Abstract
Although the primary signal for the activation of steroid hormone receptors is binding of hormone, there is increasing evidence that the activities of cell signaling pathways and the phosphorylation status of these transcription factors and their coregulators determine the overall response to the hormone. In some cases, enhanced cell signaling is sufficient to cause activation of receptors in medium depleted of steroids. Steroid receptors are targets for multiple kinases. Many of the phosphorylation sites contain Ser/Thr-Pro motifs implicating proline-directed kinases such as the cyclin-dependent kinases and the mitogen-activated kinases (MAPK) in receptor phosphorylation. Although some sites are constitutively phosphorylated, others are phosphorylated in response to hormone. Still others are only phosphorylated in response to specific cell signaling pathways. Phosphorylation of specific sites has been implicated not only in overall transcriptional activity, but also in nuclear localization, protein stability, and DNA binding. The studies of the roles of phosphorylation in coregulator function are more limited, but it is now well established that many of them are highly phosphorylated and that phosphorylation regulates their function. There is good evidence that some of the phosphorylation sites in the receptors and coregulators are targets of multiple signaling pathways. Individual sites have been associated both with functions that enhance the activity of the receptor, as well as with functions that inhibit activity. Thus, the specific combinations of phosphorylations of the steroid receptor combined with the expression levels and phosphorylation status of coregulators will determine the genes regulated and the biological response.
Introduction
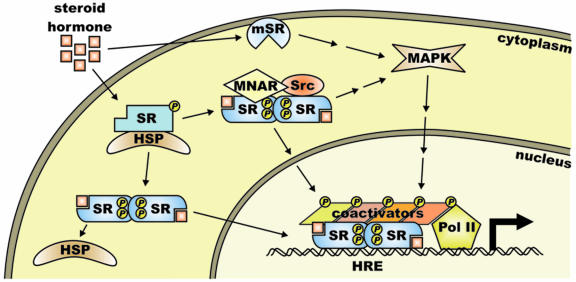
Steroid hormone receptors are hormone-activated transcription factors whose activities are also modulated by posttranslational modifications including phosphorylation [Faus and Haendler, 2006]. In the absence of hormone, receptor monomers associate with heat shock protein complexes and as a rule are minimally phosphorylated (Figure 1). Upon binding hormone, the receptors dimerize, cytoplasmic receptors translocate to the nucleus and the receptors bind to sequence-specific hormone response elements (HRE). Typically, hormone binding and localization to specific DNA binding sites is accompanied by an increase in receptor phosphorylation. The receptors recruit a series of coactivator complexes that facilitate chromatin remodeling, recruitment of Pol II (polymerase II), and transcription of specific target genes. Phosphorylation of coactivators and Pol II is also integral to regulation of transcription. Moreover, some of the proteins recruited to the chromatin by steroid receptors are themselves kinases that can modify histones or other proteins associated with chromatin.
Figure 1. Mechanism of steroid hormone action.
In the absence of hormone, steroid receptor monomers (SR) are associated with heat shock protein complexes (HSP) and are typically basally phosphorylated. Upon binding hormone, receptors dissociate from heat shock proteins, dimerize, bind to target gene-specific sites containing hormone response elements (HRE), and recruit a series of coactivator complexes to regulate target gene transcription. Site-specific phosphorylation of receptors increases subsequent to hormone binding, with some increases occurring rapidly, and others with delayed kinetics. Upon steroid binding, some receptors also interact with Src and MNAR, activating Src and downstream kinases including p42/p44 MAPK. Membrane-associated receptors (mSR) also bind hormone and initiate signaling cascades. While some of these are classical steroid receptors, others bear no homology to the steroid receptor superfamily.
In some cases, upon hormone binding, a portion of the cytoplasmic receptor associates with and activates Src kinase, leading to activation of downstream signaling [Edwards, 2005; Lange, 2004]. In addition, there is evidence that a small fraction of some of the receptors is associated with the cell membrane and hormone binding induces activation of a variety of signaling pathways.
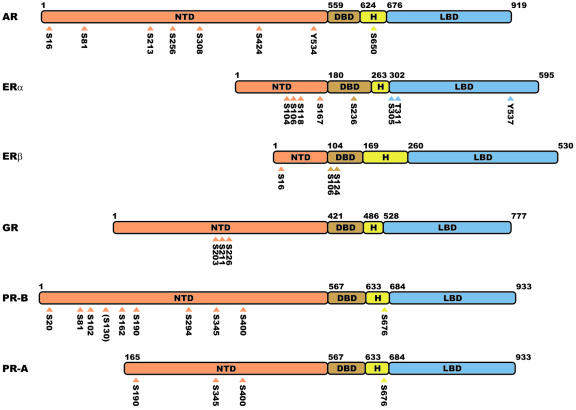
Structurally, the steroid receptors share many common features [Evans, 1988], as shown in Figure 2. The receptors all contain carboxyl terminal ligand binding domains (LBD) that include a region termed activation function 2 (AF-2), which is a site for coactivator binding and thus is important for the induction of transcriptional activity. The LBD is linked to the DNA binding domain (DBD) by a hinge region (H) that contains a nuclear localization signal. The DNA binding domains are the most highly conserved regions of the receptors and each contains two Zn++ binding motifs. The amino terminal domains (NTD) are the least conserved regions both in sequence and in length, but all contain at least one region, termed AF-1 (activation function 1), that is required for optimal transcriptional activity. Most of the phosphorylation sites identified in steroid receptors are located in the NTDs of the receptors, although many have at least one phosphorylation site in the hinge region, and there are limited reports of phosphorylation sites in the hormone and DNA binding domains. Shown in Figure 2 are the best characterized phosphorylation sites in the human steroid receptors. Others have been proposed based on in vitro studies and additional sites have been identified in steroid receptors from other species. Consequently, there are likely to be additional unidentified sites in at least some of the human steroid receptors.
Figure 2. Domain structures of steroid receptors.
The numbers of the amino acids found at the boundaries in the individual receptors between the NTD (amino-terminal domain), DBD (DNA binding domain), hinge region (H), and ligand binding domain (LBD) are indicated in the figure. Also shown are the best characterized phosphorylation sites in the human steroid receptors.
Several strategies have been used to elucidate the roles of cell signaling in steroid receptor action and in crosstalk between steroid receptors and growth factor signaling pathways. The first is simply to alter the activity of a signaling pathway using activators or inhibitors and to measure the effect on steroid receptor activity. Changes identified using this technique demonstrate a role for the pathway in regulating receptor action, but the target may be an associated protein rather than the receptor, or a combination of the receptor and other proteins. A second approach is to measure the activity of receptors containing alanine substitutions for one or more of the serine or threonine phosphorylation sites. This strategy requires knowledge of the location of the phosphorylation sites. While many have been located, others have yet to be identified. Finally, a number of studies in recent years implicate steroid receptors in the direct activation of cell signaling pathways potentially modifying receptor function, as well as inducing activation of target genes sensitive to increased kinase activity.
Modulation of cell signaling pathways alters steroid receptor activity
Ligand-independent activation
The most striking evidence for the potential importance of cell signaling in steroid receptor action was the finding that some steroid receptors can be activated in the absence of measurable levels of hormones by treatments that enhance activity of kinases or inhibit phosphatase activities. Denner et al. first showed that 8-Br cAMP treatment of cells transfected with a chicken progesterone receptor (cPR) expression vector and a PR-responsive reporter caused hormone-independent, but cPR-dependent, activation of the reporter [Denner et al., 1990b]. This activation was independent of the four characterized phosphorylation sites in cPR and 8-Br cAMP did not induce phosphorylation of PR [Bai et al., 1997]. However, subsequent studies revealed that 8-Br cAMP caused activation of p42/p44 MAPK (mitogen-activated kinase) and increased phosphorylation of the p160 coactivator, SRC-1 (steroid receptor coactivator-1); these SRC-1 phosphorylation sites contributed to the ligand-independent activation [Rowan et al., 2000]. Other signaling pathways also induce activity. Treatment of transiently transfected cells with dopamine activates a number of receptors including cPR [Power et al., 1991] and ERα (estrogen receptor α) [Smith et al., 1993] in the absence of their cognate ligands. Treatment with the phosphatase inhibitor, okadaic acid, also induces ligand-independent activation of cPR [Denner et al., 1990b]. There is evidence that these alternate activation pathways are also functional in vivo. For example, treatment with dopamine causes PR-dependent induction of lordosis in female rats and mice [Apostolakis et al., 1996; Mani et al., 1996; Mani et al., 1994; Mani et al., 2006]. As expected for a PR-dependent response, PR null mice are not responsive.
