Abstract
Distress-mediated tissue oxidative stress was examined as a model of sub-healthy condition defined in traditional Chinese medicine theory. Mice were subjected to psychologically stressful conditions by whiskers removal. Under this condition, spontaneous locomotive activity was significantly enhanced in the dark (P < 0.05 versus the control mice in three different movements), and granulocytes/lymphocytes balance shifted to granulocytes. At the same time, peroxynitrite level in blood plasma increased to ∼180% from that of the control mice at 6 h after removal of the whiskers (P < 0.01), and was maintained even after 12 h. Both protein carbonyl formation and lipid peroxidation were significantly increased under this condition in brain, heart, liver and spleen at 6 h after removal of whiskers (P < 0.05 or P < 0.01), and these levels were maximized after 12 h (increased to 120–160%, P < 0.05 or P < 0.01). The oxidative tissue injuries observed at 12 h after the removal of the whiskers were effectively prevented by two traditional Chinese medicine formula: Shengmai San (SMS) and Ling Gui Zhu Gan Tang (LGZGT), when administered for 5 days before the removal of the whiskers. Therefore, this stress model is considered useful in assessing the preventive potential of antioxidants and antioxidant-based herbal mixtures in treating the pathophysiology associated with psychological or emotional distress.
Keywords: antioxidant-based herbal medicine, oxidative stress, peroxynitrite, psychological stress, sub-healthy condition, traditional Chinese medicine
Introduction
Currently, psychological distress has attracted attention with regards to its significant negative effects in treating various diseases, especially cancer (1–4). There are a variety of stress-induced hemodynamic changes that inhibit cellular immune responses and are relevant to cancer prognosis, e.g. NK cytotoxicity and T-cell responses (1,5,6). The stressors can also induce myocardial ischemia and ventricular arrhythmias in patients with coronary artery diseases (7,8), which associate with oxidative stress. It is thus suggested that psychological stress is associated with increased oxidant production and oxidative damage, and thus long-term exposure to psychological stressors may enhance the risk of many diseases (5,9). In this sense, psychological stress-induced pathophysiology is similar to the so-called sub-healthy (or pre-disease) condition defined in the ancient theory of traditional Chinese medicine (TCM). The sub-healthy condition describes one condition that is not completely healthy but is also not ill, and therefore, Western medicine usually fails to give an appropriate diagnostic disease name. The prevention and repair of this specific condition is the basic strategy of TCM, and this idea should be re-evaluated for its importance in the current preventive treatment of lifestyle and age-related complex pathophysiologies associated with oxidative stress. Therefore, the oxidative stress associated with psychological and/or emotional stress might be an appropriate target for assessing the preventive potentiality of food supplements or TCM formulae that are used as alternative medicines.
Several animal stress models of various stressors such as immobilization, burn shock and cold-restraint have been developed, and all cause oxidative lipid, protein and DNA damages in tissues (10–12). However, almost all of them are accompanied by physical abuse in addition to psychological or emotional stressors.
In the present paper, we will show that the simple cut of whiskers causes psychological or emotional distress in mice leading to oxidative stress in tissues. Whiskers play a critical role as a locomotive sensor in mice, and thus, sensory input is directly connected to motor neurons controlling their locomotive activity (13). Therefore, the removal of whiskers may affect their locomotive behavior causing anxiety on hyperlocomotion, and thus, psychological or emotionally stressed condition. This model thus mimics a psychological or emotional stress as a sub-healthy condition.
Using this animal model, TCM formulae, Shengmai San (SMS) and Ling Gui Zhu Gan Tang (LGZGT) were examined as models of antioxidant-based alternative medicine against complex diseases associated with oxidative stress. SMS is a TCM formula comprising three herbal components: Panax ginseng, Ophiopogon japonicus and Schisandra chinensis, and is traditionally used for treating heart diseases (14–16). LGZGT, on the other hand, is composed of four herbal components, Hoelen, Cinnamomi cortex, Atractylodis rhizoma and Glycyrrhizae radix, and is often used for diseases related to edema, such as chronic bronchitis, congestive heart failure and chronic nephritis (17). Moreover, both TCM formulae were previously shown to have high potentiality for preventing cerebral oxidative stress in experimental animal models (18–23).
Materials and Methods
Reagents
Dihydrorhodamine 123 (DHR 123) and rhodamine 123 were obtained from Molecular Probes Inc., USA. Mouse monoclonal IgE against 2,4-dinitrophenylhydrazine (DNPH) was obtained from Sigma Co., Ltd, USA. Rat anti-mouse IgE conjugated to horseradish peroxidase (HRP) was obtained from SBA Inc., USA. 3,3′,5,5′-Tetramethylbenzidine (TMB) was obtained from Bio-Rad Laboratories, USA. All other reagents were purchased from Wako Pure Chemical Industries Co. Ltd, Japan.
