Abstract
Effects of changing cytosolic free Mg2+ concentration on L-type Ca2+ (I ) and Ba2+Ca currents (IBa) were investigated in rat ventricular myocytes voltage-clamped with pipettes containing 0.2 mM or 1.8 mM [Mg2+] ([Mg2+]p) buffered with 30 mM citrate acid and 10 mM ATP. Increasing [Mg2+]p from 0.2 mM to 1.8 mM reduced current amplitude and accelerated its decay under a variety of experimental conditions. To investigate the mechanism for these effects, steady-state and instantaneous current-voltage relationships were studied with two-pulse and tail current (IT) protocols, respectively. Increasing [Mg2+]p shifted the VM for half inactivation by -20 mV but dramatically decreased ICa amplitude at all potentials tested, consistent with a change in gating kinetics that decreases channel availability. This conclusion was supported by analysis of IT amplitude, but these latter experiments also suggested that, in the millimolar concentration range, [Mg2+] might also inhibit permeation through open Ca2+p channels at positive VM.
Keywords: barium current, calcium current, inactivation, tail current, voltage clamp
INTRODUCTION
Changes in cytosolic free Mg2+ concentration ([Mg2+]i) around physiologically-relevant levels have been reported to decrease peak L-type Ca2+ current, ICa [1-4], and to accelerate the rate of current decay [2]. These changes in ICa amplitude and decay rate can be interpreted as either an effect of [Mg2+]i on Ca2+ permeation and/or gating kinetics of the L-type channel. In regards to the first possible mechanism, Yamaoka and Seyama [5] showed that, at physiologic levels and lower concentrations, [Mg2+]i did not change single channel Ba2+ current amplitude in cell-attached patches. Using excised membrane patches in the presence of BayK 8644, Kuo & Hess [6] also reported that cytosolic Mg2+, at concentrations greater or equal to 5 mM, could block inwardly-directed monovalent cation flux through single L-type channels, but Carbone et al. [7] showed that Mg2+ is much less able to block divalent than monovalent cation fluxes through the same channel type. These data would seem to argue against the possibility that physiologically-relevant [Mg2+]i produce significant block of Ca2+ permeation through the L-type Ca2+ channel.
Our recent data showed that [Mg2+]i-dependent modulation on ICa is largely dependent on channel gating states [4]. Conditions that promoted channel opening, such as protein kinase A (PKA) phosphorylation, application of the channel agonist BayK8644 or truncation of the 1C subunit C-terminus, promoted inhibition of ICa around physiologically-relevant [Mg2+]i, whereas conditions lessening channel opening, such as dephosphorylation by protein phosphatase 2A or mutation of putative PKA phosphorylation sites to alanine, reduced [Mg2+]i effects on ICa. These data led us to conclude that cytosolic Mg2+ inhibition of current is enhanced under conditions that promote channel opening.
Our finding that cytosolic Mg2+ inhibition of ICa is enhanced by conditions that promote channel opening raises the possibility that Mg2+ acts by blocking ion permeation. And, in fact such a mechanism would not be surprising given that [Mg2+]i, in the 1 mM range, also produces extensive block of VM-dependent sodium and potassium channels [8,9]. For this reason, we conducted the present study to determine if cytosolic Mg2+ actions on ICa are attributable to block of divalent cation permeation through the L-type channel and/or changes in channel gating kinetics.
METHODS
Cell isolation
Adult rat cardiac ventricular myocytes were isolated enzymatically as described previously [3,10] from male Sprague-Dawley rats (200-225 g) in accordance with the procedures approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey. Following isolation, myocytes were stored in a refrigerator and used within 1 to 8 hours.
Measurement of membrane currents
Myocytes were placed in a chamber mounted on an inverted microscope (Nikon Inc., Japan) and superfused with a modified Tyrode’s or Na+- and K+-free bathing solutions (see Solutions below). Cells were voltage clamped with electrodes containing defined concentrations of Mg2+ ([Mg2+] ) and Ca2+p using standard patch clamp techniques, as described previously [3]. K+ currents were minimized by Cs+ and tetraethylammonium ions (TEA) in the pipette solution, while Na+ current was suppressed by addition of 30 μM tetrodotoxin (TTX) to the Tyrode’s solution and, in some instances, by depolarizing the membrane potential (VM) to -40 mV with ramp pulses prior to test protocols [3]. Tail currents, IT , were recorded in the presence of Na+- and K+-free bathing solutions containing 0.1 μM BayK 8644.