Estrogen receptor α
The responsiveness of receptors to cell signaling pathways in the absence of hormone differs greatly. Whereas the glucocorticoid receptor (GR) requires ligand for activation, ERα is very responsive to cell signaling pathways. Indeed, ERα in cells maintained in phenol red-free, charcoal-stripped serum, utilized to minimize/eliminate steroids, frequently displays substantial basal transcriptional activity in the absence of added ligand; this activity can be reduced by the use of a pure anti-estrogen such as ICI 182780, showing that the activity is dependent upon ERα [Smith et al., 1993]. There are multiple pathways for hormone-independent activation of ERα. One of the best characterized of the hormone-independent pathways for the activation of the ERα is the EGF (epidermal growth factor)-dependent activation of human ERα in transfected HeLa cells. EGF-dependent activation induces phosphorylation of Ser118 in the amino terminus of ER [Kato et al., 1995]. Substitution of an alanine for the serine abrogates the hormone-independent activation. However, substitution of a glutamic acid, which provides a constitutive negative charge, restores responsiveness to EGF, but does not cause the receptor to become constitutively active [Bunone et al., 1996]. Thus, EGF-dependent activation requires phosphorylation of ERα at Ser118, as well as phosphorylation of at least one other target. One possibility is Ser167 in ERα. EGF treatment typically results in activation of Rsk downstream of p42/p44 MAPK; Ser167 is a substrate for Rsk [Clark et al., 2001; Joel et al., 1998]. Other pathways are independent of Ser118 phosphorylation (or the corresponding mouser Ser122), demonstrating that there are multiple means of activating ER independent of its ligand [Patrone et al., 1998].
Androgen receptor
The androgen receptor (AR) also has the capacity to respond to cell signaling pathways in cells grown in medium depleted of hormone. Among the factors reported to induce AR activity are EGF, KGF (keratinocyte growth factor) [Culig et al., 1994], IL-6 (interleukin-6) [Hobisch et al., 1998], and forskolin [Nazareth and Weigel, 1996], an activator of protein kinase A. Overexpression of the EGF receptor family member, HER2 (Human Epidermal Growth Factor Receptor 2), also induces AR activity [Craft et al., 1999]. Activation of AR by cell signaling pathways in the absence of normal levels of androgens likely contributes to the recurrence of prostate cancer. The recurrent tumors express high levels of AR and re-express many AR target genes including PSA (prostate specific antigen) (reviewed in [Agoulnik and Weigel, 2006]). Several investigators have demonstrated that AR-positive, androgen-independent cell lines that express PSA in medium depleted of androgens still require AR for cell growth and expression of PSA [Agoulnik et al., 2005; Zegarra-Moro et al., 2002]. Because the AR sequence does not differ from that of AR in androgen-dependent cells, it is presumed that altered cell signaling is causing aberrant receptor activation.
Potentiation of partial antagonist activity
Although some receptors show minimal responsiveness to cell signaling pathways in the absence of ligand, enhanced cell signaling often potentiates receptor activity in the presence of a partial antagonist. Treatment with 8-Br cAMP causes the mammalian PR and GR antagonist, RU486 (mifepristone), to act as a GR [Nordeen et al., 1993] and a PR-B isoform [Beck et al., 1993; Sartorius et al., 1994] specific agonist. The finding that cell signaling pathways can induce hormone-independent activation, can cause receptors to be responsive to lower levels of hormone, and can cause antagonists such as tamoxifen and RU486 to have agonist activity, is particularly relevant in hormone-sensitive cancers such as breast and prostate cancer. Signaling pathways that enhance AR activity are often activated in advanced prostate cancers [Gioeli et al., 1999; Gregory et al., 2004; Gregory et al., 2005; Mellinghoff et al., 2004] and HER2 signaling combined with overexpression of AIB1 is one contributor to tamoxifen resistance in breast cancer [Shou et al., 2004].
Modulation of agonist-dependent activity
The activities of specific kinases are also required for hormone-dependent activation of steroid receptors, although in many cases the targets of receptor action have not been fully elucidated. This is an active area of research and the studies are far from comprehensive. The response of receptors to signaling pathways can be cell-specific and it is likely that many of the responses will also be target gene-specific. Although there are some recent studies examining regulation of endogenous genes, most of the information has been obtained using transfected reporters. Whereas cyclin-dependent kinases generally enhance the activity of steroid receptors, the actions of other kinases are receptor-specific. Surprisingly, some of the cyclins function as receptor coactivators or corepressors independent of their partner kinases. Only those actions that require kinase activity are discussed below. Signaling pathways that induce hormone-independent action or cause antagonist/agonist switches also potentiate the corresponding hormone-dependent activity and have been discussed above.
Human progesterone receptor
Human PR requires Cdk2 (cyclin-dependent kinase 2) for hormone-dependent activation of at least some of its target genes [Narayanan et al., 2005a]. Inhibition of Cdk activity with the cyclin-dependent kinase inhibitor roscovitine blocks hormone-dependent induction of a PR-responsive reporter or of the endogenous metallothionein gene; reducing expression of Cdk2 also strongly inhibits PR activity. The Cdk2 partner, cyclin A2, is a PR coactivator that depends on binding Cdk for its potentiation of PR activity. Although PR can be phosphorylated by cyclin A2/Cdk2 in vitro [Knotts et al., 2001], the potentiation of PR activity is independent of PR phosphorylation [Narayanan et al., 2005a]. Rather, the target is likely to be the p160 coactivator, SRC-1. In ChIP (chromatin immunoprecipitation assays), roscovitine treatment has no effect on the binding of either PR or cyclin A to an MMTV (mouse mammary tumor-like virus) promoter, but it prevents the hormone-dependent recruitment of SRC-1 [Narayanan et al., 2005a]. Consistent with the idea that cyclin A/Cdk2 is important for PR function, PR-dependent activation of the MMTV promoter is cell cycle-dependent [Narayanan et al., 2005b]. PR activity is highest in S phase, where cyclin A is most highly expressed, and lower in early G1 or in G2/M phase. In cells with low levels of the cyclin-dependent kinase inhibitor, p27, overexpression of activated Cdk2 induces hormone-independent activation of PR and this hormone-independent activation is blocked by mutation of Ser400, a PR phosphorylation site [Pierson-Mullany and Lange, 2004].
Other kinases and phosphatases also modulate PR action, although their targets have not been elucidated. Treatment with EGF, resulting in activation of p42/p44 MAPK, enhances hormone-dependent activity [Daniel et al., 2007]. In contrast, activation of p38 MAPK by MKK6 inhibits PR activity [Proia et al., 2006]. Phosphatases also potentiate PR activity. The PP1 and PP2A inhibitor, okadaic acid, stimulates PR activity [Beck et al., 1992]. In contrast, overexpression of the phosphatase, PPM1D (p53-induced serine/threonine phosphatase, protein phosphatase 1D magnesium-dependent, delta isoform), enhances PR activity; reducing expression of endogenous PPM1D in MCF7 breast cancer cells inhibits PR activity [Proia et al., 2006].
ERα and ERβ
Estrogen receptor activity is also modulated by cyclin A/Cdk2, but the activity is dependent upon the ability of the kinase to phosphorylate ERα [Rogatsky et al., 1999]. Signaling pathways that induce hormone-independent activation also potentiate hormone-dependent actions. There are additional reports of kinases regulating ER activity in specific cell types. In contrast to its effects on PR, activation of p38 MAPK enhances the activity of ERα and increases its nuclear localization in endometrial cells [Lee and Bai, 2002]. However, p38 MAPK has been reported to play a role in ERBB2/ERBB3-dependent inhibition of ERβ (estrogen receptor β) activity [St-Laurent et al., 2005]. GSK3β (glycogen synthase 3β) enhances ERα activity in neuronal cells [Mendez and Garcia-Segura, 2006]. Phosphatases also play a role in regulating activity. Overexpression of PP5 (protein phosphatase 5) reduces Ser118 phosphorylation in ERα and transcriptional activation. Moreover, reducing expression of PP5 enhances the transcriptional activity of ERα [Ikeda et al., 2004].
Glucocorticoid receptor
Studies of GR show that cyclin-dependent kinases, Cdk1 and Cdk2, can phosphorylate GR in vitro and that GR expressed in yeast lacking the corresponding kinase activity is less active than in a wild type strain [Krstic et al., 1997]. In contrast, a number of kinases inhibit GR activity. JNK (Jun N terminal kinase) inhibits rat GR activity through direct phosphorylation of GR [Rogatsky et al., 1998a]. Similarly, GSK3 inhibits rat GR through direct phosphorylation [Rogatsky et al., 1998b]; it has no effect on human GR, which lacks the corresponding phosphorylation site. Although p38 MAPK also inhibits GR activity, its effects are independent of the previously identified phosphorylation sites in GR [Szatmary et al., 2004]. Similar to ERα, PP5 is a negative regulator of GR activity [Zuo et al., 1999].
Androgen receptor
AR activity is also regulated by cyclin-dependent kinases. Similar to PR, overexpression of cyclin A enhances AR activity [Narayanan et al., 2005a]. Roscovitine reduces AR activity and expression [Chen et al., 2006]. Although roscovitine inhibits many cyclin-dependent kinases, studies with more specific inhibitors suggest that Cdk1 is the kinase required for optimal AR protein expression. Consistent with a different cyclin-dependent kinase requirement compared to PR, the cell cycle dependence of AR activity exhibits a different pattern, with a decrease in activity at the G1/S boundary [Martinez and Danielsen, 2002].