TCM prescriptions, SMS and LGZGT, which are commercially available granules, were generously provided by Iskra Co. Ltd, Japan, and by Kotaro Co. Ltd, Japan, respectively.
Animal Treatment
Male DDY 6-week-old mice were purchased from SLC Inc. (Japan). The mice were divided into four groups (control, stressed, TCM + stressed and TCM, n = 6) and habituated in a cage for a week at 24°C with a 12 h dark/light cycle (light cycle started from 7 a.m. and ended at 7 p.m.) under free access to a normal composite diet (KIC lab MR stock Co. Ltd, Japan) and water. To examine the oxidative stress induced by psychological stress, each mouse was seized by hand and the whiskers around the nose and mouth were completely cut-off with the scissors without the use of anesthesia. Yet, for the control group, the same treatment was administered without cutting the whiskers. After the removal of whiskers, the mice were kept in a cage under free access to diet and water. The mice were sacrificed under anesthesia with pentobarbital at 6, 12 and 24 h after the removal of the whiskers (whiskers were removed at 12 p.m. for a 6 h stress exposure experiment, and at 9 p.m. for the 12 and 24 h stress exposure experiments), and blood samples were withdrawn from the abdominal vein with a heparinized syringe. The brain and other organs were removed for biochemical assay immediately after blood sampling.
All the animal experiments were carried out under the guidance of NUPALS Animal Regulation Code.
To study the protective effect of TCMs on psychological stressor-mediated tissue injuries, Granulated SMS (Isukura Co. product) and LGZGT (Kotaro kampo Co. product) were dissolved in H2O to make 0.5 g ml−1 solution, and 0.5 ml of each solution was orally administered to the mice with a syringe once a day for 5 days before stress was induced by whiskers removal. The dose corresponded in an ∼20 g original dry herb per kg body weight per day. For the control mice group, the same volume of H2O was given instead of TCM samples. The dose administered was determined according to the following formula: Dm (dose per kg body weight) = Dh × Rm/Rh × (Wh/Wm)1/3 given in the textbook The methodology of Pharmacological Experiment (24), where Dm and Dh are the doses of the mouse and the human. Wh and Wm are the body weights of the human and the mouse, respectively. Rh and Rm are the coefficients for the human and the mouse, and 0.1 and 0.059, respectively.
The Measurement of Behavioral Movement Changes
The locomotive activity of mice after whiskers removal were monitored using an automatic locomotive behavior monitoring system equipped with infrared (IR) sensors (Scant Series Infrared, Co. Ltd, Japan). Three types of movement, ‘small movements (moving area smaller than body length)’, ‘large movements (moving area larger than body length)’ and ‘rearing’ were recorded with a group of 5 mice for 12 h in the dark after whiskers removal.
Measuring Peroxynitrite in the Blood Plasma after Whiskers Removal Using a DHR123 Fluorescence Probe
Peroxynitrite formation in blood plasma was determined by rhodamine 123 fluorescence, which was produced by oxidation of intravenously injected DHR 123 (25). DHR 123 (3 μmol kg−1 body weight) as a 0.2 ml saline solution was injected into the tail vein of mice at 6, 12 and 24 h after whiskers removal under anesthetized condition with pentobarbital. The same volume of saline was injected for the control group. Blood samples were then withdrawn from the abdominal vein using a heparin-rinsed syringe at 20 min after DHR 123 injection and centrifuged at 3000 r.p.m. for 5 min at 4°C. The blood plasma was placed in a 96-well micro plate and measurements of fluorescence intensity of rhodamine 123 at 527 nm were obtained using a micro plate reader (Fluoroscan Ascent) after excitation at 485 nm. Rhodamine 123 formed in plasma was quantified by a standard curve prepared with 1–50 nM rhodamine 123 mixed with an aliquot of blood plasma obtained from normal mice.
Leukocyte Analysis
Blood recovered from the abdominal vein with a heparinized syringe was immediately spread onto a glass slide, then fixed with glutaraldehyde. Leucocyte counting was carried out under light microscopy after Giemsa and peroxidase staining (26). More than 100 cells were counted per blood sample for 5 mice for each experimental group (Control, Stressed, SMS and SMS/stressed).