Currents were acquired at the digitizing rates of 10 kHz and filtered at 5 kHz. Data were analyzed using pCLAMP software, version 8.0 (Axon Instruments, Union City, CA). Displayed membrane currents are shown without linear leak subtraction or capacitance correction.
Experimental protocols
Details of voltage clamp protocols are given in the text; however, current through L-type Ca2+ channels was calculated as a 200 M CdCl2-sensitive difference current [3]. The fraction of peak current remaining at the end of a 200-ms depolarization (r200) was used to quantify the level of current decay as a function of VM. Tail currents were also calculated as 200 M CdCl2-sensitive difference currents, and they displayed a bi-exponential decay during the test pulses. The fast component of tail current had a time constant similar to that observed for charging membrane capacitance and was therefore ignored. Current amplitude was then calculated by back-extrapolating the slow component of tail current to the beginning of the test pulse.
Drugs and Chemicals
Solutions
The pipette solution was composed of (in mM): 100 cesium gluconate, 10 PIPES (cesium salt), 15 tetraethylammonium (TEA) chloride, 0.5 NaH2PO4, 0.1 Tris-GTP, 5 EGTA along with ATP, Mg-ATP, citric acid, magnesium citrate, MgCl2 and CaCl2 to produce free [Mg2+] of 0.2 and 1.8 mM, pH 7.2, at specified free [Ca2+] of 100 nM. Total citrate was 30 mM and total ATP was 10 mM. The amounts of these reagents needed to produce specified free Mg2+ and Ca2+ concentrations were calculated using a computer program (WinMAXC 2.40, http://stanford.edu/~cpatton/maxc.html). For 0.2 mM [Mg2+], solutions included (in mM) 2.7 MgCl2, 7.3 MgATP and 5.1 magnesium citrate, and for 1.8 mM, the amounts of these three reagents were 10, 9.6 and 15.4, respectively. Cells were superfused with a modified Tyrode’s solution containing (in mM): 145 NaCl, 4 KCl, 2 (or 0.5) CaCl2, 10 HEPES, 1 MgCl2, and 10 glucose, pH 7.4, or a Na+- and K+-free bathing solution containing (in mM): 150 N-methyl-D-glucamine (NMG) chloride, 0.5 CaCl2, 10 HEPES, 1 MgCl2, and 10 glucose, pH 7.4. Experiments were performed at room temperature (22 - 25°C).
Reagents
Reagents were obtained from Sigma Chemical Corp. (St. Louis, MO), unless specified otherwise. Several reagents purchased from CalBiochem (BayK8644, 3-isobutyl-1-methylxanthine - IBMX, TTX) and forskolin (FSK, BioMol) were prepared as concentrated stock solutions that were applied to bathing solutions 30 min prior to experiments. When DMSO was used as the solvent for stock solutions, the final concentration in experimental solutions was less than or equal to 0.1 %, and blank solutions containing 0.1 % DMSO were also prepared for control experiments. Okadaic acid (OA), from Calbiochem, was added to the electrode solution when indicated.
Data Analysis
Data are expressed as mean ± S.E.M for the number of cells indicated. Significance was determined using ANOVA and Student t-tests in commercial software (SigmaPlot, Jandel Scientific, and JMP IN, Duxbury, Pacific Grove, CA). A P value less than 0.05 was considered statistically significant.
RESULTS
Effect of [Mg2+]i on kinetics of L-type calcium channel current
Representative current traces of ICa are displayed in Figure 1A (left) from cells superfused with Tyrodes solution containing 2.0 mM Ca2+ and voltage-clamped with patch electrodes containing salt solutions with 0.2 mM and 1.8 mM Mg2+. Currents were elicited by step depolarizations to 0 mV after a 1 sec ramp depolarization to -40 mV from a holding potential of -70 mV. Displayed currents were recorded 5 min after establishing the whole-cell clamp configuration, as per Wang et al. [3].
Figure 1.
