Abstract
Herbal therapies are being used increasingly for the treatment of allergic rhinitis. The aim of this study was to investigate the possible pharmacological actions and cellular targets of a Chinese herbal formula (RCM-101), which was previously shown to be effective in reducing seasonal allergic rhinitis symptoms in a randomized, placebo-controlled clinical trial. Rat and guinea pig isolated tissues (trachea and aorta) were used to study the effects of RCM-101 on responses to various mediators. Production of leukotriene B4 in porcine neutrophils and of prostaglandin E2 and nitric oxide (NO) in Raw 264.7 cells were also measured. In rat and guinea pig tracheal preparations, RCM-101 inhibited contractile responses to compound 48/80 but not those to histamine (guinea pig preparations) or serotonin (rat preparations). Contractile responses of guinea pig tracheal preparations to carbachol and leukotriene C4, and relaxant responses to substance P and prostaglandin E2 were not affected by RCM-101. In rat aortic preparations, precontracted with phenylephrine, endothelium-dependent relaxant responses to acetylcholine and endothelium-independent relaxant responses to sodium nitroprusside were not affected by RCM-101. However, RCM-101 inhibited relaxations to l-arginine in endothelium-denuded rat aortic preparations, which had been pre-incubated with lipopolysaccharide. RCM-101 did not affect leukotriene B4 formation in isolated porcine neutrophils, induced by the calcium ionophore A23187; however, it inhibited prostaglandin E2 and NO production in lipopolysaccharide-stimulated murine macrophages (Raw 264.7 cells).The findings indicate that RCM-101 may have multiple inhibitory actions on the release and/or synthesis of inflammatory mediators involved in allergic rhinitis.
Keywords: allergic rhinitis, Chinese herbal medicine, histamine, inducible nitric oxide synthase, inflammation, leukotriene B4, prostaglandin E2, seasonal allergic rhinitis formula (RCM-101)
Introduction
Seasonal allergic rhinitis (SAR), also known as hay fever or pollinosis, is a common allergic condition worldwide (1–3). SAR is an immune response to a wide variety of pollens from grasses, weeds and trees (4). It involves the interaction of the allergens with specific IgE antibodies bound to high-affinity Fcɛ receptors on the surface of mast cells and basophils in the nasal mucosa (5). This induces degranulation of these cells, resulting in the release of a number of mediators, which are responsible for a cascade of symptoms. The early symptoms of SAR, such as sneezing and rhinorrhea, are due to the rapid release of particular mediators, histamine being considered the most significant (6). Other mediators, such as prostaglandins, leukotrienes and nitric oxide (NO) are generally associated with the late phase responses, which predominantly cause nasal obstruction (7).
SAR is usually treated symptomatically with histamine H1 receptor antagonists, sympathomimetic vasoconstrictors and corticosteroids. However, these agents are associated with certain undesirable side effects and, frequently, do not provide complete symptom relief (8–10). Because of the shortcomings of conventional therapies, alternatives have been sought. Certain traditional Chinese herbal formulae have long been used for treating SAR in China and other Asian countries/regions (11,12), although generally their effectiveness has not been critically evaluated. Recently, we conducted a randomized, placebo-controlled clinical trial of a traditional Chinese herbal formula for the treatment of SAR. We demonstrated that the formula, which will be referred to as RCM-101, was effective in reducing SAR symptoms (13). The present study was undertaken to identify possible cellular targets and pharmacological actions that may be involved in the beneficial effects of RCM-101 in SAR.
Methods
RCM-101
RCM-101 contains 18 herbal ingredients, selected by the principles of Chinese medicine theory. Each herb was supplied in a granulated form by the Min Tong Pharmaceutical Company, Taichong, Taiwan. The company holds Good Manufacturing Practice (GMP) certification from the Australian Therapeutic Goods Administration (TGA). Each authenticated, quality certified raw herb was tested for heavy metals and washed and dried. The dried material was extracted in boiling water for 1–1.5 h and the aqueous extract separated by filtration (100 mesh). The extract was heated (50–60°C) under reduced pressure (50–70 mm Hg) to reduce the water content to 60% (2–5 h). The concentrated extract of each herb was individually combined with an excipient (starch) and the product dried and ground into fine granules. For each preparation, 1 g of granulated product was equivalent to 5 g of the raw herb. The granulated herbal preparations were supplied, sterilized, in sealed plastic bottles.
The granulated individual herbal ingredients were combined in the proportions given in Table 1 and encapsulated (500 mg per capsule, New Product Development Pty Ltd, Queensland, Australia). The capsules provided a convenient dose-form for our clinical trial of the formula for SAR (14), such that the dosage of each individual herb was within the range recommended by the Chinese Pharmacopeia (14). For the current study, granules of the herbal formula were taken from the capsules and extracted as described below. All of the herbal ingredients of RCM-101 are listed as active raw herbs for medicines in the Australian Register of Therapeutic Goods.
Table 1.