Assessing of Protein Carbonyl and Thiobarbituric Acid Reactive Substance Formation
Tissues (brain, liver, heart and spleen) were removed immediately after blood sampling, rinsed in ice-cold 0.9% NaCl, then was frozen at −80°C until use. Tissues were suspended in ice-cold 0.05 M phosphate buffer containing 1.15% (w/v) KCl (1 g wet tissue per 9 ml), and homogenized in ice using an Ultra Turrax T8 homogenizer. The protein carbonyl contents were measured by ELISA (27). A standard curve was prepared using oxidized bovine serum albumin (BSA) obtained by oxidation of BSA with Cu2+/H2O2 (500 μM per 5 mM). The carbonyl content of the oxidized BSA standard was determined using the colorimetric method reported previously (28). Tissue homogenates were centrifuged at 4000 r.p.m. for 10 min to remove any debris and the supernatant was incubated with 1% streptomycin sulfate for 15 min. The supernatant, which was obtained, was determined for protein content using the bicinchoninic acid (BCA) method with bovine BSA as the standard (29). Protein concentration was adjusted to 1 mg ml−1 with PBS and then reacted with 10 mM DNPH in 2 N HCl at room temperature for 1 h. The protein was precipitated with 7% trichloroacetic acid (TCA), after which the precipitate was solubilized in PBS and protein concentration was determined again. A standard ELISA curve was prepared for oxidized BSA that was diluted sequentially with intact BSA at a defined ratio (0–40%). Aliquots (100 μl) of test samples and standards (4 μg as protein) were placed in 96-well plastic plates, respectively, and incubated overnight at 4°C. The plates were then washed with PBS containing 0.1% Tween 20 (PBST), and incubated with blocking buffer (1% BSA in PBST) for 2 h at room temperature. Samples were incubated for an additional 4 h with a primary antibody (anti-DNP Mouse IgE; Sigma), washed with PBST then reacted with a secondary antibody (anti-mouse IgE Rat IgG HRP conjugate; SBA) for 1 h. Peroxidase reaction was performed with the addition of TMB (Bio-Rad Lab) and stopped with the addition of 0.18 M H2SO4. Absorbance was measured at 450 nm using a Bio-Rad model 550 micro plate reader. Thiobarbituric acid reactive substance (TBARS) formation was determined as reported previously (30).
Statistical Analysis
The data were analyzed by SPSS10.0 software combined with the Duncan's multiple range test. Results are given as the means ± standard deviation (SD). The level of statistical significance was taken as P < 0.05.
Results
Effect of Whiskers Cut Stress on Locomotive Activities of Mice
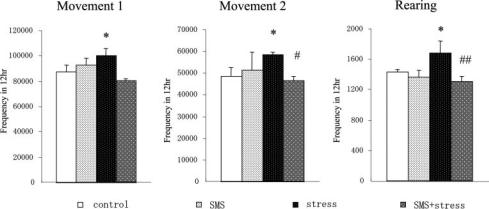
The effect of whiskers removal was first examined by observing spontaneous locomotive activity of mice since it was reported that spontaneous locomotive activity was enhanced under psychological stressed condition (31). Frequency counts of all types of behavioral locomotion (small, large and rearing) for a group of five mice recorded for 12 h in the dark after whiskers removal were significantly enhanced during the first 6 h after whiskers removal as shown in Fig. 1. The total counts of all types of locomotion of the five mice as one group increased by ∼20% after whiskers cut indicating that the mice were led to psychologically distressed condition by the deficit of locomotive sensor (13). It is interesting to note that SMS pre-administration alone did not significantly alter locomotive activity of normal mice, but inhibited hyperlocomotive activity induced by whiskers removal.
Figure 1.
Effect of whiskers cut stress and Shengmai San on locomotive activities of mice at night. SMS was orally administered to mice once a day for 5 days, after which the mice were distressed by whiskers removal. Three types of behavioral movements (small and large movements, and rearing) were then recorded in five mice for 12 h at night. Values represent the means ± SD (frequency within 12 h per five mice) of three independent experiments. N = 5. *P < 0.05, **P < 0.01 compared to the control (unstressed) mice; #P < 0.05, ##P < 0.01 compared to the stressed mice.
Change of Granulocyte and Lymphocyte Ratio under Psychological Stress-Induced by Whiskers Removal in Mice
Granulocytes play a crucial role in oxidative stress and the leukocyte profile varies depending on physiological conditions, especially under stressful conditions (32). In the present whiskers cut model, the granulocytes/lymphocytes ratio also tended to increase (Table 1), indicating that whiskers cut worked as a psychological stressor in mice.
Table 1.