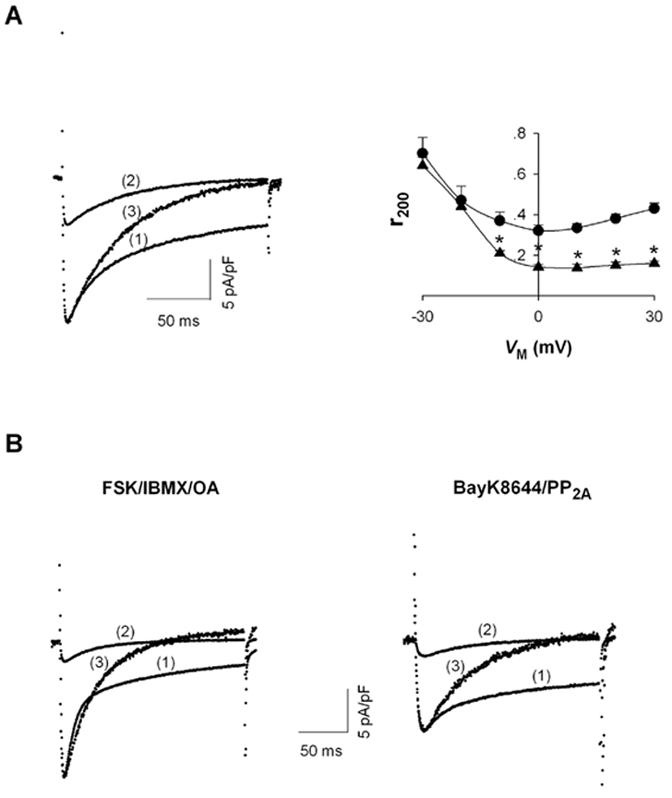
Effect of [Mg2+]p on L-type Ca2+ channel currents. A, ICa recorded during depolarizations to 0 mV in rat ventricular myocytes voltage-clamped with patch electrodes containing (1) 0.2 mM [Mg2+]p and (2) 1.8 mM [Mg2+]p. Trace (3) is ICa with 1.8 mM [Mg2+]p normalized to the amplitude of ICa with 0.2 mM [Mg2+]p (left). The fraction of peak ICa remaining at the end of 200-ms depolarizations (r200) with 0.2 mM (● , n=6) and 1.8 mM [Mg2+]p (▲ , n=5) at different test potentials (VM) (right). Significant changes of r200 between 0.2 mM [Mg2+]p and 1.8 mM [Mg2+]p are indicated by asterisks. B, tracings of ICa, as in part A, except with the addition of the indicated compounds. C, IBa recorded during depolarizations to 0 mV. Current traces (1) - (3) are indicated as in Part A (left); r200 for IBa was measured with 0.2 mM (● , n=7) and 1.8 mM [Mg2+]p (▲ , n=6) and plotted versus test potential (right).
Increasing [Mg2+]p decreased ICa amplitude and changed the kinetics of current decay. With 0.2 mM [Mg2+]p, ICa displayed bi-exponential decay kinetics with a small fast component (tau = 22.4 ± 3.7 ms, n=6) and a prominent slow component (tau = 106.8 ± 13.8 ms). In contrast, I Ca at 1.8 mM [Mg2+]p displayed mono-exponential decay kinetics (tau = 60.6 ± 1.4 ms, n=5). To illustrate this point more clearly, ICa measured with 1.8 mM [Mg2+]p was normalized to the peak current at 0.2 mM [Mg2+]p. The normalized currents showed that current decay at high [Mg2+]p was, initially, slower than or of a similar rate to the decay of current at low [Mg2+]p. Nonetheless, after a few tens of milliseconds, current amplitude with 1.8 mM [Mg2+]p continued to decline while, at low [Mg2+]p, current decay slowed to a great extent (Fig. 1A, left). To evaluate the extent of current decay, the fraction of peak current remaining at the end of 200 ms depolarizations (r200) was plotted as the function of VM (Fig. 1A, right). At 0.2 mM [Mg2+]p, r200 exhibited a characteristic “U” shape [11] with the a minimum value occurring at 0 mV, the same VM where maximal ICa was observed [3]. With 1.8 mM [Mg2+]p, r200 declined monotonically with increasing depolarization and remained unchanged at positive VM. Additionally, r200 for current at 1.8 mM [Mg2+]p was smaller than r200 at 0.2 mM [Mg2+]p for VM from -10 mV to +30 mV. These results indicated the increasing [Mg2+]i not only simplified the kinetics of ICa decay from a bi-exponential to a mono-exponential process but also resulted in an overall acceleration of current decay.