Cell signaling pathways regulate both the transcriptional activity and the stability of AR protein. Although inhibition of HER2 decreases AR protein levels and activity at low levels of hormone, the downstream kinase(s) responsible for regulating AR activity and expression has not been identified. HER2 activates Akt, but constitutively active Akt cannot compensate for inhibition of HER2 [Mellinghoff et al., 2004]. The role of Akt in AR action is controversial. Some investigators have found that Akt enhances AR activity, whereas others have reported that it inhibits AR activity [Lin et al., 2003; Lin et al., 2001; Taneja et al., 2005]. Thus, actions of Akt are likely to be context-dependent. Akt inhibits the activity of GSK-3, a kinase that has been reported to inhibit AR activity [Salas et al., 2004]. Thus, in cells with high levels of GSK-3 activity, activation of Akt through inhibition of GSK-3 may increase AR activity. Mitogen-activated protein kinases (MAPK) also modulate AR activity. MEKK1, an upstream activator of p42/p44 MAPK, increases AR activity [Abreu-Martin et al., 1999]. In contrast, inhibiting either JNK or p38 MAPK using siRNA for their upstream activators, MKK4 and MKK6, increases the expression of prostate specific antigen (PSA), an androgen-regulated gene in LNCaP prostate cancer cells [Gioeli et al., 2006].
Steroid receptor coregulators
In addition to directly modifying the receptors, there is increasing evidence that receptor activities are regulated by changes in the phosphorylation state of coactivators and corepressors. For example, the p160 coactivators are extensively phosphorylated and are targets of multiple signaling pathways. Sites in SRC-1 are important in potentiating hormone-independent activities of chicken PR and human AR [Rowan et al., 2000; Ueda et al., 2002]. Phosphorylation of SRC-1 also plays a role in the interaction between human PR and SRC-1 [Narayanan et al., 2005a]. The related p160 coactivator, TIF2 (transcription intermediary factor 2)/GRIP1 (glucocorticoid receptor interacting protein 1)/SRC-2, is phosphorylated by p42/p44 MAPK, and this phosphorylation plays a role in potentiating AR activity [Gregory et al., 2004]. Phosphorylation of GRIP1 by p38 MAPK enhances its potentiation of ERα activity [Frigo et al., 2006]. The third p160 coactivator, AIB1 (amplified in breast cancer 1)/SRC-3, plays a broader role in modulating the activities of multiple transcription factors. It is highly phosphorylated and different transcription factors, including steroid receptors, require phosphorylation of subsets of the phosphorylation sites for optimal potentiation of activity [Wu et al., 2002; Wu et al., 2004]. In some cases, the receptors themselves can alter phosphorylation of SRC-3. Estradiol treatment of ERα-containing cells increases the phosphorylation of SRC-3 at specific sites [Zheng et al., 2005].
Phosphorylation of corepressors also influences their ability to repress transcription. Phosphorylation of the corepressor, SMRT (silencing mediator of retinoid and thyroid hormone receptors), by MEKK1 (MEK kinase 1), causes nuclear export, relieving repression [Jonas and Privalsky, 2004]. MEKK1 also causes dissociation of N-CoR (nuclear receptor corepressor) complexes from androgen and estrogen receptors, enhancing transcriptional activity [Zhu et al., 2006]. Other coregulators are also phosphoproteins and altered cell signaling is likely to influence association and activities of these proteins as well.
Kinases as coactivators
The responsiveness of steroid receptor function to a variety of cell signaling pathways suggests that there may be means to preferentially enhance or inhibit the phosphorylation of components of steroid receptor pathways in addition to the changes induced by global changes in kinase/phosphatase activities. The simplest mechanism would be direct interactions of receptors or coactivators with kinases or phosphatases, enhancing proximity to other potential substrates in the pathway. Indeed, one of the first candidate coactivators identified, TIF1α, is a protein kinase whose phosphorylation is enhanced upon interaction with activated nuclear receptors [Fraser et al., 1998]. A novel nuclear protein kinase (ANPK) was identified as an AR-interacting protein that enhanced AR activity, but did not phosphorylate AR, leading to the conclusion that it acts through phosphorylation of AR coregulators or modification of chromatin proteins [Moilanen et al., 1998]. Other, better characterized kinases have also been found to be associated with nuclear receptors. AR interacts with Cdk9, a component of P-TEFb, a kinase that phosphorylates the CTD (carboxyl terminal domain) of Pol II, facilitating elongation of transcripts [Lee et al., 2001]. PR recruits cyclin A/Cdk2 to the promoter of target genes. Inhibition of its activity blocks transcription of an MMTV promoter and prevents recruitment of SRC-1, but not recruitment of another coactivator complex containing TRAP220 (thyroid receptor associated protein 220) [Narayanan et al., 2005a]. Receptors also interact with phosphatases, thereby limiting activity. AR interacts with small carboxyl terminal domain phosphatase 2, which reduces the transcriptional activity of AR [Thompson et al., 2006]. PP5 binds to ERα and ERβ; reducing PP5 expression enhances induction of a number of ERα target genes including c-myc and pS2 [Ikeda et al., 2004]. Other phosphatases and kinases are brought to receptor complexes by proteins that interact with receptors. For example, IκK (IκB kinase) associates with SRC-3, a substrate for the kinase; the kinase regulates the activity of the coactivator [Wu et al., 2002]. Kinase inhibitor studies show that Ser118 is at least in part phosphorylated by IκKα [Weitsman et al., 2006]. Thus, binding of SRC-3 to ERα may facilitate the hormone-dependent phosphorylation of Ser118. The phosphatase, PP5, interacts with GR through hsp90 complexes and reduces GR phosphorylation. Reducing expression of PP5 has target gene-specific effects on GR activity [Wang et al., 2007] ranging from no detectable effect to substantial inhibition of hormone-induced transcription.
Receptor phosphorylation
Identification of phosphorylation sites
Early attempts to identify phosphorylation sites in steroid receptors relied on radiolabeling followed by direct protein sequencing or comparisons of the radiolabeling patterns of wild type and mutant receptors [Bodwell et al., 1991; Denner et al., 1990a; Le Goff et al., 1994; Zhang et al., 1997; Zhang et al., 1995; Zhou et al., 1995]. More recent approaches to the identification of sites have included mass spectrometry [Gioeli et al., 2002; Knotts et al., 2001].
All of the steroid receptors contain multiple phosphorylation sites. The receptors are partially phosphorylated in the absence of hormone and are more highly phosphorylated after hormone treatment. Some of the sites exhibit enhanced phosphorylation in response to hormone, while phosphorylation of others is almost exclusively hormone-dependent. Most of the sites are serines or threonines in the amino terminal regions of the receptors, although there is a positionally-conserved Ser-Pro or Thr-Pro in the hinge regions of the steroid receptors. This site has been shown to be phosphorylated in chicken [Denner et al., 1990a] and human PR [Knotts et al., 2001], mouse ERα [Lahooti et al., 1995], and human AR [Zhou et al., 1995]. Whether it is also phosphorylated in other receptors has not yet been determined. Most of these sites are located in Ser/Thr-Pro motifs, indicating that the receptors are direct targets of proline-directed kinases including the cyclin-dependent kinases and the MAPK family, which is consistent with studies showing that these kinases modulate receptor activity. Sites identified as authentic in vivo sites in the human steroid receptors are shown in Figure 2.
Some of the phosphorylation sites in steroid receptors are conserved across species, whereas others are unique to specific species. There is a GSK3β phosphorylation site in rat GR that is absent in human GR [Rogatsky et al., 1998b]. Chicken PR contains four Ser-Pro phosphorylation sites [Denner et al., 1990a; Poletti and Weigel, 1993] and all are in the regions common to the PR-B and PR-A isoforms. In contrast, human PR is highly phosphorylated. There are a number of phosphorylation sites common to the PR-A and PR-B isoforms, but PR-B contains at least four additional phosphorylation sites in its unique amino-terminus [Knotts et al., 2001]. Although Ser294 is in the primary sequence of both PR-A and PR-B, studies with a phosphorylation site-specific antibody reveal that Ser294 is phosphorylated only in the PR-B isoform [Clemm et al., 2000]. The two isoforms have different biological activities and this difference may reflect unique conformations in the amino-termini of the two isoforms; alternatively, a protein may bind to the PR-A isoform, thereby occluding the site.