Change of granulocyte and lymphocyte ratio under psychological stress induced by whiskers removal in mice
| Relative % | G/L | ||
|---|---|---|---|
| Granulocyte | Lymphocyte | ||
| Control | 50.0 ± 15.0 | 50.0 ± 15.0 | 1.0 |
| Stressed | 58.0 ± 15.2 | 42.0 ± 15.2 | 1.4 |
| SMS | 60.7 ± 9.1 | 39.3 ± 9.1 | 1.5 |
| LGSGT | 58.2 ± 8.1 | 42.0 ± 15.2 | 1.4 |
Tissue of Oxidative Stress-Induced by Whisker Removal in Mice
Since many stressors are known to induce oxidative stress (10–12), tissue oxidative injuries were measured to determine whether the oxidative stress occurs as well under this psychologically distressed condition. Cellular oxidative markers for lipid (TBARS) and protein (carbonyl formation) were significantly increased in the tissues or organs at 6 h after whiskers removal. This oxidative stress was maximized at 12 h and then decreased at 24 h, probably due to adaptation (Fig. 2A and B).
Figure 2.
Tissue oxidative stress induced by whiskers removal in mice. Tissues were removed 6, 12 and 24 h after whiskers removal and then subjected to oxidative damage assays (protein carbonyl (A) and TBARS (B) formation). Data represent means ± SD (n = 6 mice). *P < 0.05 or **P < 0.01 versus the control (unstressed) mice.
Production of Peroxynitrite in Plasma of Mice after Whiskers Removal
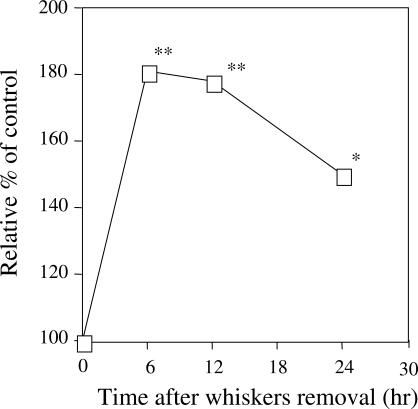
At the same time, peroxynitrite level in blood plasma increased significantly after whiskers removal, as determined by using DHR 123 as a probe that produces fluorescence when oxidized into rhodamine 123 by peroxynitrite (25). The results revealed that plasma levels of peroxynitrite increased to ∼180% to that of the control at 6 h after whiskers removal. And this level was maintained until 12 h after whisker removal. Even after 24 h, the peroxynitrite level was 150% to that of the control (Fig. 3); thus, indicating that increased plasma peroxynitrite level is one of the causative factors of multiple oxidative tissue injuries.
Figure 3.
Production of peroxynitrite in plasma of mice after whiskers removal. Peroxynitrite production in the plasma was determined at 6, 12 and 24 h after whiskers removal through DHR 123 injections 20 min before sacrificing. Data represent means ± SD (n = 6 mice). *P < 0.05 or **P < 0.01 versus the control (unstressed) mice.
Preventive Effects of TCM on Protein Carbonyl and TBARS Formation in Tissues Under Whiskers Cut Stress in Mice
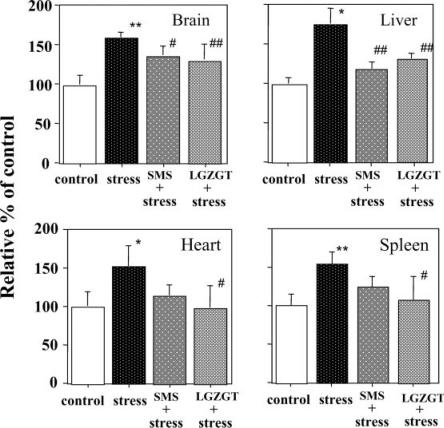
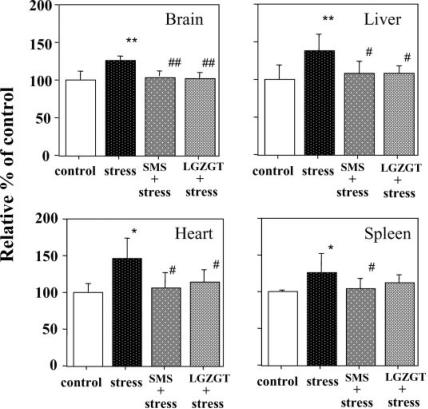
To examine the possible applicability of this stress model in evaluating therapeutic potentiality of TCM against stress-induced disorders, two TCM formulas, SMS and LGZGT, were examined at the dose of 20 g dry herbs per kg body weight per day. SMS and LGZGT were administered orally for 5 days before whiskers removal. The oxidative injuries observed in all the tissues examined were remarkably suppressed in the group of mice pre-treated with either TCM as they are shown in Figs 4 and 5 when assessed by TBARS (Fig. 4) and protein carbonyl (Fig. 5) formations.