Composition of RCM-101 (% of granulated herbs by weight*)
| Scientific name | Botanical name | Chinese name | % |
|---|---|---|---|
| Flos Magnoliae | Magnolia liliflora (Desr.) | Xin Yi | 3.81 |
| Frutus Schisandrae Chinensis | Schisandra chinensis (Turcz.) | Wu Wei Zi | 2.25 |
| Frutus Terminaliae Chebulae | Terminalia chebula Retz. | He Zi | 13.87 |
| Frutus Xanthii Sibirici | Xanthii sibirici Patr. Ex Widd. | Cang Er Zi | 7.11 |
| Herba Asari | Asarum sieboldii Miq. | Xi Xin | 3.81 |
| Herba Menthae Haplocalysis | Mentha haplocalyx Briq. | Bo He | 4.68 |
| Herba Schizonepetae Tenuifoliae | Schizonepeta tenuifolia Briq. | Jing Jie | 14.21 |
| Pericappium Citri Reticulatae | Citrus reticulata Blanco | Chen Pi | 9.36 |
| Radix Angelicae Sinensis | Angelica sinensis (Oliv.) Diels | Dang Gui | 4.68 |
| Radix Astragali Membranaceus | Astragalus membranaceus (Fisch.) Bge | Huang Qi | 4.68 |
| Radix Bupleuri | Bupleurum chinense D.C | Chai Hu | 3.81 |
| Radix Codonopsitis pilosulae | Codonopsis pilosula (Franch.) Nannf. | Dang Shen | 2.25 |
| Radix Glycyrrhizae Uralensis | Glycyrrhiza uralensis (Fisch.) | Gan Cao | 4.68 |
| Radix Saposhnikoviae Divaricata | Saposhnikovia divaricata (Turcz.) | Fang Feng | 4.51 |
| Rhizoma Atractylodis Macrocephalae | Atractylodes macrocephala Koidz | Bai Zhu | 4.68 |
| Rhizoma Cimicifugae | Cimicifuga foetida L. | Sheng Ma | 4.68 |
| Rhizoma Ligustici Chuanxiong | Ligusticum chuanxiong (Hort.) | Chuan Xiong | 4.68 |
| Semen Plantaginis | Plantago asiatica L. Wild. | Che Qian Zi | 2.25 |
*One gram of each granulated herb is equivalent to 5 g of the dry weight of the raw herb.
Reagents
5-Hydroxytryptamine (serotonin), histamine sulfate, phenylephrine hydrochloride, acetylcholine chloride, compound 48/80, sodium nitroprusside (SNP), prostaglandin E2, leukotriene C4, substance P, carbamyl-choline-chloride (carbachol), NG-nitro-l-arginine methyl ester (l-NAME), l-arginine, lipolysaccharide Escherichia coli, human recombinant interferon-γ, methysergide maleate, mepyramine maleate, calcium ionophore A23187, Hanks' balanced salt solution, RPMI 1640 medium, fetal bovine serum (FBS), gentamycin and nordihydroguaiaretic acid (NDGA) were obtained from the Sigma Chemical Company (St Louis, MO, USA). The immune-enzyme analysis (IEA) kit, arachidonic acid, prostaglandin B2 (PGB2), leukotriene B4 (LTB4), 6-trans LTB4, 6-trans-12 epi LTB4, 5-hydroxyeicosatetraenoic acid (5-HETE) and 15-hydroxyeicosatetraenoic acid (15-HETE) were obtained from the Cayman Chemical Company (Ann Arbor, MI, USA). HPLC-grade methanol was supplied by Selby-Biolab (Clayton, Victoria, Australia). All other reagents were of analytical quality and obtained from Merck Pty Ltd (Kilsyth Victoria, Australia).
The composition of the physiological salt solution (PSS) was (mM) as follows: NaCl, 118; KCl, 4.7; NaHCO3, 25; MgSO4, 0.45; KH2PO4, 1.03; and CaCl2, 2.5.
Extraction of RCM-101
The granulated combined herbal formula was extracted with distilled water (123 mg ml−1) at room temperature with continuous agitation for 4 h. The aqueous extract was collected by centrifugation (5000 r.p.m. for 10 min) and vacuum filtration. The extract was freeze-dried and the resultant powder redissolved in water in a concentration of 10 mg ml−1. Each 1000 mg of granulated herbal formula yielded a mean of 17.4 ± 2.6 mg of freeze-dried powder. This solution was stored at −20°C, being diluted to the desired concentrations on the day of use.
Functional Studies
All experimental procedures involving animals were approved by RMIT University Animal Ethics Committee and were conducted in conformity with the Australian National Health and Medical Research Council guidelines.
Rats (Sprague-Dawley, 200–300 g) and guinea pigs (Dunkin–Harley, 300–450 g) of both sexes were used. The animals were killed by asphyxiation with 95% CO2 and then decapitated. Aortae and tracheae from rats and tracheae from guinea pigs were removed and set-up in organ baths as described previously (15). In brief, each aorta and trachea was cut into four cylindrical lengths (5 mm) and each ‘ring’ was mounted, vertically, in an 8 ml organ bath containing PSS bubbled with 95% O2 and 5% CO2. The preparations were maintained at 37°C under a resting tension of 2 g wt (aortic rings) or 1 g wt (tracheal rings) and were allowed to equilibrate for 60 min before commencing experimental procedures.
Guinea Pig Tracheal Preparations
The concentration of RCM-101 extract (freeze-dried powder) used in all experiments with guinea pig tracheal preparations was 0.4 mg ml−1. The effects of RCM-101 on contractile responses to histamine, leukotriene C4 (LTC4) and compound 48/80 were investigated separately in tracheal preparations. The effects of the herbal formula on relaxant responses to substance P and prostaglandin E2 (PGE2) were investigated in tracheal preparations that had been precontracted with carbachol (10 μM), again only one agonist being used in each preparation. With LTC4 and PGE2, a submaximal response was elicited in the absence and then in the presence of RCM-101 (LTC4, 30 nM; PGE2, 1 μM). For histamine (0.1–100 μM) and substance P (0.1–10 μM), concentration response relationships were established, first in the absence and then in the presence of RCM-101.
