneously. At concentrations of AF18748 >1 nM, the binding of labeled receptor to the beads decreases, presumably due to the excess of peptide favoring a 1:1 association of peptide with both immobilized and soluble receptor. Neither IL-5 nor the monomeric IL-5 antagonist peptide, AF17121, induced the association of labeled receptor with the beads (Fig. 4A).
Figure 4.
Biochemical evidence of AF18748-induced IL-5Rα dimerization. (A) Microvolume fluorimetry. Fluorescently labeled IL-5Rα ECD was incubated with IL-5Rα ECD-coated polystyrene beads and serial dilutions of AF18748 (●), AF17121 (○), or human IL-5 (□). Binding reactions were incubated in microtiter plates overnight at room temperature, and the bead-associated fluorescence was determined by using an FMAT instrument (Perkin–Elmer). Data points represent averages of triplicate determinations from a single representative experiment. (B) Gel filtration chromatography. IL-5Rα ECD was loaded onto a Precision Superdex 200 3.2/30 column alone (dotted line) or in the presence of equimolar amounts of AF17121 (light line) or AF18748 (dark line). Peak I corresponds to free AF17121 (2.5 kDa), peak II to free AF18748 (3.9 kDa), peak III is where monomeric IL-5R ECD elutes (89.5 kDa), and peak IV corresponds to a molecular mass of 209 kDa. Results shown are from a single experiment, representative of three separate runs for each condition. (C) Analytical ultracentrifugation. IL-5Rα ECD was examined alone (Upper) or in the presence of equimolar amounts of AF18748 in both reference and sample compartments (Lower). Data were analyzed by using both ultrascan and dcdt. Data are shown as the distribution of species observed, overlaid with curves fitted to a Gaussian distribution (Upper) or two overlapping Gaussian distributions (Lower). Data shown are from a single experiment representative of three.
Second, the ability of peptides to induce receptor dimerization was studied by using gel filtration chromatography. Soluble IL-5Rα ECD eluted as a single peak with a calculated molecular mass of 89.5 kDa (Fig. 4B). Although this is considerably larger than the predicted size of the receptor, gel filtration under denaturing conditions (6 M guanidine hydrochloride) confirmed that the soluble receptor was monomeric (data not shown) and suggested that its early elution was due to extensive glycosylation. The presence of AF17121 had no effect on the elution of the IL-5Rα ECD, whereas the apparent size of the IL-5Rα ECD increased in the presence of AF18748 to ≈200 kDa, consistent with a change from monomer to dimer (Fig. 4B).
Third, analytical ultracentrifugation was used to examine the interaction of AF18748 with the IL-5Rα ECD. In velocity sedimentation experiments, the IL-5Rα ECD alone showed a Gaussian distribution of molecular species centered at 3.3 S (Fig. 4C Upper). In the presence of a molar equivalent of AF18748, the distribution shifted toward higher molecular weight complexes and became non-Gaussian. Deconvolution of this non-Gaussian distribution revealed the presence of two species, each with a Gaussian distribution, giving apparent sedimentation coefficient values of 3.9 S and 5.0 S (Fig. 4C Lower). These results are entirely consistent with peptide-induced receptor dimerization.
Last, a system was designed to analyze receptor-binding stoichiometry in a cellular context. A chimeric receptor consisting of the IL-5Rα ECD fused to the membrane-spanning and intracellular domains of the EGFR was constructed. The chimeric receptor was stably expressed in Ba/F3 cells that also contained a luciferase reporter gene under the transcriptional control of the c-fos enhancer and minimal thymidine kinase promoter. Because activation of the native EGFR by EGF-induced dimerization results in an increase in c-fos transcription (32), dimerization of the IL-5Rα/EGFR chimera could be expected to similarly increase transcription of the luciferase reporter gene. Stimulation of Ba/F3 cells expressing the chimera with AF18748 resulted in a dose-dependent increase in luciferase activity, whereas IL-5 or the monomeric peptide AF17121 had no effect (Fig. 5A). Moreover, activation of the luciferase reporter by AF18748 could be antagonized by IL-5, demonstrating that the observed effect is mediated by the chimeric receptor (Fig. 5B).
Figure 5.
