Abstract
Diabetes mellitus, DM, is commonly accompanied with various stages of hemorheologic disturbances that are the main causes of the development of chronic DM. In this study, simple Chinese material medica [yang-yin jiang-tang preparation (YYJT)] was given to alloxan-induced DM rats and analyzed to compare the changes of fasting blood glucose (FBG), fasting insulin (FINS), hemorheologic parameters and insulin-like growth factor II (IGF-II) before and after administration. The results suggested that YYJT can significantly downregulate FBG (P < 0.005), improve insulin resistance and beta-cell secretion (P < 0.05), decrease whole blood viscosity at low and high shear rates, gathering of blood index test (GIT) and fibrinogen (FIB) (P < 0.05), and enlarge the function of IGF-II (P < 0.05). We concluded that YYJT could prevent and treat hemorheologic disorder in DM rats by means of reducing glucose, improving insulin resistance and elevating IGF-II.
Keywords: Chinese material medica, diabetes mellitus rat, hemorheology, insulin resistance, insulin secretion, insulin-like growth factor II
Introduction
Patients with diabetes mellitus (DM) usually develop a range of hemorheologic disturbances due to glucolipotoxicity. Angiopathy is a risk factor of chronic DM complications (1,2). Widespread research is currently taking place in China and other countries to explore new traditional Chinese medicine and Western medicine that will improve hyperviscosity syndrome and prevent and treat chronic complications of DM (3,4). In traditional medical theory, DM is in the concept of ‘xiao-ke’ with deficiency of Yin and dryness-heat, where the in vivo blocks of blood gore leads to hemorheologic disorder. Therefore, reinforcing Qi, nourishing Yin, removing blood stasis and promoting blood circulation is the principle of DM treatment in traditional medicine (5–7). The administration of simple Chinese material medica not only has hypoglycemic effect but also improves hyperviscosity syndrome in DM, which are the advantages in the treatment of DM with TCM. Against the above background, in this study, simple Chinese material medica [yang-yin jiang-tang preparation (YYJT)], which can nourish Yin and promote blood circulation, was used in DM rats to investigate its mechanism and clinical value for improving hemorheologic disorder.
Methods
Rats
Male Sprague-Dawley rats, about 3 months old, were obtained from the Animal Center, Zhejiang Academy of Medical Science, SCXK (Shanghai, manufacture permission number 2003-0003). All rats were randomly divided into a control group, an experimental group and a YYJT-treated group, consisting 12 rats each.
Reagent
Alloxan (A-7413,Lot-36H0102) was supplied by Sigma company, Insulin radioimmunoassay reagent box (lot: 2008-10236) by the technique center, Academy of Medical Science in China, POD-PAD reagent box (lot: 03174) by the experimental center of clinical diagnostic reagent, China National Biotec Corporation, rat IGF reagent box (lot: 0305) by General Hospital of PLA and Yangyinjiangtangpian (YYJT, lot: ZZ-2970-037801) by Chiatai Qingchun Bao Pharmaceutical Co., Ltd, China.
Apparatus
We used Intelligent Radioimmuno instrument model SN-695 made by the Shanghai Research Institute of atomic nucleus, Revolving Blood Viscosity calculator LBY-N6A and NM1 models, by Beijing Plant of Medical Devices, and ACL-200 instrument, by Beckman Coulter, USA.
Animal Model
The control group was allowed free access to food and water, the other two groups were fasted for 24 h before establishing the model. Sublingual intravenous injections of Alloxan (50 mg kg−1) were given under anesthesia induced by sodium pentobarbital (40 mg kg−1) and the DM models were established 72 h later (8–10). The rats that had levels of glucose above 300 mg dl−1 for 3 days were randomly divided into the experimental (12 rats) and YYJT-treated (12 rats) groups.
Administration
The control group had free access to food and water. The experimental groups were drenched once a day via the stomach with equal amounts of normal saline solution. The YYJT-treated group was perfused via the stomach with a suspension from crude drug (4.66 g kg−1), once a day. The animals were kept in stable clean conditions (the second grade) and fed with water and rodent chow ad libitum.