Our previous studies showed that regulation of L-type Ca2+ channels by [Mg2+]i is prominent under the conditions that increase channel opening probability [4]. Therefore, we investigated how [Mg2+]i affected the L-type Ca2+ current decay under these experimental conditions. Channel opening probability was increased under channel phosphorylation conditions (60 minute preincubation with IBMX and FSK; inclusion of okadaic acid in the patch electrode solution) or by application of BayK8644, coupled with PP2A, as previously published [4]. Under high channel phosphorylation conditions, current decay exhibited bi-exponential kinetics at 0.2 mM [Mg2+]p. The fast component of current, more prominent than under basal conditions (Fig. 1B, left), is thought to be related to Ca2+-dependent inactivation of ICa, which depends on Ca2+ influx via the Ca2+ channels [12], and Ca2+ release from the sarcoplasmic reticulum [13-17]. Analogous to control conditions, increasing [Mg2+]p from 0.2 to 1.8 mM changed the current decay from a bi-exponential to a mono-exponential process and, in general, accelerated the current decay (Fig. 1B, left). Moreover, the rate of current decay with 1.8 mM [Mg2+]i was slower than the fast component of current decay at 0.2 mM [Mg2+]p. Similarly, under conditions promoting low phosphorylation but high channel opening, induced by including PP2A (1unit/ml) in the patch electrode solution and 0.1 M BayK8644 in the superfusion solution, the same increase in [Mg2+]p simplified the current decay from a bi-exponential to a mono-exponential process and produced acceleration of current decay (Fig. 1B, right). As in Fig. 1A, values of r200 were also decreased in the presence of the higher [Mg2+]p under conditions promoting greater channel opening (not shown). Thus, under all conditions tested, increasing [Mg2+]i affected kinetics of current decay in a qualitatively similar manner, accelerating current decay while simplifying that decay to an exponential process.
Changes in the kinetics of current decay might imply an alteration in Ca2+ channel inactivation. L-type Ca2+ channel inactivation is thought to be mediated via the rapid Ca2+-dependent process and a relatively slower VM-dependent process [11,18]. VM-dependent current inactivation is readily observed with minimal Ca2+-dependent inactivation by using Ba2+ as the permeating ion [19]. Thus the effects of [Mg2+]p on current decay were examined with Ba2+ as the permeant ion. As expected, Ba2+ current through L-type channels displayed a slow and incomplete decay with 0.2 mM [Mg2+]p, presumably a reflection of the time course of VM-dependent inactivation. Interestingly, increasing [Mg2+]p from 0.2 to 1.8 mM caused Ba2+ current to show much more rapid and complete decay (Fig. 1C, left), consistent with the results of Hartzell & White [2]. With 1.8 mM [Mg2+]p, Ba2+ current decayed in a mono-exponential fashion with a time constant at 0 mV of 45.8 ± 1.8 ms (n=6), similar to the time constant for decay of Ca2+ current measured with this [Mg2+]p. The value of r200 declined monotonically with increasing depolarization at both low and high [Mg2+]p but r200 values were much smaller at 1.8 mM [Mg2+]p over the VM range tested. In summary, the effects of increasing [Mg2+] on Ba2+ currents were qualitatively similar to those effects on Ca2+p currents, i.e. greatly decreasing current amplitude and accelerating current decay kinetics.
Taken together, the results above show that increasing [Mg2+]p simplified and accelerated the kinetics of current decay, irrespective of the conditions under which L-type Ca2+ channel current was measured. As presented in the Discussion, the simplest but not the only explanation for these results is that Mg2+ alters current amplitude and decay rate by acting as an open channel blocker, as has been previously shown for K+ channels [9].