Chimeric IL-5Rα/EGFR-reporter cell assay. (A) AF18748 induced activation of the IL-5Rα/EGFR chimeric receptor. Ba/F3 cells expressing a chimeric IL-5Rα/EGFR and containing a luciferase reporter gene were treated with serial dilutions of AF18748 (●), AF17121 (○), or IL-5 (□). Data points represent mean ± SEM for three separate experiments performed in triplicate. The data has been normalized to the stimulation induced by 1 μM AF18748 in each experiment. (B) Inhibition of AF18748-induced activation of the IL-5Rα/EGFR chimeric receptor by IL-5. Cells were treated with 200 pM AF18748 in the presence of increasing concentrations of IL-5. Data points represent the average of triplicate determinations from a single representative experiment.
Discussion
In this paper, we describe the identification and characterization of two series of peptides that interact with the IL-5 receptor and prevent ligand binding. The first series of peptides identified comprises a set of disulfide-cyclized peptides that compete with IL-5 for binding to the IL-5Rα chain. The most potent peptide in this series, AF17121, blocks IL-5-dependent activation of human eosinophils with an IC50 of 50 nM. The second, more potent, series of peptides bind with high affinity to the IL-5Rα chain as disulfide-linked dimers. The best-characterized peptide from this series, AF18748, a 4-kDa peptide, binds to the IL-5 receptor with an affinity equal to that of IL-5, a glycoprotein of approximately 40 kDa (33). Furthermore, over the same range of concentrations as it competes with IL-5 for receptor binding, AF18748 is a potent and specific antagonist of IL-5 function in an eosinophil adhesion assay.
Although neither series of antagonist peptides share any primary sequence similarity to IL-5, it is tempting to speculate that the peptides adopt a structure that presents the appropriate functional epitopes in the correct spatial orientation to mimic the key contact points on IL-5 important for IL-5Rα binding. Point mutants of IL-5 have been described that are potent and specific antagonists of the IL-5 receptor (16, 17). In addition, small molecule isothiazolone compounds have been reported to specifically block IL-5 binding by covalent modification of the IL-5 receptor; however, no functional data were presented (34). We are not aware of any other low molecular weight functional antagonists of IL-5 that exhibit selectivity for the IL-5 receptor over the closely related receptors for GM-CSF and IL-3.
Because the peptides of the clone 2 series all contained three cysteines in the linear sequence, and could therefore adopt a number of potential disulfide bond arrangements, it was surprising that AF17362 spontaneously formed a single dimer structure in solution. However, NMR studies demonstrated that the intramolecular bond between C-15 and C-18 forms before other potential disulfide bonds. It is tempting to speculate that the presence of a turn-inducing proline between these two cysteines promotes the rapid formation of this disulfide bond, resulting in the remarkable uniformity of structure that was observed. This bias toward a single active structure could explain why proline was so strongly favored in this position during the affinity maturation process.
The biochemical and biophysical data presented in this paper provide strong evidence that, in solution, the dimeric peptide, AF18748, exhibits a unique mechanism of antagonist action in binding to two IL-5Rα chains simultaneously. Furthermore, the use of the IL-5Rα/EGFR chimeric receptor demonstrates that AF18748 displays bivalent receptor binding in a cellular context. Using a similar library screening protocol, we have previously described the identification of small dimeric peptide mimetics of erythropoietin and thrombopoietin (21, 22). In these instances the peptides mimic the natural ligand in inducing receptor homodimerization, a prerequisite for receptor activation. In contrast, activation of the IL-5 receptor involves the heterodimerization of the ligand-binding α chain with the βc signaling chain. Therefore, the ability of AF18748 to occupy two IL-5R α chains leads to high potency functional antagonism by sequestration of the α chains, thereby preventing IL-5 binding and the subsequent receptor heterodimerization required to initiate signal transduction.
Like AF18748, IL-5 is a disulfide-linked homodimer, but there are clear differences in the nature of their interactions with the IL-5 receptor. Analytical ultracentrifugation and gel filtration techniques have been previously used to demonstrate that each IL-5 dimer binds to only a single IL-5Rα in solution (35, 36), an observation fully supported by the microvolume fluorimetry binding studies described here (Fig. 4A). We have extended these observations into a cellular system, where the inability of IL-5 to induce activation of the IL-5Rα/EGFR chimera demonstrates that it is unable to bring two receptor subunits together in the context of a membrane. However, like the native IL-5Rα, the chimeric receptor does bind IL-5 monovalently, which is why addition of IL-5 antagonizes the transcriptional up-regulation induced by AF18748 (Fig. 5B). The binding of IL-5 also reduces the basal level of luciferase expression (Fig. 5), presumably by sterically hindering the ligand-independent receptor dimerization, and subsequent transcriptional activation, which is frequently observed in overexpression studies.