Detection of Laboratory Parameters
The serum samples were collected 1 day before administration and at 10 days after administration from the tail in all three groups. Fasting blood glucose (FBG) was determined by methods of glucose oxidase: FBG (mmol l−1) = assay tube absorbance/standard tube absorbance 5.55. Fasting insulin (FINS, μU ml−1) was measured under the instructions of the reagent box. Whole blood viscosity at low and high shear rates (LS, HS), plasma viscosity, hematocrit (HCT) in the blood, gathering of blood index test (GIT) and fibrinogen (FIB) in plasma were tested for hemorheology. Insulin-like growth factor II (IFG-II, ng ml−1) was measured using the instructions on the reagent box. Insulin resistance index (Homa-IR) and insulin secretion index (Homa-IS) were detected by HOMA to evaluate insulin resistance and beta-cell secretion. Homa-IR = FINS × FBG/22.5; Homa-IS = FINS × 20 (FBG−3.5) (11–14).
Statistical Analysis
Statistical analysis was done with 4Steps Excel (Statcel2) software. The data are presented as the mean ± standard deviation. Statistical significance was determined using analysis of variance, ANOVA and Fisher's PSLD.
Results
FBG Decreased Significantly in the YYJT Group
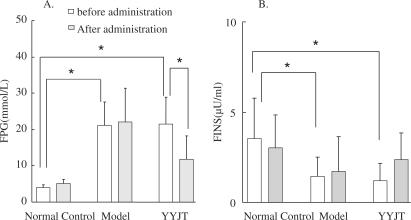
The levels of FBG in the YYJT and experimental groups were significantly elevated (P < 0.001) compared to the control group after the model was established, but no significant difference was noted between the experimental and YYJT groups. At 10 days after administration, the level of FBG in the YYJT group significantly decreased (P < 0.005) but was still higher than that in the control group (P < 0.01). There was no change of FBG in the control and experimental groups (Fig. 1A).
Figure 1.
Levels of FPG and FINS in the three treatment groups. Picture (A) shows the changes of FPG levels. Picture (B) shows the changes of FINS levels. *P-value <0.05. FPG, fasting plasma glucose; FINS, fasting insulin.
After establishing the model, the levels of FINS in the YYJT and experimental groups were significantly lower than in the control group (P < 0.05), but no significant difference was noted in the former two groups. No difference was marked before administration or 10 days after in all three groups (Fig. 1B).
Homa-IR Decreased, Homa-IS Increased Significantly in the YYJT Group
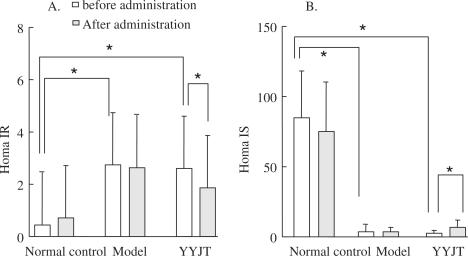
The levels of Homa-IR in the YYJT and experimental groups were significantly increased (P < 0.001) compared to the control group after the model was established, but no significant difference was noted between the experimental and YYJT groups. This suggests that insulin resistance occurred in the DM rats. Ten days after administration, the level of Homa-IR in the YYJT group significantly decreased (P < 0.005), but it was still higher than that in the control group (P < 0.01). There was no change of Homa-IR in the control and experimental groups (Fig. 2A).
Figure 2.
Levels of Homa-IR and Homa-IS in the three treatment groups. Picture (A) shows the changes of Homa-IR levels. Picture (B) shows the changes of Homa-IS levels. *P-value <0.05. Homa-IR, Homa insulin resistance; Homa-IS, insulin secretion index.
The levels of Homa-IS in the YYJT and experimental groups were significantly lower than those in the control group after the model was established (P < 0.05), but no significant difference was noted in the former two groups. This might indicate that beta-cell destruction developed in the DM rats. The level of Homa-IS significantly increased (P < 0.05) in the YYJT group administration, but it was still lower than that in the control group (P < 0.005). No changes of Homa-IS occurred before administration or 10 days after in the control or experimental groups (Fig. 2B).
Most of Hemorheologic Parameters Decreased Significantly in the YYJT Group
The post-administration parameters in the experimental group largely increased in comparison with those in the control group (P < 0.05), showing that distinct hemorheologic obstruction occurred in the DM rats. Compared to the experimental group, LS, HS, GIT and FIB decreased significantly in the YYJT group (P < 0.05) at 10 days administered, while plasma viscosity and HCT were stable (P > 0.05, Table 1).