To study the effects of RCM-101 on contractions of guinea pig trachea induced by compound 48/80, in each tracheal preparation, a near maximal response to histamine (30 μM) was first established and then the preparation was challenged once with compound 48/80 (0.125 mg ml−1) (16) in the presence of either RCM-101 extract, vehicle or the H1-histamine antagonist, mepyramine (100 μM). In each case the contractile response to compound 48/80 was expressed as a percentage of the maximal histamine response.
Rat Tracheal Preparations
To investigate the effect of RCM-101 on contractile responses of rat tracheal preparations to serotonin, responses to serotonin (10 μM) were obtained first in the absence and then in the presence of RCM-101 extract. To study the effects of the herbal formula on contractions induced by compound 48/80, in each tracheal preparation, a near maximal response to 10 μM serotonin was established and then the preparation was challenged once with compound 48/80 (0.125 mg ml−1) (16), in the presence of either RCM-101 extract, vehicle or the serotonin antagonist methysergide (100 μM). In each case, the contractile response to compound 48/80 was expressed as a percentage of the response to serotonin. As with guinea pig tracheal preparations, the concentration of RCM-101 extract used in all experiments with rat tracheal preparations was 0.4 mg ml−1.
Rat Aortic Preparations
To investigate the effect of RCM-101 on endothelium-dependent relaxations, cumulative concentration–response relationships to the relaxant actions of acetylcholine (0.03–10 μM) were established in phenylephrine (1 μM)-contracted aortic rings in the absence and then presence of RCM-101 extract (0.04, 0.1 or 0.4 mg ml−1) or vehicle. RCM-101 or vehicle was introduced into the PSS 15 min before re-establishing the concentration–response relationship.
To investigate the effect of RCM-101 on endothelium-independent relaxations, the vascular endothelium was removed by gently rubbing the vessel lumen with a pair of forceps (17). The preparations were then set-up in organ baths as described above, and contracted with 1 μM phenylephrine. Successful endothelium removal was confirmed by the failure of acetylcholine (10 μM) to produce relaxation. Cumulative concentration–response relationships to the relaxant actions of SNP (0.001–1 μM) were established in the absence and then presence of RCM-101 extract (0.04, 0.1 or 0.4 mg ml−1) or vehicle (15 min incubation).
In some preparations, the effect of RCM-101 on inducible nitric oxide synthase (iNOS)-dependent relaxations was investigated as described previously (18). Briefly, endothelium-denuded preparations, as described above, were incubated with lipopolysaccharide (LPS, 100 μg ml−1) for 6 h, and then responses to l-arginine (10, 30 and 100 μM, added cumulatively) were established in phenylephrine (1 μM) contracted preparations, in the absence or presence of RCM-101 extract (0.1, 0.4 mg ml−1) or vehicle. In these experiments RCM-101 was present throughout the incubation with LPS and l-arginine challenges, being introduced into the bathing solution 15 min before contracting the preparations with phenylephrine (except where indicated otherwise).
Cell Culture Studies
Leukotriene B4 Production
Synthesis of leukotriene B4 (LTB4) was induced in neutrophils as described previously (19). Porcine blood was collected from a local abattoir. Neutrophils were isolated using a Percoll gradient and suspended in Hanks' buffer, containing 5 mM HEPES. Suspended neutrophils (2.8 × 106 cells per ml) were incubated (37°C) with arachidonic acid (2.5 μM) together with RCM-101 extract, NDGA or vehicle, for 5 min. Formation of LTB4 was then initiated by adding the calcium ionophore A23187 (2.5 μM) and, 5 min later, the reaction was terminated by adjusting the pH to 3 with citric acid. Prostaglandin B2 (PGB2, 45 ng) and 15-HETE (83 ng) were then added as internal standards. The reaction mixture was extracted with 5 ml of chloroform/methanol (7:3, v/v) and dried under vacuum. The residue was dissolved in 120 μl of the HPLC mobile phase (methanol/water/acetic acid, 76/34/0.08, v/v/v, pH 3.0) and leukotriene metabolites were separated using a Waters HPLC system equipped with an auto sampler, a multisolvent delivery system and a Waters 996 Photodiode Array Detector. Standard curves were prepared for LTB4 (10–200 ng) and 5-HETE (500–800 ng) (in neutrophil suspensions).
Prostaglandin E2 Production
Murine macrophages (Raw 264.7 cells; American Type Culture Collection, Rockville, MD, USA) were grown in RPMI 1640 medium, supplemented with 10% heat-inactivated FBS, 100 μg ml−1 gentamycin, 1.5 g l−1 sodium bicarbonate and 10 mM HEPES, at 37°C, in an atmosphere containing 5% CO2. Cells were subcultured once a week by harvesting them with trypsin/EDTA and seeding them in 75 cm2 flasks. Once confluent, the cells were seeded in 24-well plates (1 × 105 cells per well), in serum-free RPMI medium and then incubated with LPS (1 μg ml−1) for 24 h, in the absence or presence of RCM-101 extract (1, 100 or 1000 μg ml−1). The supernatant was collected and PGE2 was detected using an EIA kit. The assay depends on competition between PGE2 and PGE2–acetylcholinesterase conjugate (PGE2-tracer) for a limited amount of monoclonal PGE2 antibody. The assays were carried out according to the manufacture's protocol, in triplicate. PGE2 release was calculated using software supplied by the kit manufacturer.
NO Production
Raw 264.7 cells were cultured as described above, except that interferon-γ (10 ng ml−1) was added to the wells during incubation with LPS/RCM-101. NO production was measured from the amount of the stable metabolite of NO, nitrite ion. Aliquots of 5 μl of cell culture medium were added to 1 ml of 0.03 M potassium iodide solution, acidified to pH with 0.07 M H2SO4. Nitrite ion was then determined by an electrochemical method using an amiNO-700 sensor and an inNO system (Innovative Instruments Inc., USA). The apparatus was calibrated with acidified nitrite solution, over the range of 50–200 nM, in accordance with the manufacturer's specification. The sensitivity of the sensor was determined to be 123 ± 8.9 pA nM−1 (n = 25).