In addition to the Ser/Thr-Pro sites, sites in other consensus sequences have been identified. Additional receptor sites include Ser167 in ERα, a casein kinase II consensus site [Arnold et al., 1994] that is also phosphorylated by Rsk [Joel et al., 1998], as well as by Akt [Shah and Rowan, 2005], and a site in PR (Ser81), which is in a consensus CKII site and can be phosphorylated by CKII in vitro [Zhang et al., 1994]. Additional sites are detected when specific cell signaling pathways are activated. For example, activation of p38 MAPK causes phosphorylation of Thr311 in ERα [Lee and Bai, 2002] and activation of Protein Kinase A (PKA) induces phosphorylation of Ser305 in ERα [Al-Dhaheri and Rowan, 2007]. In cells with high Akt activity, AR Ser213 is phosphorylated in response to DHT (dihydrotestosterone); this phosphorylation is inhibited by the PI-3K inhibitor, LY294002, suggesting that the phosphorylation is Akt-dependent [Taneja et al., 2005]. In addition to the Ser/Thr phosphorylation sites in steroid receptors, there is limited evidence to support tyrosine phosphorylation. In ERα, phosphorylation of Tyr537 in the hormone binding domain has been described, although others have not detected phosphorylation of this site, suggesting that the stoichiometry of phosphorylation may be low and/or it is only phosphorylated under specific conditions [Al-Dhaheri and Rowan, 2006; Arnold et al., 1995]. Two recent studies have shown that Src can phosphorylate AR on Tyr534 in the amino-terminus of AR and there is some evidence that other tyrosines can be phosphorylated to a lesser extent [Guo et al., 2006; Kraus et al., 2006]. Consistent with the failure to detect tyrosine phosphorylation of AR previously, an analysis of AR in LNCaP cells revealed that tyrosine phosphorylation in response to hormone was a rapid and transient event. Using a phosphorylation site-specific antibody, Guo et al. have shown that the site is phosphorylated in AR in prostate tumors [Guo et al., 2006].
Applications of phosphorylation site-specific antibodies
Because each receptor is multiply phosphorylated, identifying the kinases that phosphorylate the individual sites in vivo has been challenging. Site-specific antibodies have been made for a number of the sites and some are now commercially available. Studies with these antibodies reveal that some sites are targets for multiple kinases, allowing the receptors to respond to a variety of stimuli. For example, Ser118 in ERα is phosphorylated by p42/p44 MAPK as a result of EGF treatment, but cyclin H/Cdk7 phosphorylates the site in response to hormone treatment [Chen et al., 2000; Chen et al., 2002]. Similarly, p42/p44 MAPK phosphorylates Ser294 in PR-B in response to EGF, but hormone-dependent phosphorylation of the site occurs despite blocking p42/p44 MAPK activation [Narayanan et al., 2005b].
Site-specific antibodies have also been useful in resolving whether candidate sites are authentic in vivo sites. Initial studies in LNCaP prostate cancer cells failed to identify Ser213, a consensus Akt phosphorylation site in AR, as an authentic in vivo phosphorylation site [Gioeli et al., 2002]; however, Ser213 is phosphorylated by Akt in vitro [Lin et al., 2001]. Subsequent studies with a site-specific antibody revealed that Ser213 is phosphorylated in wild type AR, but in a cell type-specific manner [Taneja et al., 2005]. Interestingly, Ser213 is phosphorylated in AR in LAPC-4 prostate cancer cells. The LNCaP AR contains an amino acid mutation in the ligand binding domain (T877A). Surprisingly, when wild type and mutant ARs were expressed by transient transfection, the wild type receptor displayed much more Ser213 phosphorylation than did the mutant [Taneja et al., 2005]. Thus, the AR in LNCaP cells likely is minimally phosphorylated on this site, consistent with a failure to detect phosphorylation by direct analyses of phosphopeptides.
Phosphorylation site-specific antibodies to phosphorylated Ser118 and Ser167 in ERα have been used to examine breast cancer specimens. Some studies show a correlation between phosphorylation of Ser118 and a favorable response to Tamoxifen [Murphy et al., 2004], as well as a correlation between Ser167 phosphorylation and response to endocrine therapy [Yamashita et al., 2005]. The studies to date are limited and not all studies have found such correlations (reviewed in [Murphy et al., 2006]). Phosphorylation of Ser118 also correlated with PR expression, suggesting that the phosphorylation is an indication that the ERα is active in those samples and that an ERα antagonist is likely to be beneficial.
Phosphorylation and receptor function
Transcriptional activation
Initially, studies of the role of phosphorylation in receptor function were limited to measuring transcriptional activity of transiently transfected reporters. More recent studies have revealed that receptor phosphorylation contributes to a variety of functions including receptor stability, nuclear localization, transcriptional activity, interaction with coregulators, and other activities such as splicing. In some cases, the phosphorylation may determine whether a subsequent posttranslational modification will occur. As better assays are developed, it is probable that additional functions will be identified.
Using transient transfection assays to measure the activities of wild type and mutant receptors, Bai and Weigel found that an amino-terminal site in chicken PR (Ser211) was required for optimal transcriptional activity and that the hinge site was required for response to low levels of hormone [Bai et al., 1994; Bai and Weigel, 1996]. Analyses of human PR phosphorylation site mutants revealed modest reductions in transcriptional activity [Takimoto et al., 1996]. In contrast, an Ala294 PR-B mutant stably transfected into a PR-negative breast cancer cell line was much less active than the corresponding line containing a stably transfected wild type receptor, suggesting that cell context and/or stable rather than transient transfection alters responsiveness [Daniel et al., 2007; Qiu and Lange, 2003]. Transient transfection assays of the activities of ERα and AR phosphorylation mutants also revealed some decreases in activity [Le Goff et al., 1994; Zhou et al., 1995].
Additional roles for receptor phosphorylation
Analyses of individual receptor functions have revealed more specific roles for phosphorylation. Mutation of the phosphorylation sites in GR increases receptor stability [Webster et al., 1997]. A GR-interacting protein, TSG101, preferentially interacts with hypophosphorylated GR and protects it from degradation [Ismaili et al., 2005]. Substitution of an alanine for Ser294 in human PR increases receptor stability. PR is degraded by the proteasome pathway and elimination of the phosphorylation site decreases ubiquitination of PR, suggesting that the phosphorylation may serve as a signal for ubiquitination [Lange et al., 2000]. In ERβ, phosphorylation of Ser16 enhances ER degradation [Cheng et al., 2000]. This serine is also a site for O-GlcNAc modification [Cheng et al., 2000; Cheng and Hart, 2001]. The competing modifications likely regulate the stability of ERβ.
Phosphorylation can enhance or inhibit protein/protein interactions. For example, phosphorylations in the amino terminus of ERβ induced by p42/p44 MAPK enhance SRC-1 association and induce ligand-independent activation [Tremblay et al., 1999]. In contrast, activation of Akt reduces interaction between ERβ and CBP. Mutation of Ser255 in ERβ restores CBP binding and blocks inhibition of ERβ activity by this signaling pathway [Sanchez et al., 2007].
Phosphorylation also plays a role in regulating nuclear localization of receptors. Phosphorylation of Thr311 in ERα increases nuclear localization [Lee and Bai, 2002]. In contrast, stress kinase-induced phosphorylation of Ser650 in AR enhances cytoplasmic localization [Gioeli et al., 2006]. Phosphorylation of Ser294 is required for EGF-induced nuclear localization of human PR-B, but not for hormone-dependent nuclear localization [Qiu et al., 2003]. Phosphatase activity is also required for GR relocalization to the nucleus, although the target of the phosphatase is unknown [Dean et al., 2001; Galigniana et al., 1999]. Thus, phosphorylation plays a role in subcellular distribution of most of the steroid receptors.
Phosphorylation and ERα function
The role of phosphorylation in the regulation of receptor functions has been most extensively characterized for ERα and these studies highlight the wide range of kinases that can phosphorylate a receptor and the diverse roles for phosphorylation in regulating receptor function. Eight phosphorylation sites in ERα (serines 104, 106, 118, 167, 236, and 305, Thr311 and Tyr537), distributed throughout the receptor (see Figure 2), have been identified and antibodies have been prepared to study their phosphorylation [Al-Dhaheri and Rowan, 2006]. Phosphorylation of serines 104, 106, and 118 has been implicated in optimal interaction with several coactivators [Dutertre and Smith, 2003; Likhite et al., 2006].
Phosphorylation of Ser118 appears to be particularly important for physical and/or functional interactions with a variety of coregulators. It has been implicated in the interaction with (SF)3a p120, a splicing factor, and the potentiation of splicing [Masuhiro et al., 2005]. On the other hand, phosphorylation of this site enhances interaction with the estrogen receptor repressor SPBP (stromelysin-1 platelet-derived growth factor-responsive element-binding protein) [Gburcik et al., 2005]. Ser167 phosphorylation plays a role in optimal DNA binding in vitro and in binding to endogenous promoters in vivo [Castano et al., 1997; Likhite et al., 2006; Shah and Rowan, 2005]. Ser236 phosphorylation has been implicated in inhibition of hormone-independent dimerization and DNA binding, but this inhibition is overcome by the addition of estradiol [Chen et al., 1999]. Phosphorylation of Ser305 increases transcriptional activity and has been implicated in preventing acetylation of Lys303 [Cui et al., 2004].