Table 1.
Data in hemorheology after administration in rats (X ± S)
| N | LS (mpa s) | HS (mpa s) | PV (mpa s) | HCT (%) | GIT index | FIB (g l−1) | |
|---|---|---|---|---|---|---|---|
| Normal control | 12 | 10.51 ± 1.89 | 6.11 ± 0.83 | 1.46 ± 0.17 | 48.20 ± 5.55 | 1.20 ± 0.14 | 2.29 ± 0.59 |
| Model | 12 | 15.61 ± 1.77Δ | 7.83 ± 0.97Δ | 1.83 ± 0.19Δ | 54.30 ± 5.55Δ | 1.68 ± 0.11Δ | 4.34 ± 0.75Δ |
| YYJT | 12 | 10.35 ± 1.57* | 6.82 ± 0.52# | 1.89 ± 0.19 | 53.82 ± 6.32 | 1.32 ± 0.15* | 2.53 ± 0.65* |
Homa-IR, Homa insulin resistance; LS, low shear rate; HS, high shear rate; PV, plasma viscosity; HCT, hematocrit; GIT, gathering of index test; FIB, fibrinogen.
ΔP-value <0.01 compared with normal control group; *P-value <0.001 compared with model group; #P-value <0.05 compared with model group.
Table 2 illustrates the relationship between Homa-IR and hemorheologic parameters before administration and 10 days after. The statistically analyzed results explained that Homa-IR had a correlation with LS and GIT (r = 0.56, 0.88, P < 0.05) after administration. There was no marked relationship in the other groups.
Table 2.
Correlation between Homa-IR and hemorheology
| Time | LS (mpa s) | HS (mpa s) | GIT index | FIB (g l−1) | |
|---|---|---|---|---|---|
| Normal control | Before | 0.2505 | 0.2463 | 0.3276 | −0.1318 |
| After | 0.2053 | 0.0778 | 0.3226 | 0.1961 | |
| Model | Before | 0.2935 | 0.2599 | 0.2694 | 0.1432 |
| After | 0.3041 | 0.0466 | 0.1932 | 0.1781 | |
| YYJT | Before | 0.3063 | 0.1394 | 0.1412 | 0.1532 |
| After | 0.5591* | 0.2471 | 0.8797Δ | 0.3009 |
Homa-IR, Homa insulin resistance; LS, low shear rate; HS, high shear rate; GIT, gathering of index test; FIB, fibrinogen.
ΔP-value <0.001; *P-value <0.05.
IFG-II Increased Significantly in the YYJT Group
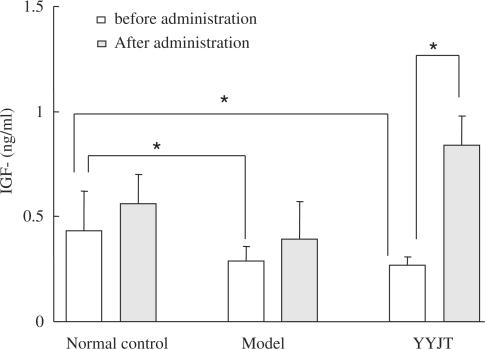
Compared with the control group, IFG-II declined significantly in the DM rats (P < 0.05) and had a negative relationship to HCT (r = −0.69). No difference was noted in the experimental and YYJT groups. After administration, IFG-II increased significantly (P < 0.05) in YYJT group while no changes happened in the other two groups (Fig. 3).
Figure 3.
Levels of IGF-II in the three study groups. *P-value <0.05.
Discussion
In this study, we observed the effects of simple traditional YYJT preparation on FBG, FINS, hemorheology and IGF-II. YYJT preparation (troche) consists of milk vetch, dangshen, Chinese wolfberry fruit, chuanxiong, root of kudzuvine, root of zhejiang figwort and rehmannia. All these components can reinforce Qi, nourish Yin, remove blood gore and promote blood circulation. YYJT is available on the market. The results of this study proved YYJT to be functional in reducing glucose, ameliorating insulin resistance, accelerating beta-cell secretion, downregulating whole blood viscosity at low and high shear rates, GIT and FIB, and upregulating IFG-II.