Statistical Analysis
Data are expressed as means ± standard errors of the mean (SEM). The statistical significance of differences between means was determined by unpaired, two-tailed Student's t-test or, for more than two groups, by first testing for global differences by one- or two-way analysis of variance (ANOVA) and then testing for differences between predetermined pairs of means by Dunnet's test. Probability levels less than 0.05 (P < 0.05) were taken to indicate significant differences. For the 5-lipoxygenase assay, the data were analyzed using Water Millenium Software, Version 3.2, results being expressed as percentage inhibition, the vehicle control being taken as 0% inhibition.
Results
Contractile Responses Induced by Compound 48/80 Markedly Reduced in Guinea Pig Trachea
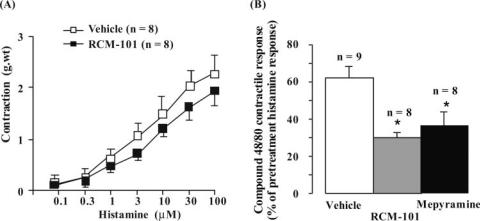
RCM-101 extract (0.4 mg ml−1) did not significantly affect contractile responses to histamine (Fig. 1A). Similarly, contractile responses to carbachol (0.1–30 μM) and LTC4 (30 ng ml−1) were unaltered by RCM-101 in the same concentration (data not shown). Relaxant responses of tracheal preparations, precontracted with 10 μM carbachol, to PGE2 (1 μM) and substance P (0.1–10 μM) were also unaltered by RCM-101 (data not shown). In contrast, as shown in Fig. 1B, contractile responses induced by compound 48/80 (125 μg ml−1) were markedly reduced by RCM-101 (0.4 mg ml−1). The histamine H1 antagonist (100 μM) mepyramine, reduced responses to 48/80 to about the same extent as RCM-101 (Fig. 1B).
Figure 1.
Guinea pig tracheal preparations. (A) Histamine-induced contractions were unaffected by RCM-101 (0.4 mg ml−1). Data are means ± SEM (n = number of preparations). (B) Inhibition of compound 48/80-induced contractions by RCM-101 (0.4 mg ml−1) and mepyramine (100 μM). Responses are expressed as percentages of the response to 30 μM histamine. Data are means ± SEM. *P < 0.05, Student's unpaired t-test.
Contractile Responses Induced by Compound 48/80 Markedly Reduced in Rat Trachea
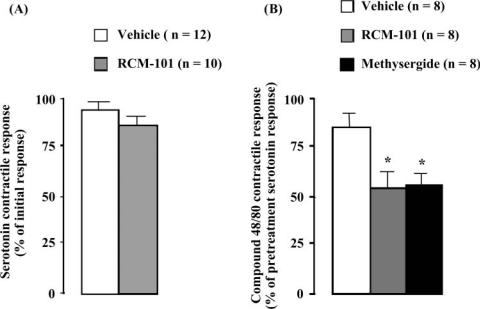
Contractile responses to 10 μM serotonin were not significantly affected by 0.4 mg ml−1 RCM-101 extract (Fig. 2A). However, as with guinea pig tracheal preparations, contractions of rat tracheal preparations induced by compound 48/80 (125 μg ml−1) were significantly reduced by 0.4 mg ml−1 RCM-101 (Fig. 2B). RCM-101 also slowed the rate of onset of contraction of rat tracheal preparations to compound 48/80, the maximum tension developing in 2.6 ± 0.2 min (n = 12) in the vehicle-treated group and in 6.2 ± 0.5 min (n = 8) in the presence of RCM-101. Compound 48/80 responses were also reduced by 100 μM methysergide (Fig. 2B).
Figure 2.
Rat tracheal preparations. (A) Contractions to serotonin (10 μM) were unaffected by RCM-101 (0.4 mg ml−1). Data are means ± SEM (n = number of preparations). (B) Inhibition of compound 48/80-induced contractions by RCM-101 (0.4 mg ml−1) and methysergide (100 μM). Responses are expressed as percentages of pre-treatment serotonin response. Data are means ± SEM. *P < 0.05, Student's unpaired t-test.
Inhibition of iNOS-Mediated Relaxations of Endothelium-Denuded Rat Aortic Preparations to l-Arginine
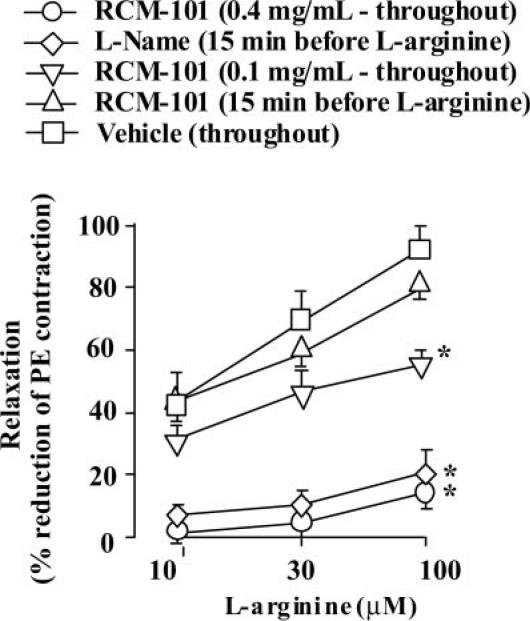
In LPS-treated endothelium-denuded aortic preparations, precontracted with phenylephrine (1 μM), l-arginine (10, 30 and 100 μM) produced concentration-dependent relaxation. The presence of RCM-101 extract (0.4 mg ml−1) during the 6 h incubation with LPS markedly reduced the responses to l-arginine (Fig. 3). A lower concentration of RCM-101 (0.1 mg ml−1) introduced for the LPS incubation, produced smaller reductions in l-arginine responses (Fig. 3). If RCM-101 was only introduced into the tissue bathing solution 15 min before the first exposure to l-arginine, the responses to l-arginine were not significantly different from control responses (Fig. 3). In contrast, l-NAME (100 μM), introduced 15 min before the first exposure to l-arginine, markedly reduced the l-arginine responses (Fig. 3).