In summary, we report the identification of potent and selective peptide antagonists of the IL-5 receptor. We also define a unique mechanism by which antagonists of multisubunit cytokine receptors can function, namely the sequestration of ligand-binding chains by using multivalent ligands. Determination of the structure of the peptide–receptor complex by using NMR and x-ray crystallographic methods will help us to understand the precise molecular characteristics of this interaction and would represent a significant step on the way toward the identification or design of nonpeptidic small molecule antagonists. Additionally, metabolically stable analogs of the peptides described here, when combined with appropriate delivery technology, have the potential of being useful therapeutic agents in the treatment of allergic asthma, and other atopic disorders.
Acknowledgments
We thank E. Tate, A. Dobbs, N. Alvarado, and Y.-F. Feng for cell culture, R. Andregg for peptide MS, and C.-W. Chung for peptide NMR.
Abbreviations
- ECD
extracellular domain
- IL-5Rα
interleukin-5 receptor α chain
- EGFR
epidermal growth factor receptor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.110053997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.110053997
References
- 1.Sanderson C J. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 2.Denburg J A, Silver J E, Abrams J S. Blood. 1991;77:1462–1468. [PubMed] [Google Scholar]
- 3.Yamaguchi Y, Suda T, Ohta S, Tominaga K, Miura Y, Kasahara T. Blood. 1991;78:2542–2547. [PubMed] [Google Scholar]
- 4.Walsh G M, Hartnell A, Wardlaw A J, Kurihara K, Sanderson C J, Kay A B. Immunology. 1990;71:258–265. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson C J, Gleich G J. J Immunol. 1990;144:642–646. [PubMed] [Google Scholar]
- 6.Clutterbuck E J, Hirst E M, Sanderson C J. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 7.Lee J J, Mcgarry M P, Farmer S C, Denzler K L, Larson K A, Carrigan P E, Brenneise I E, Horton M A, Haczku A, Gelfand E W, et al. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwama T, Nagai H, Tsuruoka N, Koda A. Clin Exp Allergy. 1993;23:32–38. doi: 10.1111/j.1365-2222.1993.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 9.Shi H Z, Xiao C Q, Zhong D, Qin S M, Liu Y, Liang G R, Xu H, Chen Y Q, Long X M, Xie Z F. Am J Respir Crit Care Med. 1998;157:204–209. doi: 10.1164/ajrccm.157.1.9703027. [DOI] [PubMed] [Google Scholar]
- 10.Akutsu I, Kojima T, Kariyone A, Fukuda T, Makino S, Takatsu K. Immunol Lett. 1995;45:109–116. doi: 10.1016/0165-2478(94)00241-i. [DOI] [PubMed] [Google Scholar]
- 11.Van Oosterhout A J, Ladenius A R, Savelkoul H F, Van Ark I, Delsman K C, Nijkamp F P. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 12.Mauser P J, Pitman A M, Fernandez X, Foran S K, Adams G K, Kreutner W, Egan R W, Chapman R W. Am J Respir Crit Care Med. 1995;152:467–472. doi: 10.1164/ajrccm.152.2.7633694. [DOI] [PubMed] [Google Scholar]
- 13.Foster P S, Hogan S P, Ramsay A J, Matthaei K I, Young I G. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavernier J, Devos R, Cornelis S, Tuypens T, Van der Heyden J, Fiers W, Plaetinck G. Cell. 1991;66:1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 15.Monahan J, Siegel N, Keith R, Caparon M, Christine L, Compton R, Cusik S, Hirsch J, Huynh M, Devine C, et al. J Immunol. 1997;159:4024–4034. [PubMed] [Google Scholar]