Effect of [Mg2+]i on VM dependence of ICa steady-state inactivation
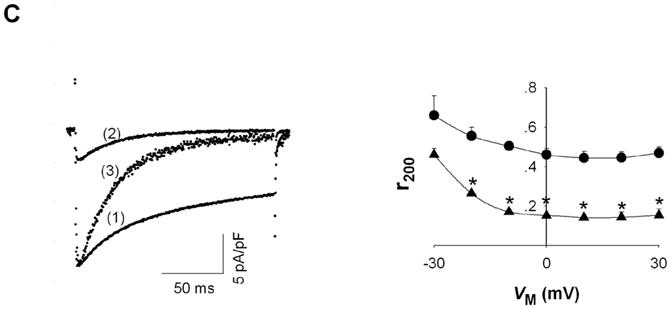
Is there any evidence that cytosolic Mg2+ modulation of L-type Ca2+ channels might not depend on block of ion permeation? To address this issue, the effect of [Mg2+]p on steady state current inactivation was investigated, since this channel property depends on closed and inactivated, i.e. nonconducting, channels. To study steady state inactivation, a two pulse protocol was used in which a prepulse between -90 and 0 mV (in 10 mV increments) was elicited prior to a 200 msec test depolarization to 0 mV (Fig. 2, top). Preliminary experiments (not shown) indicated that recovery from inactivation required approximately 13 seconds. For this reason, the duration of the prepulse was set to 20 sec to insure that channels had reached steady state prior to the test depolarization.
Figure 2.
Effect of [Mg2+]p on the VM dependence of steady state ICa inactivation. Two-pulse inactivation protocol with a 20 sec prepulse and 200 msec test depolarization to 0 mV (top). Typical currents with a prepulse of -30 mV where traces (1) and (2) were recorded with 0.2 and 1.8 mM [Mg2+]p, respectively (middle). ICa plotted versus prepulse potential (VM) for myocytes voltage-clamped at 0.2 (● , n=6) and 1.8 mM [Mg2+]p (▲ , n=6) (bottom).
Representative current tracings at prepulse of -30 mV with low and high [Mg2+]p are displayed in the middle panel of Figure 2. Current amplitude during the prepulse was initially similar. With 0.2 mM [Mg2+]p, current was essentially unchanged during the 20 sec prepulse but, with 1.8 mM [Mg2+]p, current decayed very slowly. Consequently, during the test depolarization to 0 mV, ICa was greatly reduced with 1.8 mM [Mg2+]p. The currents induced by the test depolarization are plotted as the function of the prepulse potential (Fig. 2, bottom). The potentials for half-maximal inactivation of ICa with 0.2 mM and 1.8 mM [Mg2+]p were -31.4 ± 2.4 mV (n=4) and -51.5 ± 1.3 mV (n=6), respectively, i.e. increasing [Mg2+]p shifted inactivation to more negative VM by -20.1 ± 1.3 mV (n=4). These results suggested that [Mg2+]p can affect the VM-dependence of channel inactivation. Perhaps a more interesting result is that ICa amplitude with high [Mg2+]p was much smaller than with low [Mg2+]p at all VM. Even at the negative VM range where the current during the test depolarization was not affected by prepulse potential, increasing [Mg2+]p from 0.2 mM to 1.8 mM decreased ICa amplitude by approximately 60%. Thus, in addition to any effects on the VM dependence of channel gating, [Mg2+]i reduced I in a VM-independent manner that suggested [Mg2+]i might affect Ca2+ Ca channel availability, in other words, channel gating.