Over 90% of all DM cases are diagnosed as type 2, caused mostly by insulin resistance and beta-cell destruction. WHO illustrated the onset mechanism of type 2 insulin resistance with insulin hyposecretion and insulin hyposecretion with or without insulin resistance (15,16). Gerich (17), however, considered that beta-cell destruction occurred before insulin resistance in nosogenesis of type 2 DM. In this study, DM was induced in all the rats with Alloxan, which damaged beta-cells, declined insulin secretion and then produced hyperglycosemia. The hyperglycosemia further destroyed insulin function and then insulin resistance formed, leading to worse beta-cell function. Thus, a vicious circle began. All DM rats in this study followed the above onset mechanism of type 2 diabetes mellitus and then were treated with YYJT. Later, their glucose and Homa-IR significantly decreased and beta-cell secretion was improved. This may indicate that YYJT can decrease glucose in association with promoting beta-cell function and improving insulin resistance.
Hemorheologic disorder is the outcome of interaction in the metabolism disorders of glucose, lipid and protein in DM, which accelerates the combined vasculopathy after DM (18–21). The hemorheologic disorder in DM patients is ∼20% higher than that in non-DM patients. The hemorheologic parameters vary in different types and clinical phases of DM, displaying aggregation of erythrocyte and decrease of erythrocyte deformability (22), especially whole blood viscosity at low and high shear rates and HCT. The increase of FIB causes ropier plasma viscosity (23,24). In TCM theory, DM is in the concept of ‘xiao-ke’ with deficiency of Yin and dryness-heat, where the in vivo blocks of blood gore leads to hemorheologic disorder. Such hemokinetic disturbance is defined as pathological changes in TCM. In this study, the hemorheologic parameters were much higher in DM rats than those in the control group before administration, which expressed DM rats had hemokinetic pathological changes. After YYJT administration, the whole blood viscosity at low and high shear rates, GIT and FIB significantly decreased. This might indicate that YYJT could improve hemorheologic disorder through removing blood gore and promoting blood circulation. Insulin resistance is the main factor of secondary angiopathy (25). To discuss the correlation between hemorheology and insulin resistance, we analyzed the relationship of Homa-IR and blood viscosity index. The outcome showed that there was a correlation between Homa-IR and whole blood viscosity at low and high shear rates and GIT, which suggest that the pharmaceutical effect of promoting blood circulation and removing blood stasis of YYJT could regulate insulin resistance to alleviate blood viscosity.
Insulin-like growth factor (IGF) is a polypeptide, classified into IFG-I and IFG-II. It adjusts the proliferation and differentiation of cells with insulin-like metabolism and nutrition (26). The beta-cells in the pancreas of humans and rats contains IFG-II and the alpha-cells contain IFG-I (27). The gene of IFG-II from man and rodents and the gene of insulin are homogenous (28), which signifies that IFG-II plays an important role in beta-cell self-secretion and regulation. We monitored IFG-II during the study, which decreased in DM rats and was negatively related with HCT, indicating its close relation with beta-cell function and blood viscosity. After YYJT administration, the level of IFG-II increased. We assumed that YYJT might promote the secretion of IFG-II to repair or regulate the damaged beta-cells, the exact mechanism however is still unclear and needs further study.
Conclusions
In this study, we showed that traditional medicine with Yin nourishing and promoting blood circulation function can decrease glucose, develop beta-cell function and correct hemorheologic disorder to positively prevent and treat chronic complications of DM in DM rat models, but the long-term efficiency and mechanism is uncertain.