- 16.McKinnon M, Page K, Uings I, Banks M, Fattah D, Proudfoot A E I, Graber P, Arod C Y, Fish R, Wells T N C, Solari R. J Exp Med. 1997;186:121–129. doi: 10.1084/jem.186.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavernier J, Tuypens T, Verhee A, Plaetinck G, Devos R, Van der Heyden J, Guisez Y, Oefner C. Proc Natl Acad Sci USA. 1995;92:5194–5198. doi: 10.1073/pnas.92.11.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher J H, O'Garra A, Shrader B, van Kimmenade A, Bond M W, Mosmann T R, Coffman R L. J Immunol. 1988;141:1576–1581. [PubMed] [Google Scholar]
- 19.Graber P, Proudfoot A E I, Talabot F, Bernard A R, McKinnon M, Banks M, Fattah D, Solari R, Peitsch M C, Wells T N C. J Biol Chem. 1995;270:15762–15769. doi: 10.1074/jbc.270.26.15762. [DOI] [PubMed] [Google Scholar]
- 20.Yanofsky S D, Baldwin D N, Butler J H, Holden F R, Jacobs J W, Balasubramanian P, Chinn J P, Cwirla S E, Peters-Bhatt E, Whitehorn E A, et al. Proc Natl Acad Sci USA. 1996;93:7381–7386. doi: 10.1073/pnas.93.14.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrighton N C, Farrell F X, Chang R, Kashyap A K, Barbone F P, Mulcahy L S, Johnson D L, Barrett R W, Jolliffe L K, Dower W J. Science. 1996;273:458–464. doi: 10.1126/science.273.5274.458. [DOI] [PubMed] [Google Scholar]
- 22.Cwirla S E, Balasubramanian P, Duffin D J, Wagstrom C R, Gates C M, Singer S C, Davis A M, Tansik R L, Mattheakis L C, Boytos C M, et al. Science. 1997;276:1696–1699. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 23.Whitehorn E A, Tate E, Yanofsky S D, Kochersperger L, Davis A, Mortensen R B, Yonkovich S, Bell K, Dower W J, Barrett R W. Bio/Technology. 1995;13:1215–1219. doi: 10.1038/nbt1195-1215. [DOI] [PubMed] [Google Scholar]
- 24.Cull M G, Miller J F, Schatz P J. Proc Natl Acad Sci USA. 1992;89:1865–1869. doi: 10.1073/pnas.89.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatz P J, Cull M G, Martin E L, Gates C M. Methods Enzymol. 1996;267:171–191. doi: 10.1016/s0076-6879(96)67012-8. [DOI] [PubMed] [Google Scholar]
- 26.Tam J P, Wu C-R, Liu W, Zhang J-W. J Am Chem Soc. 1991;113:6657. [Google Scholar]
- 27.Tamamura H, Matsumoto F, Sakano K, Otaka A, Ibuka T, Fujii N. Chem Commun. 1998;1:151. doi: 10.1006/bbrc.1996.1858. [DOI] [PubMed] [Google Scholar]
- 28.Banks M, Graber P, Proudfoot A E I, Arod C Y, Allet B, Bernard A R, Sebille E, McKinnon M, Wells T N C, Solari R. Anal Biochem. 1995;230:321–328. doi: 10.1006/abio.1995.1481. [DOI] [PubMed] [Google Scholar]
- 29.Fattah D, Page K R, Bezbaruah S, Preist R, Horgan C M, Solari R. Cytokine. 1996;8:248–259. doi: 10.1006/cyto.1996.0034. [DOI] [PubMed] [Google Scholar]
- 30.Gates C M, Stemmer W P, Kaptein R, Schatz P J. J Mol Biol. 1996;255:373–386. doi: 10.1006/jmbi.1996.0031. [DOI] [PubMed] [Google Scholar]
- 31.Martens C, Bakker A, Rodriguez A, Mortensen R B, Barrett R W. Anal Biochem. 1999;273:20–31. doi: 10.1006/abio.1999.4184. [DOI] [PubMed] [Google Scholar]
- 32.Hazzalin C A, Cuenda A, Cano E, Cohen P, Mahadevan L C. Oncogene. 1997;15:2321–2331. doi: 10.1038/sj.onc.1201403. [DOI] [PubMed] [Google Scholar]
- 33.Kinashi T, Harada N, Severinson E, Tanabe T, Sideras P, Konishi M, Azuma C, Tominaga A, Bergstedt-Lindqvist S, Takahashi M. Nature (London) 1986;324:70–73. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- 34.Devos R, Guisez Y, Plaetinck G, Cornelis S, Tavernier J, Van der Heyden J, Foley L H, Scheffler J E. Eur J Biochem. 1994;225:635–640. doi: 10.1111/j.1432-1033.1994.00635.x. [DOI] [PubMed] [Google Scholar]
- 35.Johanson K, Appelbaum E, Doyle M, Hensley P, Zhao B, Abdel-Meguid S S, Young P, Cook R, Carr S, Matico R. J Biol Chem. 1995;270:9459–9471. doi: 10.1074/jbc.270.16.9459. [DOI] [PubMed] [Google Scholar]
- 36.Devos R, Guisez Y, Cornelis S, Verhee A, Van der Heyden J, Manneberg M, Lahm H W, Fiers W, Tavernier J, Plaetinck G. J Biol Chem. 1993;268:6581–6587. [PubMed] [Google Scholar]