Effects of [Mg2+]i on voltage dependence of L-type Ca2+ tail currents
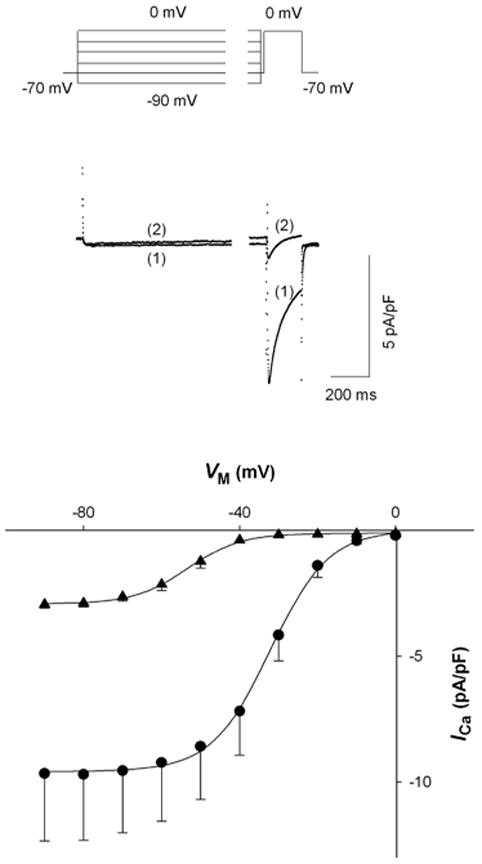
Our conclusion that cytosolic Mg2+ affects L-type Ca2+ channel availability could be complicated if cytosolic Mg2+ can block ion permeation through open Ca2+ channels during the test depolarization to 0 mV (see Discussion). Whether and to what extent Mg2+ blocks ion permeation through the open channel at this VM is unknown. However, our published data [4] and data in Fig. 1 may be consistent with cytosolic Mg2+ acting as an open channel blocker. Thus, the possibility that cytosolic Mg2+, at the concentrations used, might block open Ca2+ channels was evaluated by comparing L-type Ca2+ channel tail currents (IT) with 0.2 and 1.8 mM [Mg2+]p. Cells were first depolarized to +10 mV for 10 ms to maximally open Ca2+ channels with a minimum of inactivation. The membrane was then stepped immediately to test potentials ranging from -80 mV to +30 mV for 50 ms to elicit IT (Fig. 3A). Figure 3B shows representative current tracings at a test potential of -20 mV for IT from ventricular myocytes at 0.2 mM and 1.8 mM [Mg2+]p. Currents were recorded during superfusion with a Na+- and K+-free (substituted with equimolar NMG) Tyrodes solution containing 0.1 μM BayK8644. As expected, IT was considerably smaller in the cell voltage-clamped with 1.8 mM [Mg2+]p.
Figure 3.
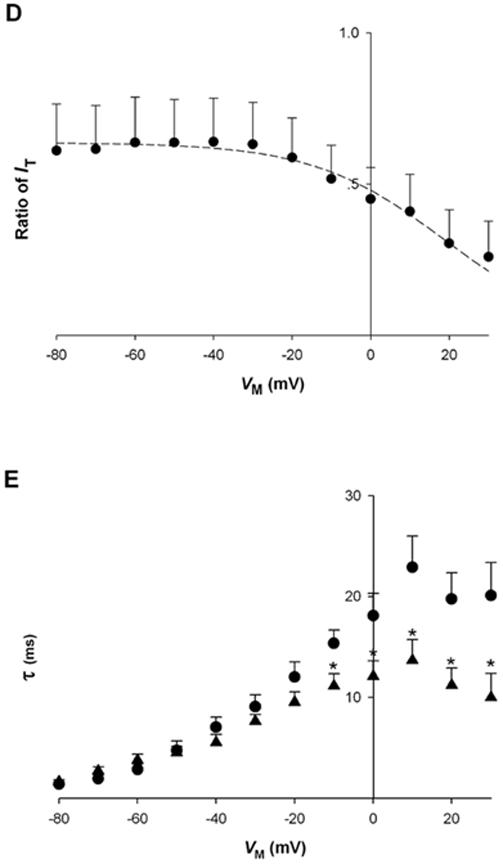
Effect of [Mg2+]p on L-type calcium channel tail currents (IT).A, voltage protocol used to measure instantaneous IT-VM relationships. B, sample records of IT in cardiac myocytes dialyzed with 0.2 mM (1) and 1.8 mM [Mg2+]p (2) at a test potential of -20 mV. IT was recorded in the presence of 0.1 μM BayK8644, 5 min after establishing the whole-cell patch-clamp. C, instantaneous IT-VM relationships in cells dialyzed with 0.2 mM (●, n=5) and 1.8 mM [Mg2+]p (▲ , n=5). D, the ratio of IT, defined as peak IT recorded with 1.8 mM [Mg2+]p at one VM divided by that with 0.2 mM [Mg2+]p, is plotted against VM (n=5). The dashed curve was fit to the data with an arbitrary function. E, time constant of IT decay ( τ) plotted against VM for myocytes dialyzed with 0.2 mM (● , n=5) and 1.8 mM [Mg2+]p (▲ , n=5). Time constants were determined by fitting the change in current during the 50 msec test pulse at each VM without accounting for current levels observed in the presence of 200 M CdCl2. Asterisks indicate significant changes of τ between 0.2 and 1.8 mM [Mg2+]p.