Figure 3.
Endothelium-denuded, phenylephrine-contracted rat aortic preparations in which NO synthase was induced by incubation for 6 h with lipopolysaccharide (LPS). RCM-101 (0.1 and 0.4 mg ml−1), when present throughout LPS incubation, inhibited responses to l-arginine. The responses were unaffected by RCM-101 (0.4 mg ml−1) when it was introduced 15 min before the first l-arginine challenge. In contrast, l-NAME (100 μM), introduced 15 min before the first l-arginine challenge, inhibited the responses. Data plotted are means ± SEM (n = number of preparations). *P < 0.05, two-way ANOVA.
The inhibitory effects of RCM-101 (present during LPS incubation) and of l-NAME (introduced after LPS incubation) were only partly reversed by repeated exchanges of the tissue bathing solution over a 5 min period (data not shown).
RCM-101 extract (0.04, 0.1 and 0.4 mg ml−1) did not significantly affect endothelium-dependant relaxations to acetylcholine (0.03–10 μM) or endothelium-independent relaxations to SNP (0.001–1 μM) (data not shown).
LPS-Stimulated Prostaglandin E2 Production in Murine Macrophages Blocked by RCM-101
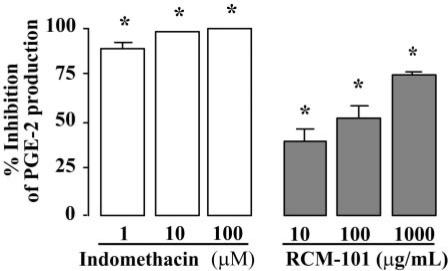
Unstimulated murine macrophage (Raw 264.7) cells incubated in serum-free RPMI medium for 24 h had a baseline concentration of PGE2 of 52 ± 10 pg ml−1. Incubating the cells with LPS (1 μg ml−1) increased the PGE2 level to 7442.4 ± 766 pg ml−1 (n = 6). This was reduced in a concentration-dependent manner by RCM-101 extract (10, 100 and 1000 μg ml−1), when present during incubation with LPS. Indomethacin, present during LPS incubation, in each of the concentrations tested (1, 10 and 100 μM), completely blocked PGE2 production. The data are shown in Fig. 4.
Figure 4.
Inhibition of PGE2 production in LPS-stimulated Raw 264.7 cells by RCM-101 and indomethacin. Data plotted are mean (±SEM) percentage inhibition of control PGE2 production (n = 6 in each case). *P < 0.05, one-way ANOVA and Dunnet's test.
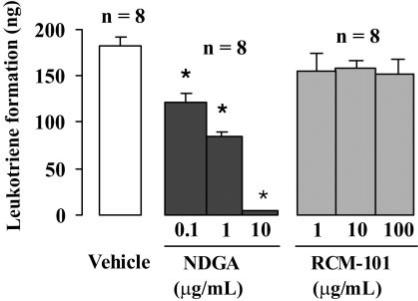
RCM-101 Did Not Affect LTB4 Synthesis in Porcine Neutrophils
As shown in Fig. 5, LTB4 formation in porcine neutrophils was not significantly affected by RCM-101 extract in concentrations of 1, 10 and 100 μg ml−1. In contrast, NGDA (0.1–10 μ g ml−1, produced concentration-dependent inhibition of LTB4 production.
Figure 5.
LTB4 formation in porcine neutrophils was unaffected by RCM-101 (1, 10 and 100 μg ml−1). Nordihydroguaiaretic acid (NDGA; 0.1, 1 and 10 μg ml−1) produced concentration-dependent inhibition of LTB4 formation. Data plotted are means ± SEM (n = 8 in each case). *P < 0.05, one-way ANOVA and Dunnet's test.
Inhibition of LPS/Interferon-γ-Induced NO Production in Murine Macrophages
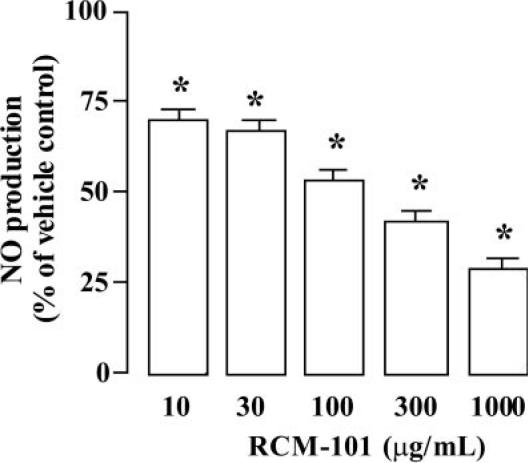
The concentration of NO in the culture medium of unstimulated murine macrophage (Raw 264.7) cells was 46.31 ± 8.0 nM (n = 6). The concentration in the cell culture medium after incubation of RAW 264.6 with LPS and interferon-γ was 768.8 ± 125.5 nM. As shown in Fig. 6, RCM-101 extract (10–1000 μg ml−1) reduced the NO level in a concentration-dependent manner.