References
- 1.Shen Z. Chronic Complications of Diabetes Mellitus. Shanghai: Shanghai Medical University Press; 1999. pp. 23–6. [Google Scholar]
- 2.Zhu W, Xu J, Song Y. Effect of dengzhanhua injection on capillary dynamics abnormality in patients with diabetes mellitus. J Pract Chinese Mod Med. 2003;3:335–6. [Google Scholar]
- 3.Liu J, Shi D. Study of Chinese herbal drugs in affecting hemorheology promoting blood circulation and removing blood stasis. Chinese J Hemorheol. 2004;14:133–7. [Google Scholar]
- 4.Punitha ISR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ning Y. The clinical research of blood stasis in diabetes mellitus. J Tradit Chin Med. 1991;8:50–3. [Google Scholar]
- 6.Xing M, Kong D, Yan S, Zhu Z. The relation of endotheliotoxin and differentiation of symptoms and signs in “Xiao-ke”. Chinese J Med Drugs. 2000;15:27–30. [Google Scholar]
- 7.Song J. The relation of sputum and gore from biochemical aspect. China J Tradit Chin Med. 2002;8:40–3. [Google Scholar]
- 8.Jing Y, Guan X, Lin W, Zhao J. Changes of hemorheology in diabetes in rats. J Chinese Microcirc. 2002;6:34. [Google Scholar]
- 9.Xu SY, Bian RL, Chen X. Methodology in Pharmacological Experiments. Beijing: People's Medical Publishing House; 1994. [Google Scholar]
- 10.Xi T, Wang LL. Efficiencies studies of Ginkgo biloba extract on diabetes. J China Pharm Univ. 2000;31:285–8. [Google Scholar]
- 11.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 12.Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the Moma model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–41. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- 13.Guan-wei L, Ying-hua S, Wen-ying W, et al. Effect of insulin resistance and insulin secretion on the incidence of type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi. 1998;37:600–4. [Google Scholar]
- 14.Li GW. Function analysis of insulin β cells. Int J Endocrinol Metab. 2003;23:159–63. [Google Scholar]
- 15.Alberti KGM, Zimmet PZ, for the WHO Consultation Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus, provisional report of a WHO consultation. Diabet Med. 1998;15:539. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Luo JZ, Luo L. American ginseng stimulates insulin production and prevents apoptosis through regulation of uncoupling protein-2 in cultured beta cells. Evid Based Complement Alternat Med. 2006;3:365–72. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerich JF. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19:491–503. doi: 10.1210/edrv.19.4.0338. [DOI] [PubMed] [Google Scholar]
- 18.Lin SQ, Fung YC. Changes in the rheological properties of blood vessel tissue remodeling in the course of development of diabetes. Biorheology. 1992;29:443. doi: 10.3233/bir-1992-295-605. [DOI] [PubMed] [Google Scholar]
- 19.Tsushima N. Microcirculation in diabetic microangiopathy. Clin Hemorheol. 1991;11:217. [Google Scholar]
- 20.Sao K, Wang Y, Lu H. Analysis on hemorheological changes in patients with diabetes. Chinese J Hemorheol. 2003;13:77. [Google Scholar]
- 21.Dou Z, Lu Y, Meng L. Analysis of hemorheology in patients with diabetes incorporated with cerebrovascular disease. Mod J Integr Tradit Chin West Med. 2004;13:1454–5. [Google Scholar]
- 22.Tsukada K, Sekizuka E, Oshio C, Minamitani H. Direct measurement of erythrocyte deformability in diabetes mellitus with a transparent microchannel capillary model and high-speed video camera system. Microvasc Res. 2001;61:231–9. doi: 10.1006/mvre.2001.2307. [DOI] [PubMed] [Google Scholar]
- 23.Qi Hua JZ. Metabolic Syndrome. Beijing: People's Medical Publishing House; 2003. pp. 468–9. [Google Scholar]
- 24.Ge-qun Yu JA. Analysis of hemorheology and correlation factors in the Uygurs and Hans with type 2 diabetes. Chinese J Hemorheol. 2004;14:199–210. [Google Scholar]
- 25.Haffner SM. Epidemiology of insulin resistance and its relation to coronary disease. Am J Cardiol. 1999;84:11J–14J. doi: 10.1016/s0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- 26.Near SL, Whalen RL, Miller JA, Ishii DN. Insulin-like growth factor stimulates motor nerve regeneration. Proc Natl Acad Sci USA. 1992;89:11716–20. doi: 10.1073/pnas.89.24.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maake C, Reinecke M. Immunohistochemical localization of insulin-like growth factor1 and 2 in the endocrine pancreas of rat, dog, and man, and their coexistence with classical islet hormones. Cell Tissue Res. 1993;273:249–59. doi: 10.1007/BF00312826. [DOI] [PubMed] [Google Scholar]
- 28.Hansson HA, Edwall D, Lowenadler B, Norstedt G, Paleus S, Skottner A. Isulin-like growth factor in the pancreas of normal and diabetic adult rats. Acta Physiol Scand. 1988;132:569–76. doi: 10.1111/j.1748-1716.1988.tb08367.x. [DOI] [PubMed] [Google Scholar]