Interestingly, Lys303 is often mutated to Arg in breast cancer and this mutation has been associated with hypersensitivity to estrogen [Fuqua et al., 2000]. As discussed above, phosphorylation of Thr311 enhances nuclear translocation [Lee and Bai, 2002]. The role of phosphorylation of Tyr537 has been controversial [Yudt et al., 1999]. Mutation of the site alters estradiol binding kinetics and some amino acid substitutions promote hormone-independent activation [Weis et al., 1996]. However, these changes may not be a reflection of the phosphorylation states. In vitro phosphorylation with Src, which phosphorylates Tyr537, as well as at least one other tyrosine, enhances affinity for estradiol [Likhite et al., 2006]. As more sophisticated means of measuring receptor functions are developed, it is likely that additional roles for site-specific receptor phosphorylation will be identified.
Activation of cell signaling pathways by steroid hormones
There is abundant evidence that steroids can activate a variety of cell signaling pathways that influence transcription and enzyme activities independent of the genomic activities of the receptors [Watson and Lange, 2005] and, as discussed above, these pathways also modulate receptor function. These actions are mediated by steroid receptor family members, as well as by other proteins that respond to steroids. A detailed discussion of this aspect of steroid action is beyond the scope of this review. However, these pathways offer a potential means of autoregulation of the transcriptional activity of the receptors. For example, AR and ER interact with Src and MNAR (modulator of nongenomic actions of the estrogen receptor) [Haas et al., 2005; Wong et al., 2002]. Hormone binding results in activation of Src and the downstream activation of p42/p44 MAPK, kinases that regulate receptor function through phosphorylation of coactivators and, in some cases, phosphorylation of the receptors themselves. PR directly interacts with Src family tyrosine kinases through a proline-rich motif in the NTD of PR, which also activates a kinase cascade [Boonyaratanakornkit et al., 2001]. PR also induces long term activation of p42/p44 MAPK through induction of Wnt-1 and the resulting activation of the EGF receptor [Faivre and Lange, 2007].
Summary
Cell signaling pathways that regulate phosphorylation of steroid receptors and their coactivators are critical factors in determining the activities of steroid receptors under different physiological conditions. While many of the phosphorylation sites in the steroid receptors and some of the sites in the coregulators have been identified, others are still unknown. In particular, there are likely to be sites that are phosphorylated only in response to a specific signaling pathway; these phosphorylations may be transient, but important for specific biological responses. A number of additional candidate sites have been identified by in vitro phosphorylation studies. Some of these sites likely are authentic targets under some circumstances, while others may be artifacts of the conditions (high concentrations of purified receptor and kinase with no competing substrates) and are not authentic in vivo phosphorylation sites. Moreover, because phosphorylation studies using high specific activity γ[32P] ATP can yield a substantial signal despite a low percent or stoichiometry for the phosphorylation, some signals may be due to phosphorylation of a small amount of denatured receptor. The sites shown in Figure 2 are restricted to those that have been shown to be phosphorylated in receptors expressed in mammalian or insect cells. As additional antibodies are generated for candidate sites, it is likely that others will be established as genuine sites.
Some sites are targets of multiple kinases, thereby permitting integration of signals from multiple pathways. Studies under special conditions (for example, serum-free medium or activation of a kinase pathway through a stress signal) can identify a pathway or kinase capable of phosphorylating a specific site, but these experiments should be interpreted with caution. Under other circumstances, another kinase may be the dominant regulator of the site. Although roles for some phosphorylation sites have been identified, many others have not been characterized. Roles for specific sites may also be context-dependent. This is suggested by the role of Ser118 not only in recruiting proteins that contribute to ERα activation, but also in the recruitment of a repressor of ERα activity. It is likely that there are phosphorylation “codes” dependent upon the signaling pathways activated under a specific physiological condition that determine the function of the receptor. These alterations will determine the phosphorylation of specific subsets of receptor phosphorylation sites, as well as the level of phosphorylation of coregulators.
While some functions may depend upon a single phosphorylation, others will have more complex requirements. For example, EGF-dependent activation of ERα requires Ser118 phosphorylation [Bunone et al., 1996]; although substitution of a negatively-charged glutamic acid restores responsiveness to EGF, the Glu118 mutant is not constitutively active. Therefore, at least one additional EGF-induced modification of receptor or other proteins is required. In the case of AR, mutation of Ser650 in the hinge region of AR has been reported to reduce AR transcriptional activity by about 30% [Zhou et al., 1995], but this site is also the target of JNK phosphorylation [Gioeli et al., 2006] and in the context of activated JNK, phosphorylation of this site reduces AR activity. As better assays for individual receptor functions are devised and more phosphorylation site-specific antibodies for receptors and coregulators are developed, it is likely that many additional roles for phosphorylation in receptor function will be identified.
Studies of the regulation of endogenous target genes by phosphorylation site mutants are likely to reveal target- and perhaps tissue-specific requirements for phosphorylations. In contrast to simple transiently transfected reporters with multiple hormone response elements, natural targets require receptor interactions with a variety of other transcription factors and coregulators to appropriately modify chromatin and induce transcription. The contributions of individual phosphorylation sites to overall biological function can be tested for conserved sites by generating mice with individual amino acid substitutions and examining their phenotypes. There is evidence that many, but not all, phosphorylation sites are conserved. Thus, differential phosphorylation is also a potential regulator of species-specific actions of receptors.
Acknowledgments
This work was supported in part by R01 CA57539.
Abbreviations
- AF-1
activation function 1
- AF-2
activation function 2
- AIB1
amplified in breast cancer 1
- AR
androgen receptor
- CBP
CREB (cyclic AMP response element binding protein) binding protein
- Cdk2
cyclin-dependent kinase 2
- ChIP
chromatin immunoprecipitation
- cPR
chicken progesterone receptor
- DBD
DNA binding domain
- DHT
dihydrotestosterone
- EGF
epidermal growth factor
- ERα
estrogen receptor α
- ERβ
estrogen receptor β
- GR
glucocorticoid receptor
- GRIP1
(glucocorticoid receptor interacting protein 1)
- GSK3β
(glycogen synthase kinase 3 β)
- HER2
human epidermal growth factor receptor 2
- HRE
hormone response element
- IκK
IκB kinase
- JNK
jun N terminal kinase
- KGF
keratinocyte growth factor
- LBD
ligand binding domain
- MAPK
mitogen-activated protein kinase
- MEKK1
MEK kinase 1
- MMTV
mouse mammary tumor-like virus
- MNAR
modulator of nongenomic action of estrogen receptor
- N-CoR
nuclear receptor corepressor
- NTD
amino terminal domains
- Pol II
polymerase II
- PP5
protein phosphatase 5
- PPM1D
p53-induced serine/threonine phosphatase, protein phosphatase 1D magnesium-dependent, delta isoforms
- PR
progesterone receptor
- PSA
prostate specific antigen
- SMRT
silencing mediator of retinoid and thyroid hormone receptors
- SPBP
stromelysin-1 platelet-derived growth factor-responsive element-binding protein
- SRC-1
steroid receptor coactivator-1
- TIF2
transcription intermediary factor 2
- TRAP220
thyroid receptor associated protein 220
References
- Abreu-Martin M. T., Chari A., Palladino A. A., Craft N. A., Sawyers C. L. Mitogen-activated protein kinase kinase kinase 1 activates androgen receptor-dependent transcription and apoptosis in prostate cancer. Mol Cell Biol. 1999;19:5143–54. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoulnik I. U., Weigel N. L. Androgen receptor action in hormone-dependent and recurrent prostate cancer. J Cell Biochem. 2006;99:362–72. doi: 10.1002/jcb.20811. [DOI] [PubMed] [Google Scholar]
- Agoulnik I. U., Vaid A., Bingman W. E., 3rd, Erdeme H., Frolov A., Smith C. L., Ayala G., Ittmann M. M., Weigel N. L. Role of SRC-1 in the promotion of prostate cancer cell growth and tumor progression. Cancer Res. 2005;65:7959–67. doi: 10.1158/0008-5472.CAN-04-3541. [DOI] [PubMed] [Google Scholar]