Figure 4.
Preventive effects of TCM on protein carbonyl formation in tissues under whiskers cut stress in mice. SMS or LGZGT was orally administered once a day for 5 days before whiskers removal. At 12 h after whiskers removal, tissues were removed to determine protein carbonyl (gray bar) and TBARS (black bar) formation. Data represent means ± SD (n = 6 mice). *P < 0.05 or **P < 0.01 versus the untreated control mice. #P < 0.05 or ##P < 0.01 versus stressed mice.
Figure 5.
Preventive effects of TCM on TBARS formation in tissues under whiskers cut stress in mice. SMS or LGZGT were the same as in Fig. 4. At 12 h after whiskers removal, tissues were removed to determine TBARS. Data represent means ± SD (n = 6 mice). *P < 0.05 or **P < 0.01 versus the untreated control mice. #P < 0.05 or ##P < 0.01 versus stressed mice.
Inhibition of Stress-Induced Plasma Peroxynitrite Production by TCM
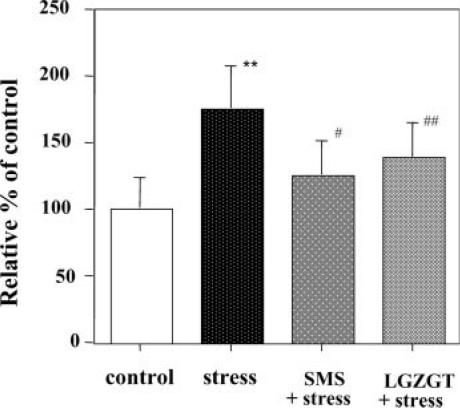
At the same time, the plasma peroxynitrite level was also maintained low in mice pre-treated with both TCM formulas (Fig. 6). These results indicated the usefulness of these formulae in modulating a physiological antioxidant potential against the oxidative abuse caused by psychological stress.
Figure 6.
Inhibition of stress-induced plasma peroxynitrite production by TCM. SMS or LGZGT was orally administered once a day for 5 days. At 12 h after whiskers removal, plasma was subjected to a DHR 123 oxidation assay for peroxynitrites. Data represent means ± SD (n = 6 mice). *P < 0.05 or **P < 0.01 versus the untreated control mice. #P < 0.05 or ##P < 0.01 versus stressed mice.
Discussion
According to the ancient theory of TCM, a so-called sub-healthy (pre-disease) condition was defined as a condition that was neither ill nor completely healthy, and the treatment of this condition was recognized as more important than treating the endpoint disease (33). This idea seems quite important in the current preventive treatment of lifestyle and age-related diseases. Although this sub-healthy condition is not physiologically yet fully understood, we assumed this condition is associated with oxidative stress. This is because the pathophysiology to which TCMs are applied are usually associated with oxidative stress, and the herbal components of TCM formulas generally have a high antioxidant potential (19–23,34).
On the other hand, it is well understood that stressors are the causative factor of oxidative stress and that chronic exposure to such stressors leads to serious diseases (5,9). Therefore, stressor-mediated oxidative stress is similar to the sub-healthy condition in terms of the disease initiating stage. Of all the stresses, psychological or emotional stress-mediated oxidative stress seems to be a more reliable model of the sub-healthy condition defined by the TCM theory, since such stressors reportedly modulate immune responses in cancer patients and control the therapeutic potential of cancer treatment (1–6).
In the present study, we developed a simple model for studying psychological or emotional stress-induced oxidative stress in mice after whiskers removal. Whiskers removal was shown to induce oxidative stress in tissues, so that protein carbonyl and TBARS formation were significantly increased as seen in other stress models (10–12). Plasma peroxynitrite levels were also significantly increased indicating plasma peroxinitrite production, which is one of the causative factors for the oxidative tissue injury in multiple organs. Since physical abuse might contribute less to this model than in other stress models, such as immobilization (10), electric shock (11) and water-immersion (12), the oxidative stress observed here might be mainly due to the psychological or emotional stress induced by whiskers removal, which leads to certain distressed condition as was reflected in locomotive behavioral activity changes and plasma leucocytes profile (Fig. 1 and Table 1).