Figure 6.
Inhibition of LPS/interferon-γ-stimulated NO production (determined as the stable metabolite nitrite) in Raw 264.7 cells by RCM-101. Data plotted are mean percentage of control NO production (±SEM; n = 6 in each case). *P < 0.05, one-way ANOVA and Dunnet's test.
Discussion
In SAR, mast cell-derived mediators, such as histamine, are generally considered to be responsible for the acute allergic symptoms (20). These mediators act on the smooth muscle cells of small blood vessels, blood platelets, mucous glands and on sensory nerve endings to produce symptoms such as nasal congestion, nasal and throat itching, sneezing and hypersecretion (21).
The present study has demonstrated that a Chinese herbal formula RCM-101, which is effective in the symptomatic treatment of SAR (13), inhibits the contractile responses of rat and guinea pig tracheal preparations to the mast cell activator, compound 48/80. However, the aqueous extract of RCM-101 did not affect contractile responses of tracheal preparations elicited by exogenous histamine (guinea pig preparations) or serotonin (rat preparations), most likely this indicates that RCM-101 inhibits the release of histamine and serotonin in the respective tracheal preparations. The involvement of histamine and serotonin in compound 48/80-induced responses is evidenced by the findings with specific histamine and serotonin antagonists (22). It is noted, however, that although mepyramine and methysergide markedly reduced the effects of compound 48/80 they did not abolish the responses, indicating that mediators other than histamine or serotonin may also be involved. Indeed, given that mast cells contain a variety of inflammatory mediators, in addition to histamine (or serotonin), it is possible that the inhibitory action of RCM-101 on the responses to compound 48/80 may have been due to the blockade of the contractile action of an agent or agents other than histamine (or serotonin).
Previous studies suggest that prostaglandins and leukotrienes are likely to be involved in the late phase symptoms of SAR (8). For example, serotonin induces sneezing and rhinorrhea without congestion while leukotrienes and prostaglandins cause congestion without rhinorrhea (9). Among different leukotrienes, LTB4 is released during the immediate phase response by infiltrating neutrophils (23).
PGE2 has been shown to be involved in both the early and late phases of SAR. In the early phase, PGE2 is released from mast cells whilst, in the late phase, it is released from basophils and eosinophils (20,24). In the present study, in which production of PGE2 by murine macrophages was initiated by LPS, RCM-101 inhibited PGE2 production. The exact mechanism involved in the inhibition was not investigated; however, it may involve modification of cyclooxygenase (COX) activity or of expression of the enzyme. There are two isoforms of COX, and the isoform responsible for the induced PG production is COX-2. Previous studies have shown that the herbs Rhizome Cimicifugae and Radix Glycyrrhizae, which are constituents of RCM-101, have potent COX2 inhibitory effects (25). In addition, Radix Glycyrrhizae is known to have anti-inflammatory activity. Both topical and oral administration glycyrrhetinic acid, a constituent of Radix Glycyrrhizae, prevent ear edema and to inhibits PGE2 and LTC4 formation in mice, induced by arachidonic acid, with approximately the same potency as NDGA (26).
NO is an important mediator in various inflammatory processes (27–30). In SAR, the level of NO in the parasinus is dependent on iNOS (31), and there is evidence that exhaled NO is elevated in subjects with allergic rhinitis (32,33). It has been suggested that increased production of NO in the nasal mucosa may promote vasodilatation and modulate the responsiveness/activity of sensory nerves, leading to nasal congestion, rhinorrhea and sneezing (30). The present finding of inhibition by RCM-101 of l-arginine-induced vasodilatation in LPS-treated aortic rings indicates that RCM-101 may inhibit iNOS-dependent NO production, since the responses to exogenous NO were not affected by RCM-101. The finding that RCM-101 was without effect on responses of rat aortic preparations when it was introduced after the preparations had been incubated for a prolonged period with LPS (15 min before l-arginine), indicates that unlike l-NAME (NOS inhibitor), it does not interfere with the action of preformed iNOS. Previous studies in denuded rat aorta have demonstrated induction of NO synthase by LPS, leading to increased production of NO (34). The finding that the herbal formula inhibited LPS/interferon-γ-induced NO production by murine macrophage cells further supports the suggestion that RCM-101 inhibits iNOS-dependent NO production. Further studies will be necessary to elucidate the exact mechanism involved in inhibition of iNOS-mediated NO production by RCM-101, which may involve modification of existing enzyme activity or altered expression of iNOS protein or its gene.
The present findings with RCM-101 are consistent with previous studies on some of the individual herbal ingredients of the formula. Naturally occurring furanocoumarins (imperatorin and deltoin), isolated from Radix Saposhnikoviae, inhibited iNOS protein expression but not the enzyme itself (35). Polysaccharides from Angelica sinensis have also been shown to reduce the levels of endogenous NO and the expression of iNOS (36). Luteolin, which is present in Herba Schizonepetae (37), markedly lowers NO production by reducing iNOS enzyme expression without inhibiting the enzyme activity (38). Polyacetylenes isolated from Radix Saposhnikoviae were found to inhibit NO production induced by LPS/interferon-γ in macrophages (35). In addition, costunolide, found in Magnolia grandiflora, reduced NO production by downregulating iNOS mRNA. It also reduced iNOS expression by inhibiting binding of the transcription factor nuclear factor-kappa B (NF-κB). Costunolide was also shown to inhibit phosphorylation of IκB (39), thereby enhancing its inhibitory action on NF-κB. Radix Glycyrrhizae increases blood cortisone levels (40), which may, in turn, inhibit iNOS gene transcription by decreasing NF-κB activity and inducing IκB-α (41). Thus, it is likely that multiple ingredients of RCM-101 may be involved in its inhibition of iNOS-dependent NO production. RCM-101 did not inhibit eNOS-dependent relaxations to acetylcholine or direct relaxations to the NO donor, SNP. The inhibition of the iNOS pathway by RCM-101 indicates that it selectively interferes with inflammatory processes in the late phase of SAR (26).