- Al-Dhaheri M. H., Rowan B. G. Application of phosphorylation site-specific antibodies to measure nuclear receptor signaling: characterization of novel phosphoantibodies for estrogen receptor α. Nucl Recept Signal. 2006;4:e007. doi: 10.1621/nrs.04007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhaheri M. H., Rowan B. G. Protein kinase A exhibits selective modulation of estradiol-dependent transcription in breast cancer cells that is associated with decreased ligand binding, altered estrogen receptor α promoter interaction, and changes in receptor phosphorylation. Mol Endocrinol. 2007;21:439–56. doi: 10.1210/me.2006-0059. [DOI] [PubMed] [Google Scholar]
- Apostolakis E. M., Garai J., Fox C., Smith C. L., Watson S. J., Clark J. H., O'Malley B. W. Dopaminergic regulation of progesterone receptors: brain D5 dopamine receptors mediate induction of lordosis by D1-like agonists in rats. J Neurosci. 1996;16:4823–34. doi: 10.1523/JNEUROSCI.16-16-04823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Jaffe H., Notides A. C. Phosphorylation of the human estrogen receptor on tyrosine 537 in vivo and by src family tyrosine kinases in vitro. Mol Endocrinol. 1995;9:24–33. doi: 10.1210/mend.9.1.7539106. [DOI] [PubMed] [Google Scholar]
- Arnold S. F., Obourn J. D., Jaffe H., Notides A. C. Serine 167 is the major estradiol-induced phosphorylation site on the human estrogen receptor. Mol Endocrinol. 1994;8:1208–14. doi: 10.1210/mend.8.9.7838153. [DOI] [PubMed] [Google Scholar]
- Bai W., Rowan B. G., Allgood V. E., O'Malley B. W., Weigel N. L. Differential phosphorylation of chicken progesterone receptor in hormone-dependent and ligand-independent activation. J Biol Chem. 1997;272:10457–63. doi: 10.1074/jbc.272.16.10457. [DOI] [PubMed] [Google Scholar]
- Bai W., Weigel N. L. Phosphorylation of Ser211 in the chicken progesterone receptor modulates its transcriptional activity. J Biol Chem. 1996;271:12801–6. doi: 10.1074/jbc.271.22.12801. [DOI] [PubMed] [Google Scholar]
- Bai W., Tullos S., Weigel N. L. Phosphorylation of Ser530 facilitates hormone-dependent transcriptional activation of the chicken progesterone receptor. Mol Endocrinol. 1994;8:1465–73. doi: 10.1210/mend.8.11.7877616. [DOI] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Edwards D. P. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol Endocrinol. 1992;6:607–20. doi: 10.1210/mend.6.4.1316549. [DOI] [PubMed] [Google Scholar]
- Beck C. A., Weigel N. L., Moyer M. L., Nordeen S. K., Edwards D. P. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc Natl Acad Sci U S A. 1993;90:4441–5. doi: 10.1073/pnas.90.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodwell J. E., Orti E., Coull J. M., Pappin D. J., Smith L. I., Swift F. Identification of phosphorylated sites in the mouse glucocorticoid receptor. J Biol Chem. 1991;266:7549–55. [PubMed] [Google Scholar]
- Boonyaratanakornkit V., Scott M. P., Ribon V., Sherman L., Anderson S. M., Maller J. L., Miller W. T., Edwards D. P. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–80. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- Bunone G., Briand P. A., Miksicek R. J., Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. Embo J. 1996;15:2174–83. [PMC free article] [PubMed] [Google Scholar]
- Castano E., Vorojeikina D. P., Notides A. C. Phosphorylation of serine-167 on the human oestrogen receptor is important for oestrogen response element binding and transcriptional activation. Biochem J. 1997;326 ( Pt 1):149–57. doi: 10.1042/bj3260149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Riedl T., Washbrook E., Pace P. E., Coombes R. C., Egly J. M., Ali S. Activation of estrogen receptor α by S118 phosphorylation involves a ligand-dependent interaction with TFIIH and participation of CDK7. Mol Cell. 2000;6:127–37. [PubMed] [Google Scholar]
- Chen S., Xu Y., Yuan X., Bubley G. J., Balk S. P. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc Natl Acad Sci U S A. 2006;103:15969–74. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X., Hart G. W. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor β: post-translational regulation of turnover and transactivation activity. J Biol Chem. 2001;276:10570–5. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- Cheng X., Cole R. N., Zaia J., Hart G. W. Alternative O-glycosylation/O-phosphorylation of the murine estrogen receptor β. Biochemistry. 2000;39:11609–20. doi: 10.1021/bi000755i. [DOI] [PubMed] [Google Scholar]
- Chen D., Washbrook E., Sarwar N., Bates G. J., Pace P. E., Thirunuvakkarasu V., Taylor J., Epstein R. J., Fuller-Pace F. V., Egly J. M., Coombes R. C., Ali S. Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene. 2002;21:4921–31. doi: 10.1038/sj.onc.1205420. [DOI] [PubMed] [Google Scholar]
- Chen D., Pace P. E., Coombes R. C., Ali S. Phosphorylation of human estrogen receptor α by protein kinase A regulates dimerization. Mol Cell Biol. 1999;19:1002–15. doi: 10.1128/mcb.19.2.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. E., Poteet-Smith C. E., Smith J. A., Lannigan D. A. Rsk2 allosterically activates estrogen receptor α by docking to the hormone-binding domain. Embo J. 2001;20:3484–94. doi: 10.1093/emboj/20.13.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemm D. L., Sherman L., Boonyaratanakornkit V., Schrader W. T., Weigel N. L., Edwards D. P. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- Craft N., Shostak Y., Carey M., Sawyers C. L. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- Cui Y., Zhang M., Pestell R., Curran E. M., Welshons W. V., Fuqua S. A. Phosphorylation of estrogen receptor α blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64:9199–208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- Culig Z., Hobisch A., Cronauer M. V., Radmayr C., Trapman J., Hittmair A., Bartsch G., Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- Daniel A. R., Qiu M., Faivre E. J., Ostrander J. H., Skildum A., Lange C. A. Linkage of progestin and epidermal growth factor signaling: Phosphorylation of progesterone receptors mediates transcriptional hypersensitivity and increased ligand-independent breast cancer cell growth. Steroids. 2007;72:188–201. doi: 10.1016/j.steroids.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. A., Urban G., Aragon I. V., Swingle M., Miller B., Rusconi S., Bueno M., Dean N. M., Honkanen R. E. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol. 2001;2:6. doi: 10.1186/1471-2121-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner L. A., Schrader W. T., O'Malley B. W., Weigel N. L. Hormonal regulation and identification of chicken progesterone receptor phosphorylation sites. J Biol Chem. 1990b;265:16548–55. [PubMed] [Google Scholar]
- Denner L. A., Weigel N. L., Maxwell B. L., Schrader W. T., O'Malley B. W. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990a;250:1740–3. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- Dutertre M., Smith C. L. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor-α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Edwards D. P. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–76. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre E. J., Lange C. A. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–80. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faus H., Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60:520–8. doi: 10.1016/j.biopha.2006.07.082. [DOI] [PubMed] [Google Scholar]
- Fraser R. A., Heard D. J., Adam S., Lavigne A. C., Le Douarin B., Tora L., Losson R., Rochette-Egly C., Chambon P. The putative cofactor TIF1alpha is a protein kinase that is hyperphosphorylated upon interaction with liganded nuclear receptors. J Biol Chem. 1998;273:16199–204. doi: 10.1074/jbc.273.26.16199. [DOI] [PubMed] [Google Scholar]
- Frigo D. E., Basu A., Nierth-Simpson E. N., Weldon C. B., Dugan C. M., Elliott S., Collins-Burow B. M., Salvo V. A., Zhu Y., Melnik L. I., Lopez G. N., Kushner P. J., Curiel T. J., Rowan B. G., McLachlan J. A., Burow M. E. p38 mitogen-activated protein kinase stimulates estrogen-mediated transcription and proliferation through the phosphorylation and potentiation of the p160 coactivator glucocorticoid receptor-interacting protein 1. Mol Endocrinol. 2006;20:971–83. doi: 10.1210/me.2004-0075. [DOI] [PubMed] [Google Scholar]
- Fuqua S. A., Wiltschke C., Zhang Q. X., Borg A., Castles C. G., Friedrichs W. E., Hopp T., Hilsenbeck S., Mohsin S., O'Connell P., Allred D. C. A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Res. 2000;60:4026–9. [PubMed] [Google Scholar]
- Galigniana M. D., Housley P. R., DeFranco D. B., Pratt W. B. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J Biol Chem. 1999;274:16222–7. doi: 10.1074/jbc.274.23.16222. [DOI] [PubMed] [Google Scholar]
- Gburcik V., Bot N., Maggiolini M., Picard D. SPBP is a phosphoserine-specific repressor of estrogen receptor α. Mol Cell Biol. 2005;25:3421–30. doi: 10.1128/MCB.25.9.3421-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioeli D., Mandell J. W., Petroni G. R., Frierson H. F., Jr., Weber M. J. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59:279–84. [PubMed] [Google Scholar]