Although the mechanisms of stress-induced oxidative damage are complex and remain unclear, for example, as to how stress causes oxidant production and oxidative damage via imbalances of hormones, neurotransmitters, oxidants and other stress mediators (5,9), granulocyte activation is expected to be involved in the stress-induced oxidative injury of tissues. Under certain stress conditions including that of psychological stress, physical exercise and shear stress significantly alter immune functions, changes such as in subsets of peripheral blood cells (35–38). There, the granulocyte macrophage-colony stimulating factor (GM-CSF) increases a percentage of granulocytes in a shorter period than 6 h after stressor stimuli, and thus reactive oxygen species (ROS) both in the human body and the human derived cell line (37,38). In the present study, that a relative percent of granulocytes tended to increase while lymphocytes tended to decrease after whiskers removal, and thus G/L ratio became greater even though change in the tendency was not statistically defined (Table 1). This might also be caused by GM-CSF induction through emotional stress and this leads to the overproduction of ROS to damage tissues.
Using this model, we examined the protective effects of TCM formulae on mental and emotional stress-induced oxidative stress. Currently, TCM has attracted attention as an alternative for the treatment of complex diseases that Western medicines are unable to treat. In TCM, multicomponent prescriptions are usually used for treating diseases. Therefore, complex interactions, such as additive, synergistic, restraint and antagonistic interactions are involved in the activities of the component herbs. These interactions are, however, considered essential in improving their therapeutic potentiality and in reducing the side effects of certain toxic ingredients (39). Thus, the underlying molecular mechanism is waiting to be clarified for routine usage of TCM. At the same time, an appropriate system needs to be developed to allow evaluation of the preventive and/or ameliorative potential of such complex formulae against sub-healthy pathological conditions.
The present study revealed that a simple whiskers removal model is applicable for this purpose. Two TCM formulae, SMS and LGZGT, markedly suppressed the stress-induced oxidative stress of the distressed mice. Previously, we showed that SMS has a stronger scavenging activity towards hydroxyl radicals rather than superoxide radicals (18–23). LGZGT, on the other hand, scavenges superoxide radicals rather than hydroxyl radicals (18). In the present study, it was further revealed that both formulae inhibited peroxynitrite level increased under the whiskers removal stress as shown in Fig. 6. Thus, the radical scavenging property might play a critical role in preventing effect of these formulae against oxidative stress. Since ginseng, one of the constituent herbs of SMS, is reported to have an anti-stress activity, such as the inhibition of enhanced lipid peroxidation in mouse brains (40), it might also have played a major role in the protective action of SMS against the stressor studied here.
The modulation of physiological antioxidant systems by TCM is thought to be a more probable mechanism for the preventative ability of SMS on stressor-mediated oxidative stress, since we previously reported that SMS prevented the loss of GPX activity in the brain after ischemia reperfusion in rats (18–23) in addition to its strong radical scavenging activity. This is also supported by our recent observation that SMS enhanced GPX activity expression in cultured myoblasts both at transcriptional and post-transcriptional levels (paper in preparation). Similar modulation effects of SMS on antioxidant defense enzymes have been reported elsewhere, for example, hepatic glutathione (GSH) content increase in SMS-administered rats following CCl4 intoxication (16) and P. ginseng, one of the component herbs of SMS, enhanced SOD activity (41) and activated the Cu/Zn-SOD gene at a transcriptional level (42). It is thus suggested that TCM formulae modulate the antioxidant potential of mice either by acting as an antioxidant and/or free radical scavenger, or by modulating physiological antioxidant defense mechanisms. Further investigations are underway to clarify the precise effect of TCM on the modulation of antioxidant defense mechanisms or other multiple pathways that modulate stress-mediated oxidative stress as a model of sub-healthy condition.
Acknowledgments
This study was supported in part by a grant from the promotion and mutual aid corporation for private schools in Japan and also AOB in Japan. We would also like to thank Iskura Co. Ltd for providing Shengmai San preparation (Bakumi-San granules) and Kotaro Co. Ltd for the LGZGT preparation.