In conclusion, the results obtained in this study indicate that RCM-101 may have multiple pharmacological actions including inhibition of histamine release from mast cells, inhibition prostaglandin formation and inhibition of inducible NO synthase-mediated NO production/response in target cells. The exact inhibitory mechanisms and which herbal constituents contribute to these effects require further investigation.
Acknowledgments
The authors would like to acknowledge RMIT University for providing a Postgraduate Research Scholarship for G.L.
References
- 1.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific quality of life questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 2.Crown WH, Olufade A, Smith MW, Nathan R. Seasonal versus perennial allergic rhinitis: drug and medical resource use patterns. Value Health. 2003;6:448–56. doi: 10.1046/j.1524-4733.2003.64231.x. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58:691–706. doi: 10.1034/j.1398-9995.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 4.van Cauwenberge P. Management of rhinitis—the specialist's opinion. Clin Exp Allergy. 1998;28(Suppl 6):29–33. doi: 10.1046/j.1365-2222.1998.0280s6029.x. [DOI] [PubMed] [Google Scholar]
- 5.Baraniuk JN. Pathogenesis of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S763–72. doi: 10.1016/s0091-6749(97)70125-8. [DOI] [PubMed] [Google Scholar]
- 6.Van Cauwenberge P, Watelet JB, Bachert C. New insights in the pathogenesis of allergic rhinitis. Curr Probl Dermatol. 1999;28:95–101. doi: 10.1159/000060605. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly A, Casale TB. Nedocromil sodium is rapidly effective in the therapy of seasonal allergic rhinitis. J Allergy Clin Immunol. 1993;91:997–1004. doi: 10.1016/0091-6749(93)90212-x. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo M, Castel-Branco MG, Oliveira JF, Ramos E, Delgado L, Almeida J. Double-blind comparison of levocabastine eye drops with sodium cromoglycate and placebo in the treatment of seasonal allergic conjunctivitis. Clin Exp Allergy. 1991;21:689–94. doi: 10.1111/j.1365-2222.1991.tb03197.x. [DOI] [PubMed] [Google Scholar]
- 9.Davies RJ, Bagnall AC, McCabe RN, Calderon MA, Wang JH. Antihistamines: topical vs oral administration. Clin Exp Allergy. 1996;26:11–7. doi: 10.1111/j.1365-2222.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 10.Andersson M, Berglund R, Greiff L, Hammarlund A, Hedbys L, Malcus I, et al. A comparison of budesonide nasal dry powder with fluticasone propionate aqueous nasal spray in patients with perennial allergic rhinitis. Rhinology. 1995;33:18–21. [PubMed] [Google Scholar]
- 11.But P, Chang C. Chinese herbal medicine in the treatment of asthma and allergies. Clin Rev Allergy Immunol. 1996;14:253–69. doi: 10.1007/BF02802218. [DOI] [PubMed] [Google Scholar]
- 12.Kaphle K, Wu LS, Yang NY, Lin JH. Herbal medicine research in Taiwan. Evid Based Complement Alternat Med. 2006;3:149–55. doi: 10.1093/ecam/nek016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue CC, Thien FC, Zhang JJ, Da Costa C, Li CG. Treatment for seasonal allergic rhinitis by Chinese herbal medicine: a randomized placebo controlled trial. Altern Ther Health Med. 2003;9:80–7. [PubMed] [Google Scholar]
- 14.Pharmacopoeia of The People's Republic of China. Beijing: Chemical Industry Press; 2000. [Google Scholar]
- 15.Li CG, Rand MJ. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991;102:91–4. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikawati Z, Hayashi M, Nose M, Maeyama K. The lack of compound 48/80-induced contraction in isolated trachea of mast cell-deficient Ws/Ws rats in vitro: the role of connective tissue mast cells. Eur J Pharmacol. 2000;402:297–306. doi: 10.1016/s0014-2999(00)00482-9. [DOI] [PubMed] [Google Scholar]
- 17.Griffiths MJ, Messent M, MacAllister RJ, Evans TW. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993;110:963–8. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binko J, Meachem S, Majewski H. Endothelium removal induces iNOS in rat aorta in organ culture, leading to tissue damage. Am J Physiol. 1999;276:E125–34. doi: 10.1152/ajpendo.1999.276.1.E125. [DOI] [PubMed] [Google Scholar]
- 19.Betts WH, Hurst NP, Murphy GA, Cleland LG. Auranofin stimulates LTA hydrolase and inhibits 5-lipoxygenase/LTA synthase activity of isolated human neutrophils. Biochem Pharmacol. 1990;39:1233–7. doi: 10.1016/0006-2952(90)90268-p. [DOI] [PubMed] [Google Scholar]
- 20.Naclerio RM. Allergic rhinitis. N Engl J Med. 1991;325:860–9. doi: 10.1056/NEJM199109193251206. [DOI] [PubMed] [Google Scholar]
- 21.Kuby J. Immunology. 2nd edn. New York, USA: W.H. Freemen and Company; 1994. [Google Scholar]