Figure 3C shows that, as the test potential was depolarized from -80 mV to +30 mV, IT decreased in amplitude. The curvilinear relationship between current amplitude and test potential suggests that IT behaves as predicted by the constant field equation [20], as has been observed previously for Ca2+ currents reviewed in Hagiwara & Byerly [21]. In addition, IT was decreased at 1.8 mM [Mg2+]p for the all test potentials.
Instantaneous IT amplitude in any condition reflects the permeability properties of the open channel under investigation, independent of channel gating properties. However, in comparing two different experimental conditions, the a priori assumption that the number of open channels is the same cannot be made. Nonetheless, even if the absolute number of open Ca2+ channels changes with [Mg2+]p, the relative number of channels should be constant. Therefore, if cytosolic Mg2+ has no effect of channel permeability properties, the ratio of IT should be constant at all VM, but the ratio should change if ion permeability is affected by Mg2+ (see Discussion).
To investigate this point, tail current amplitude measured with 1.8 mM [Mg2+]p was divided by that at 0.2 mM [Mg2+]p and plotted as the function of VM (Fig. 3D). The ratio of IT was not VM-dependent with VM from -80 mV to -20 mV, but at more positive VM, the ratio decreased progressively. These results suggest that increasing [Mg2+]p from 0.2 to 1.8 mM does affect the permeability properties of the open Ca2+ channel, but only at VM approaching or exceeding 0 mV. Over a broad range of negative VM, this change in [Mg2+]p had no effect on the ratio of IT and, therefore, did not appear to affect ion permeability properties of the channel. Interestingly, the maximal ratio of IT observed at these negative VM, approximately 0.6, was significantly less than 1, a clear demonstration that increasing [Mg2+]p inhibited Ca2+ channel opening independent of VM. This VM-independent effect on IT cannot reflect open channel permeability properties and therefore must reflect a change in the number of channels that are open at the different [Mg2+]p (see Discussion). In other words, increasing [Mg2+]i decreased Ca2+ channel availability. These results also suggest that the decrease in ICa observed in Fig. 2 reflects, for the most part, a true reduction in Ca2+ channel availability with increased [Mg2+]p.
Another observation in these experiments was the effect of increasing [Mg2+]i on the decay kinetics of the tail currents. Figure 3E shows that the time constant for tail current decay was unchanged at those negative VM where ratio of tail currents was constant; however, at more positive VM, increasing [Mg2+]i accelerated current decay in addition to reducing the tail current ratio.
DISCUSSION
The most prominent effect on L-type Ca2+ current of changing [Mg2+]i around its physiological concentration range is a marked modulation of current amplitude shown here and in previous papers [1-5,22,23]. This modulation in current amplitude might be explained by the change in channel gating and/or permeation rate through the ion channels. The motivation for the present study was to establish whether [Mg2+]i, under the conditions of our experiments, was having a prominent effect on ion permeation rate.
The results of previous studies suggest that the concentrations of cytosolic Mg2+ used in our experiments would not likely block Ca2+ permeation through the L-type Ca2+ channel pore. Yamaoka and Seyama [5] measured single channel Ba2+ currents with cell-attached patch electrodes while whole-cell voltage-clamping cardiac myocytes with patch-electrodes containing micromolar Mg2+ concentrations. During the time course of the experiment, in which [Mg2+]i presumably declined towards the micromolar level, single channel Ba2+ current amplitude did not change. These results suggested that [Mg2+]i up to those approaching physiologic levels did not block Ba2+ permeation. One caveat in extrapolating these results to the present data or other published whole cell current data is the high concentration of Ba2+ used in the cell-attached patch electrode solution (90 mM). This high concentration of permeant cation might complicate comparisons to whole cell current recordings where the permeant ion concentration is in the neighborhood of 1 millimolar in the extracellular solution.