References
- 1.Andersen BL, Farrar WB, Golden-Kreutz D, Kutz LA, MacCallum R, Courtney ME, et al. Stress and immune responses after surgical treatment for regional breast cancer. J Natl Cancer Inst. 1998;90:30–6. doi: 10.1093/jnci/90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iribarren J, Prolo P, Neagos N, Chiappelli F. Post-traumic stress disorder: evidence-based research for the third millennium. Evid Based Complement Alternat Med. 2005;2:503–12. doi: 10.1093/ecam/neh127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Wang Z. Effects of social isolation stress on immune response and survival time of mouse with liver cancer. World J Gastroenterol. 2005;11:5902–4. doi: 10.3748/wjg.v11.i37.5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. J Clin Oncol. 2005;23:6440–1. doi: 10.1200/JCO.2005.02.3259. [DOI] [PubMed] [Google Scholar]
- 5.Kelly GS. Nutritional and botanical interventions to assist with the adaptation to stress. Altern Med Rev. 1999;4:249–65. [PubMed] [Google Scholar]
- 6.Sieber WJ, Rodin J, Larson L, Ortega S, Cammings N, Levy S, et al. Modulation of human natural killer cell activity by exposure to uncontrollable stress. Brain Behav Immun. 1992;6:141–56. doi: 10.1016/0889-1591(92)90014-f. [DOI] [PubMed] [Google Scholar]
- 7.Sant'anna ID, de Sousa EB, de Moraes AV, Loures DL, Mesquita ET, da Nobrega AC. Cardiac function during mental stress: cholinergic modulation with pyridostigmine in healthy subjects. Clin Sci (Lond) 2003;105:161–5. doi: 10.1042/CS20030064. [DOI] [PubMed] [Google Scholar]
- 8.Kawachi I, Sparrow D, Spiro A, III, Vokona SP, Weiss ST. A prospective study of anger and coronary heart disease. The Normative Aging Study. Circulation. 1996;94:2090–5. doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- 9.Liu JK, Mori A. Stress, aging, brain oxidative damage. Neurochem Res. 1999;24:1479–97. doi: 10.1023/a:1022597010078. [DOI] [PubMed] [Google Scholar]
- 10.Liu JK, Wang XY, Shigenaga MK, Yeo HC, Mori A. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10:1532–8. [PubMed] [Google Scholar]
- 11.Yoshikawa T, Yoshida N, Miyagawa H, Takemura T, Tanigawa T, Sugino S, et al. Role of lipid peroxidation in gastric mucosal lesions induced by burn shock in rats. J Clin Biochem. 1987;2:163–70. [Google Scholar]
- 12.Kovacs P, Juranek I, Stankovicova T, Svec P. Lipid peroxidation during acute stress. Pharmazie. 1996;51:51–3. [PubMed] [Google Scholar]
- 13.Talwar SK, Xu S, Hawley ES, Weiss Sa, Moxon KA, Chapin JK. Rat navigation guided by remote control. Nature. 2002;417:37–8. doi: 10.1038/417037a. [DOI] [PubMed] [Google Scholar]
- 14.Li PC, Poon KT, Ko KM. Schisandra chinensis-dependent myocardial protective action of Sheng-Mai-San in rats. Am J Chin Med. 1996;24:255–62. doi: 10.1142/S0192415X96000311. [DOI] [PubMed] [Google Scholar]
- 15.Wang NY, Minatoguchi S, Arai M, Uno Y, Nishida Y, Hashimoto K, et al. Sheng-Mai-San is protective against post-ischemic myocardial dysfunction in rats through its opening of the mitochondrial KATP channels. Circ J. 2002;66:763–8. doi: 10.1253/circj.66.763. [DOI] [PubMed] [Google Scholar]
- 16.Ko KM, Yick PK, Poon MKT, Che CT, Ng KH, Kong YC. Schisandra chinensis-derived antioxidant activities in ‘Sheng Mai San’, a compound formulation, in vivo and in vitro. Phytother Res. 1995;9:203–6. [Google Scholar]
- 17.Song ZH, Feng D, Xu JB, Bi KS. Study on the compatibility and therapeutical basis of composite herbal medicines of Lingguizhugan decoction. Chin Tradit Pat Med. 2003;25:132–7. [Google Scholar]
- 18.Tetsuya K. Prevention of cerebral oxidative stress using traditional Chinese medicines: a model of antioxidant-based composite formula. In: Hiramatsu M, Yoshikawa T, editors. Molecular Interventions in Lifestyle-Delated Diseases. Florida: Taylor & Francis; 2005. [Google Scholar]
- 19.Wang XJ, Magara T, Konishi T. Prevention and repair of cerebral ischemia-reperfusion injury by Chinese herbal medicine, Shengmai San, in rats. Free Radic Res. 1999;31:449–55. doi: 10.1080/10715769900301011. [DOI] [PubMed] [Google Scholar]