- Gioeli D., Ficarro S. B., Kwiek J. J., Aaronson D., Hancock M., Catling A. D., White F. M., Christian R. E., Settlage R. E., Shabanowitz J., Hunt D. F., Weber M. J. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–14. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- Gioeli D., Black B. E., Gordon V., Spencer A., Kesler C. T., Eblen S. T., Paschal B. M., Weber M. J. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol Endocrinol. 2006;20:503–15. doi: 10.1210/me.2005-0351. [DOI] [PubMed] [Google Scholar]
- Gregory C. W., Fei X., Ponguta L. A., He B., Bill H. M., French F. S., Wilson E. M. Epidermal growth factor increases coactivation of the androgen receptor in recurrent prostate cancer. J Biol Chem. 2004;279:7119–30. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- Gregory C. W., Whang Y. E., McCall W., Fei X., Liu Y., Ponguta L. A., French F. S., Wilson E. M., Earp H. S., 3rd. Heregulin-induced activation of HER2 and HER3 increases androgen receptor transactivation and CWR-R1 human recurrent prostate cancer cell growth. Clin Cancer Res. 2005;11:1704–12. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- Guo Z., Dai B., Jiang T., Xu K., Xie Y., Kim O., Nesheiwat I., Kong X., Melamed J., Handratta V. D., Njar V. C., Brodie A. M., Yu L. R., Veenstra T. D., Chen H., Qiu Y. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–19. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Haas D., White S. N., Lutz L. B., Rasar M., Hammes S. R. The modulator of nongenomic actions of the estrogen receptor (MNAR) regulates transcription-independent androgen receptor-mediated signaling: evidence that MNAR participates in G protein-regulated meiosis in Xenopus laevis oocytes. Mol Endocrinol. 2005;19:2035–46. doi: 10.1210/me.2004-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobisch A., Eder I. E., Putz T., Horninger W., Bartsch G., Klocker H., Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–5. [PubMed] [Google Scholar]
- Ikeda K., Ogawa S., Tsukui T., Horie-Inoue K., Ouchi Y., Kato S., Muramatsu M., Inoue S. Protein phosphatase 5 is a negative regulator of estrogen receptor-mediated transcription. Mol Endocrinol. 2004;18:1131–43. doi: 10.1210/me.2003-0308. [DOI] [PubMed] [Google Scholar]
- Ismaili N., Blind R., Garabedian M. J. Stabilization of the unliganded glucocorticoid receptor by TSG101. J Biol Chem. 2005;280:11120–6. doi: 10.1074/jbc.M500059200. [DOI] [PubMed] [Google Scholar]
- Joel P. B., Smith J., Sturgill T. W., Fisher T. L., Blenis J., Lannigan D. A. pp90rsk1 regulates estrogen receptor-mediated transcription through phosphorylation of Ser-167. Mol Cell Biol. 1998;18:1978–84. doi: 10.1128/mcb.18.4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas B. A., Privalsky M. L. SMRT and N-CoR corepressors are regulated by distinct kinase signaling pathways. J Biol Chem. 2004;279:54676–86. doi: 10.1074/jbc.M410128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Endoh H., Masuhiro Y., Kitamoto T., Uchiyama S., Sasaki H., Masushige S., Gotoh Y., Nishida E., Kawashima H., Metzger D., Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- Knotts T. A., Orkiszewski R. S., Cook R. G., Edwards D. P., Weigel N. L. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J Biol Chem. 2001;276:8475–83. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- Kraus S., Gioeli D., Vomastek T., Gordon V., Weber M. J. Receptor for activated C kinase 1 (RACK1) and Src regulate the tyrosine phosphorylation and function of the androgen receptor. Cancer Res. 2006;66:11047–54. doi: 10.1158/0008-5472.CAN-06-0596. [DOI] [PubMed] [Google Scholar]
- Krstic M. D., Rogatsky I., Yamamoto K. R., Garabedian M. J. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–54. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahooti H., White R., Hoare S. A., Rahman D., Pappin D. J., Parker M. G. Identification of phosphorylation sites in the mouse oestrogen receptor. J Steroid Biochem Mol Biol. 1995;55:305–13. doi: 10.1016/0960-0760(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Lange C. A. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol Endocrinol. 2004;18:269–78. doi: 10.1210/me.2003-0331. [DOI] [PubMed] [Google Scholar]
- Lange C. A., Shen T., Horwitz K. B. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97:1032–7. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff P., Montano M. M., Schodin D. J., Katzenellenbogen B. S. Phosphorylation of the human estrogen receptor. Identification of hormone-regulated sites and examination of their influence on transcriptional activity. J Biol Chem. 1994;269:4458–66. [PubMed] [Google Scholar]
- Lee D. K., Duan H. O., Chang C. Androgen receptor interacts with the positive elongation factor P-TEFb and enhances the efficiency of transcriptional elongation. J Biol Chem. 2001;276:9978–84. doi: 10.1074/jbc.M002285200. [DOI] [PubMed] [Google Scholar]
- Lee H., Bai W. Regulation of estrogen receptor nuclear export by ligand-induced and p38-mediated receptor phosphorylation. Mol Cell Biol. 2002;22:5835–45. doi: 10.1128/MCB.22.16.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhite V. S., Stossi F., Kim K., Katzenellenbogen B. S., Katzenellenbogen J. A. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol. 2006;20:3120–32. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- Lin H. K., Yeh S., Kang H. Y., Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci U S A. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. K., Hu Y. C., Yang L., Altuwaijri S., Chen Y. T., Kang H. Y., Chang C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J Biol Chem. 2003;278:50902–7. doi: 10.1074/jbc.M300676200. [DOI] [PubMed] [Google Scholar]
- Mani S. K., Reyna A. M., Chen J. Z., Mulac-Jericevic B., Conneely O. M. Differential response of progesterone receptor isoforms in hormone-dependent and -independent facilitation of female sexual receptivity. Mol Endocrinol. 2006;20:1322–32. doi: 10.1210/me.2005-0466. [DOI] [PubMed] [Google Scholar]
- Mani S. K., Allen J. M., Lydon J. P., Mulac-Jericevic B., Blaustein J. D., DeMayo F. J., Conneely O., O'Malley B. W. Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol. 1996;10:1728–37. doi: 10.1210/mend.10.12.8961281. [DOI] [PubMed] [Google Scholar]
- Mani S. K., Blaustein J. D., Allen J. M., Law S. W., O'Malley B. W., Clark J. H. Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology. 1994;135:1409–14. doi: 10.1210/endo.135.4.7925102. [DOI] [PubMed] [Google Scholar]
- Martinez E. D., Danielsen M. Loss of androgen receptor transcriptional activity at the G(1)/S transition. J Biol Chem. 2002;277:29719–29. doi: 10.1074/jbc.M112134200. [DOI] [PubMed] [Google Scholar]
- Masuhiro Y., Mezaki Y., Sakari M., Takeyama K., Yoshida T., Inoue K., Yanagisawa J., Hanazawa S., O'Malley B W., Kato S. Splicing potentiation by growth factor signals via estrogen receptor phosphorylation. Proc Natl Acad Sci U S A. 2005;102:8126–31. doi: 10.1073/pnas.0503197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinghoff I. K., Vivanco I., Kwon A., Tran C., Wongvipat J., Sawyers C. L. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–27. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Mendez P., Garcia-Segura L. M. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–39. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- Moilanen A. M., Karvonen U., Poukka H., Janne O. A., Palvimo J. J. Activation of androgen receptor function by a novel nuclear protein kinase. Mol Biol Cell. 1998;9:2527–43. doi: 10.1091/mbc.9.9.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. C., Niu Y., Snell L., Watson P. Phospho-serine-118 estrogen receptor-α expression is associated with better disease outcome in women treated with tamoxifen. Clin Cancer Res. 2004;10:5902–6. doi: 10.1158/1078-0432.CCR-04-0191. [DOI] [PubMed] [Google Scholar]
- Murphy L. C., Weitsman G. E., Skliris G. P., Teh E. M., Li L., Peng B., Davie J. R., Ung K., Niu Y. L., Troup S., Tomes L., Watson P. H. Potential role of estrogen receptor α (ERalpha) phosphorylated at Serine118 in human breast cancer in vivo. J Steroid Biochem Mol Biol. 2006;102:139–46. doi: 10.1016/j.jsbmb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Adigun A. A., Edwards D. P., Weigel N. L. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol Cell Biol. 2005a;25:264–77. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R., Edwards D. P., Weigel N. L. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol Cell Biol. 2005b;25:2885–98. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazareth L. V., Weigel N. L. Activation of the human androgen receptor through a protein kinase A signaling pathway. J Biol Chem. 1996;271:19900–7. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K., Bona B. J., Moyer M. L. Latent agonist activity of the steroid antagonist, RU486, is unmasked in cells treated with activators of protein kinase A. Mol Endocrinol. 1993;7:731–42. doi: 10.1210/mend.7.6.8395651. [DOI] [PubMed] [Google Scholar]
- Patrone C., Gianazza E., Santagati S., Agrati P., Maggi A. Divergent pathways regulate ligand-independent activation of ER α in SK-N-BE neuroblastoma and COS-1 renal carcinoma cells. Mol Endocrinol. 1998;12:835–41. doi: 10.1210/mend.12.6.0114. [DOI] [PubMed] [Google Scholar]