- 22.Joos GF, Lefebvre RA, Bullock GR, Pauwels RA. Role of 5-hydroxytryptamine and mast cells in the tachykinin-induced contraction of rat trachea in vitro. Eur J Pharmacol. 1997;338:259–68. doi: 10.1016/s0014-2999(97)81929-2. [DOI] [PubMed] [Google Scholar]
- 23.Bousquet J, Vignola AM, Campbell AM, Michel FB. Pathophysiology of allergic rhinitis. Int Arch Allergy Immunol. 1996;110:207–18. doi: 10.1159/000237289. [DOI] [PubMed] [Google Scholar]
- 24.Mygind N, Dahl R, Pedersen S, Thestrup-Pedersen K. Essential Allergy. 2nd edn. Carlton: Blackwell Science; 1996. [Google Scholar]
- 25.Kim SJ, Kim MS. Inhibitory effects of Cimicifugae rhizoma extracts on histamine, bradykinin and COX-2 mediated inflammatory actions. Phytother Res. 2000;14:596–600. doi: 10.1002/1099-1573(200012)14:8<596::aid-ptr731>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Inoue H, Mori T, Shibata S, Koshihara Y. Inhibitory effect of glycyrrhetinic acid derivatives on arachidonic acid-induced mouse ear oedema. J Pharm Pharmacol. 1988;40:272–7. doi: 10.1111/j.2042-7158.1988.tb05242.x. [DOI] [PubMed] [Google Scholar]
- 27.Durland WF, Lane AP, Durland KW, Smith TL, Johnson KL, Prazma J, et al. Nitric oxide is a mediator of the late-phase response in an animal model of nasal allergy. Otolaryngol Head Neck Surg. 2000;122:706–11. doi: 10.1016/S0194-5998(00)70201-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawamoto H, Takeno S, Yajin K. Increased expression of inducible nitric oxide synthase in nasal epithelial cells in patients with allergic rhinitis. Laryngoscope. 1999;109:2015–20. doi: 10.1097/00005537-199912000-00023. [DOI] [PubMed] [Google Scholar]
- 29.Watkins DN, Lewis RH, Basclain KA, Fisher PH, Peroni DJ, Garlepp MJ, et al. Expression and localization of the inducible isoform of nitric oxide synthase in nasal polyp epithelium. Clin Exp Allergy. 1998;28:211–9. doi: 10.1046/j.1365-2222.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- 30.Arnal JF, Didier A, Rami J, M'Rini C, Charlet JP, Serrano E, et al. Nasal nitric oxide is increased in allergic rhinitis. Clin Exp Allergy. 1997;27:358–62. [PubMed] [Google Scholar]
- 31.Kharitonov SA, Rajakulasingam K, O'Connor B, Durham SR, Barnes PJ. Nasal nitric oxide is increased in patients with asthma and allergic rhinitis and may be modulated by nasal glucocorticoids. J Allergy Clin Immunol. 1997;99:58–64. doi: 10.1016/s0091-6749(97)70301-4. [DOI] [PubMed] [Google Scholar]
- 32.Martin U, Bryden K, Devoy M, Howarth P. Increased levels of exhaled nitric oxide during nasal and oral breathing in subjects with seasonal rhinitis. J Allergy Clin Immunol. 1996;97:768–72. doi: 10.1016/s0091-6749(96)80154-0. [DOI] [PubMed] [Google Scholar]
- 33.Arnal JF, Flores P, Rami J, Murris-Espin M, Bremont F, Pasto IAM, et al. Nasal nitric oxide concentration in paranasal sinus inflammatory diseases. Eur Respir J. 1999;13:307–12. doi: 10.1034/j.1399-3003.1999.13b15.x. [DOI] [PubMed] [Google Scholar]
- 34.Moritoki H, Hisayama T, Kida K, Kondoh W, Inoue S, Takaishi Y. Inhibition by triptoquinone-A of LPS and IL-1 beta-primed induction of NO synthase in rat thoracic aorta. Life Sci. 1996;59:L49–54. doi: 10.1016/0024-3205(96)00283-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang CC, Chen LG, Yang LL. Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett. 1999;145:151–7. doi: 10.1016/s0304-3835(99)00248-7. [DOI] [PubMed] [Google Scholar]
- 36.Ding H, Peng R, Yu J. [Modulation of Angelica sinensis polysaccharides on the expression of nitric oxide synthase and Bax, Bcl-2 in liver of immunological liver-injured mice] Zhonghua Gan Zang Bing Za Zhi. 2001;9(Suppl):50–2. [PubMed] [Google Scholar]
- 37.Zhou J, Yan X, Xie G. Traditional Chinese Medicine: Molecular Structures, Natural Sources and Application. Hampshire, England: Ashgate; 2003. [Google Scholar]
- 38.Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem Pharmacol. 1999;58:759–65. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 39.Koo TH, Lee JH, Park YJ, Hong YS, Kim HS, Kim KW, et al. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-kappa B by targeting I kappa B phosphorylation. Planta Med. 2001;67:103–7. doi: 10.1055/s-2001-11503. [DOI] [PubMed] [Google Scholar]
- 40.Kase Y, Saitoh K, Makino B, Hashimoto K, Ishige A, Komatsu Y. Relationship between the antidiarrhoeal effects of Hange-Shashin-To and its active components. Phytother Res. 1999;13:468–73. doi: 10.1002/(sici)1099-1573(199909)13:6<468::aid-ptr504>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 41.Matsumura M, Kakishita H, Suzuki M, Banba N, Hattori Y. Dexamethasone suppresses iNOS gene expression by inhibiting NF-kappaB in vascular smooth muscle cells. Life Sci. 2001;69:1067–77. doi: 10.1016/s0024-3205(01)01196-1. [DOI] [PubMed] [Google Scholar]