- 20.Wang XJ, Ichikawa H, Konishi T. Antioxidant potential of Qizhu Tang, a Chinese herbal medicine, and the effect on cerebral oxidative damage after ischemia reperfusion in rats. Biol Pharm Bull. 2001;24:558–63. doi: 10.1248/bpb.24.558. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa H, Konishi T. In vitro antioxidant potentials of traditional Chinese medicine, Shengmai San and their relation to in vivo protective effect on cerebral oxidative damage in rats. Biol Pharm Bull. 2002;25:898–903. doi: 10.1248/bpb.25.898. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa H, Wang X, Konishi T. Role of component herbs in antioxidant activity of Shengmai San—a traditional Chinese medicine formula preventing cerebral oxidative damage in rat. Am J Chin Med. 2003;31:509–21. doi: 10.1142/S0192415X03001193. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Nishida H, Ogawa Y, Konishi T. Prevention of oxidative injury in PC12 cell by traditional Chinese medicine, Shengmai San as a model of an antioxidant based composite formula. Biol Pharm Bull. 2003;26:1000–4. doi: 10.1248/bpb.26.1000. [DOI] [PubMed] [Google Scholar]
- 24.Xu S-Y, Bian RL, Chen X. The Methodology of Pharmacological Experiment. People's Medicinal Publishing House; 2003. pp. 202–4. [Google Scholar]
- 25.Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994;16:149–56. doi: 10.1016/0891-5849(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 26.Kamei K. All of Haematohistology. Ishiyaku Publishing Co. Ltd, 1984; 1984. p. 140. [Google Scholar]
- 27.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–6. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 28.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–63. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 29.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. J Physiol. 1993;460:221–9. doi: 10.1113/jphysiol.1993.sp019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abo T, Kawamura T. Immunomodulation by the autonomic nervous system: therapeutic approach for cancer, collagen diseases, and inflammatory bowel diseases. Ther Apher. 2002;6:348–57. doi: 10.1046/j.1526-0968.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q. Traditional Chinese medicine will make new contributions to mankind in treating sub-health conditions in the 21 century. J Beijing Univ TCM. 2001;24:1–4. [Google Scholar]
- 34.Boxin OU, Huang D, Hampsch-Woodili M, Flanagan JA. When east meets west; the relationship between yin-yang and antioxidation-oxidation. FASEB J. 2003;17:127–9. doi: 10.1096/fj.02-0527hyp. [DOI] [PubMed] [Google Scholar]
- 35.Bargellini A, Piccinini L, De Palma M, Giacobazzi P, Scaltriti S, Mariano M, et al. Trace elements, anxiety and immune parameters in patients affected by cancer. J Trace Elem Med Biol. 2003;17:3–9. [PubMed] [Google Scholar]
- 36.Kittner JM, Jacobs R, Pawlak CR, Heijnen CJ, Schedlowski M, Schmidt RE. Adrenaline-induced immunological changes are altered in patients with rheumatoid arthritis. Rheumatology. 2002;41:1031–9. doi: 10.1093/rheumatology/41.9.1031. [DOI] [PubMed] [Google Scholar]
- 37.Kosaki K, Ando J, Korenaga R, Kurokawa T, Kamiya A. Fluid shear stress increases the production of granulocyte-macrophage colony-stimulating factor by endothelial cells via mRNA stabilization. Circ Res. 1998;82:794–802. doi: 10.1161/01.res.82.7.794. [DOI] [PubMed] [Google Scholar]
- 38.Carcamo JM, Borquez-Ojeda O, Golde DW. Vitamin C inhibits granulocyte macrophage-colony stimulating factor induced signaling pathway. Blood. 2002;99:3205–12. doi: 10.1182/blood.v99.9.3205. [DOI] [PubMed] [Google Scholar]
- 39.Cheng JT. Review: drug therapy in Chinese traditional medicine. J Clin Pharmacol. 2000;40:445–50. doi: 10.1177/00912700022009198. [DOI] [PubMed] [Google Scholar]
- 40.Yobimoto K, Matsumoto K, Huong NT, Kasai R, Yamasaki K, Watanabe H. Suppressive effects of Vietnamese ginseng saponin and its major component majonoside-R2 on psychological stress-induced enhancement of lipid peroxidation in the mouse brain. Pharmacol Biochem Behav. 2000;66:661–5. doi: 10.1016/s0091-3057(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 41.Chu GX, Chen X. Anti-lipid peroxidation and protection of ginsenosides against cerebral ischemia-reperfusion injuries in rats. Acta Pharmacol Sin. 1990;11:119–23. [PubMed] [Google Scholar]
- 42.Kim YH, Park KH, Rho HM. Transcriptional activation of the Cu, Zn-superoxide dismutase gene through the AP2 site by ginsenoside Rb2 extracted from a medicinal plant, Panax ginseng. J Biol Chem. 1996;271:24539–43. doi: 10.1074/jbc.271.40.24539. [DOI] [PubMed] [Google Scholar]