- Pierson-Mullany L. K., Lange C. A. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activation of cyclin-dependent protein kinase 2. Mol Cell Biol. 2004;24:10542–57. doi: 10.1128/MCB.24.24.10542-10557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletti A., Weigel N. L. Identification of a hormone-dependent phosphorylation site adjacent to the DNA-binding domain of the chicken progesterone receptor. Mol Endocrinol. 1993;7:241–6. doi: 10.1210/mend.7.2.8469237. [DOI] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991;254:1636–9. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Proia D. A., Nannenga B. W., Donehower L. A., Weigel N. L. Dual roles for the phosphatase PPM1D in regulating progesterone receptor function. J Biol Chem. 2006;281:7089–101. doi: 10.1074/jbc.M511839200. [DOI] [PubMed] [Google Scholar]
- Qiu M., Lange C. A. MAP kinases couple multiple functions of human progesterone receptors: degradation, transcriptional synergy, and nuclear association. J Steroid Biochem Mol Biol. 2003a;85:147–57. doi: 10.1016/s0960-0760(03)00221-8. [DOI] [PubMed] [Google Scholar]
- Qiu M., Olsen A., Faivre E., Horwitz K. B., Lange C. A. Mitogen-activated protein kinase regulates nuclear association of human progesterone receptors. Mol Endocrinol. 2003b;17:628–42. doi: 10.1210/me.2002-0378. [DOI] [PubMed] [Google Scholar]
- Rogatsky I., Logan S. K., Garabedian M. J. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci U S A. 1998a;95:2050–5. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogatsky I., Waase C. L., Garabedian M. J. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem. 1998b;273:14315–21. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- Rogatsky I., Trowbridge J. M., Garabedian M. J. Potentiation of human estrogen receptor α transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J Biol Chem. 1999;274:22296–302. doi: 10.1074/jbc.274.32.22296. [DOI] [PubMed] [Google Scholar]
- Rowan B. G., Garrison N., Weigel N. L., O'Malley B. W. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000;20:8720–30. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas T. R., Kim J., Vakar-Lopez F., Sabichi A. L., Troncoso P., Jenster G., Kikuchi A., Chen S. Y., Shemshedini L., Suraokar M., Logothetis C. J., DiGiovanni J., Lippman S. M., Menter D. G. Glycogen synthase kinase-3 β is involved in the phosphorylation and suppression of androgen receptor activity. J Biol Chem. 2004;279:19191–200. doi: 10.1074/jbc.M309560200. [DOI] [PubMed] [Google Scholar]
- Sanchez M., Sauve K., Picard N., Tremblay A. The hormonal response of estrogen receptor β is decreased by the phosphatidylinositol 3-kinase/Akt pathway via a phosphorylation-dependent release of CREB-binding protein. J Biol Chem. 2007;282:4830–40. doi: 10.1074/jbc.M607908200. [DOI] [PubMed] [Google Scholar]
- Sartorius C. A., Groshong S. D., Miller L. A., Powell R. L., Tung L., Takimoto G. S., Horwitz K. B. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors: only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 1994;54:3868–77. [PubMed] [Google Scholar]
- Shah Y. M., Rowan B. G. The Src kinase pathway promotes tamoxifen agonist action in Ishikawa endometrial cells through phosphorylation-dependent stabilization of estrogen receptor (α) promoter interaction and elevated steroid receptor coactivator 1 activity. Mol Endocrinol. 2005;19:732–48. doi: 10.1210/me.2004-0298. [DOI] [PubMed] [Google Scholar]
- Shou J., Massarweh S., Osborne C. K., Wakeling A. E., Ali S., Weiss H., Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Conneely O. M., O'Malley B. W. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci U S A. 1993;90:6120–4. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Laurent V., Sanchez M., Charbonneau C., Tremblay A. Selective hormone-dependent repression of estrogen receptor β by a p38-activated ErbB2/ErbB3 pathway. J Steroid Biochem Mol Biol. 2005;94:23–37. doi: 10.1016/j.jsbmb.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Szatmary Z., Garabedian M. J., Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–15. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- Takimoto G. S., Hovland A. R., Tasset D. M., Melville M. Y., Tung L., Horwitz K. B. Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J Biol Chem. 1996;271:13308–16. doi: 10.1074/jbc.271.23.13308. [DOI] [PubMed] [Google Scholar]
- Taneja S. S., Ha S., Swenson N. K., Huang H. Y., Lee P., Melamed J., Shapiro E., Garabedian M. J., Logan S. K. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280:40916–24. doi: 10.1074/jbc.M508442200. [DOI] [PubMed] [Google Scholar]
- Thompson J., Lepikhova T., Teixido-Travesa N., Whitehead M. A., Palvimo J. J., Janne O. A. Small carboxyl-terminal domain phosphatase 2 attenuates androgen-dependent transcription. Embo J. 2006;25:2757–67. doi: 10.1038/sj.emboj.7601161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A., Tremblay G. B., Labrie F., Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–9. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- Ueda T., Mawji N. R., Bruchovsky N., Sadar M. D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J Biol Chem. 2002;277:38087–94. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen W., Kono E., Dang T., Garabedian M. J. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–34. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- Watson C. S., Lange C. A. Steadying the boat: integrating mechanisms of membrane and nuclear-steroid-receptor signalling. EMBO Rep. 2005;6:116–9. doi: 10.1038/sj.embor.7400336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster J. C., Jewell C. M., Bodwell J. E., Munck A., Sar M., Cidlowski J. A. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–93. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- Weis K. E., Ekena K., Thomas J. A., Lazennec G., Katzenellenbogen B. S. Constitutively active human estrogen receptors containing amino acid substitutions for tyrosine 537 in the receptor protein. Mol Endocrinol. 1996;10:1388–98. doi: 10.1210/mend.10.11.8923465. [DOI] [PubMed] [Google Scholar]
- Weitsman G. E., Li L., Skliris G. P., Davie J. R., Ung K., Niu Y., Curtis-Snell L., Tomes L., Watson P. H., Murphy L. C. Estrogen receptor-α phosphorylated at Ser118 is present at the promoters of estrogen-regulated genes and is not altered due to HER-2 overexpression. Cancer Res. 2006;66:10162–70. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- Wong C. W., McNally C., Nickbarg E., Komm B. S., Cheskis B. J. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99:14783–8. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu R. C., Qin J., Hashimoto Y., Wong J., Xu J., Tsai S. Y., Tsai M. J., O'Malley B. W. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I κ B kinase. Mol Cell Biol. 2002;22:3549–61. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. C., Qin J., Yi P., Wong J., Tsai S. Y., Tsai M. J., O'Malley B. W. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell. 2004;15:937–49. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Yamashita H., Nishio M., Kobayashi S., Ando Y., Sugiura H., Zhang Z., Hamaguchi M., Mita K., Fujii Y., Iwase H. Phosphorylation of estrogen receptor α serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast Cancer Res. 2005;7:R753–64. doi: 10.1186/bcr1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudt M. R., Vorojeikina D., Zhong L., Skafar D. F., Sasson S., Gasiewicz T. A., Notides A. C. Function of estrogen receptor tyrosine 537 in hormone binding, DNA binding, and transactivation. Biochemistry. 1999;38:14146–56. doi: 10.1021/bi9911132. [DOI] [PubMed] [Google Scholar]
- Zegarra-Moro O. L., Schmidt L. J., Huang H., Tindall D. J. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- Zhang Y., Beck C. A., Poletti A., Edwards D. P., Weigel N. L. Identification of a group of Ser-Pro motif hormone-inducible phosphorylation sites in the human progesterone receptor. Mol Endocrinol. 1995;9:1029–40. doi: 10.1210/mend.9.8.7476977. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Beck C. A., Poletti A., Edwards D. P., Weigel N. L. Identification of phosphorylation sites unique to the B form of human progesterone receptor. In vitro phosphorylation by casein kinase II. J Biol Chem. 1994;269:31034–40. [PubMed] [Google Scholar]
- Zhang Y., Beck C. A., Poletti A., Clement J. P. th, Prendergast P., Yip T. T., Hutchens T. W., Edwards D. P., Weigel N. L. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol Endocrinol. 1997;11:823–32. doi: 10.1210/mend.11.6.0006. [DOI] [PubMed] [Google Scholar]
- Zheng F. F., Wu R. C., Smith C. L., O'Malley B. W. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25:8273–84. doi: 10.1128/MCB.25.18.8273-8284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. X., Kemppainen J. A., Wilson E. M. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9:605–15. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- Zhu P., Baek S. H., Bourk E. M., Ohgi K. A., Garcia-Bassets I., Sanjo H., Akira S., Kotol P. F., Glass C. K., Rosenfeld M. G., Rose D. W. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124:615–29. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Zuo Z., Urban G., Scammell J. G., Dean N. M., McLean T. K., Aragon I., Honkanen R. E. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–57. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]