Kuo & Hess [6] also studied the effect of Mg2+ on ion permeation through L-type Ca2+ channels using excised inside-out membrane patches exposed to the Ca2+ channel agonist, Bay K8644. Their studies showed that Mg2+ on the intracellular side of the membrane could block ion permeation at high (micromolar) and low affinity sites. However, with inwardly directed currents, Mg2+ did not appear to block the channel at the high affinity sites unless the current-carrying ion was Li+, which interacts with the channel at low affinity [24]. Mg2+ interacting with the channel at its low affinity sites was observed to decrease single channel Li+ current amplitude, but the concentration that produced a half-maximal decrease in permeation rate was calculated to be 10 mM at 0 mV [6]. The low affinity Mg2+ site also appeared to be relatively non-selective for cations, so their estimated value of Mg2+ affinity might be relevant to the situation where Ca2+ is the permeating ion. In any case, the affinity of Mg2+ for the low affinity site in the L-type channel permeation pathway would seem to indicate that the highest [Mg2+]i used in the present study, 1.8 mM, would not have a significant effect on ion permeation. Nonetheless, given the assumptions and extrapolations needed to arrive at this conclusion, it is important to experimentally test whether [Mg2+]i could be blocking ion permeation under the conditions of our experiments [3,4], that are similar to those of other reports in which [Mg2+]i has been reported to decrease whole-cell Ca2+ currents [1,5,22,23,25].
Are there data suggesting that [Mg2+]i used here could block ion permation? Wang & Berlin [4] studied that effect of [Mg2+]i on wild-type and mutant L-type Ca2+ channels and came to the conclusion that experimental maneuvers producing an increase in channel opening also promoted the ability of cytosolic Mg2+ to inhibit current. This finding could be consistent with Mg2+ acting as an open channel blocker. The findings of White & Hartzell [1] and Pelzer et al. [25] that channel phosphorylation promotes the reduction of Ca2+ current by cytosolic Mg2+ current might also be consistent with Mg2+ block of ion permeation through the open channel. This possibility motivated the present experiments to determine if [Mg2+]i used here could decrease current in a manner consistent with permeation block.
We used instantaneous I-V relationships to investigate whether [Mg2+]i could be acting as an open channel blocker. Instantaneous I-V relationships, measured from the peak amplitude of tail currents, provide information on the permeation properties of an ion channel, independent of time-dependent changes in gating. The results showed that tail currents through Ca2+ channels display a curvilinear dependence on test potential at both [Mg2+]p tested, consistent with the predictions of the constant field equation [21]. Taking the ratio of tail current amplitudes measured at each VM allowed us to look at relative changes in ion permeation through open Ca2+ channels with 0.2 and 1.8 mM [Mg2+]p. However, in order to interpret the tail current ratio, it is necessary to assume that Mg2+ block of ion permeation in an open channel would occur at a site in the membrane electric field. This assumption is based on the results of Kuo & Hess (1993) showing that low-affinity intracellular Mg2+ block occurs at a site located 30% of the way through the membrane electric field from the inside. Then, using eqns. 1 and 2 from Kuo & Hess [6], it is possible to predict the relative degree of Ca2+ channel current at two different [Mg2+]i , [Mg2+]a and [Mg2+]b, as a function of VM:
where KD0 is the Mg2+ binding affinity for the blocking site in the permeation pathway at 0 mV, Θ is a proportionality factor and F, R and T have their usual meanings. This equation predicts that, at negative VM, the current ratio will asymptotically approach a value of 1, i.e. no significant block of ion permeation occures, but the ratio will approach the value of [Mg2+]a/[Mg2+]b at positive VM, in this instance a value of 0.11.
We observed that the ratio of tail currents was constant at a value of approximately 0.6 for negative VM, but progressively decreased at VM approaching and exceeding 0 mV. Thus, the data clearly do not fit with the predictions for a purely VM-dependent permeation blocker. Instead, two mechanisms, one VM-independent and the other VM-dependent, are necessary to explain the properties of the current ratio. Given the design of the protocol, in which peak tail current amplitude is determined by the number of open channels and the channel conductance, we can conclude that the VM-independent reduction of current ratio must reflect a decrease in the number of channels open in the presence of 1.8 mM [Mg2+] , i.e. Ca2+p channel availability is decreased, as seen in Fig. 2, is presumably due to altered gating kinetics.
Given this outcome, our experiments suggest that the predominant mechanism of current inhibition on increasing [Mg2+]i from 0.2 to 1.8 mM is a decrease in channel availability, except at VM equal or exceeding +30 mV. Thus, block of ion permeation would not seem to be the major mechanism underlying the reduction of Ca2+ current amplitude, and it probably plays a less prominent role in reducing current amplitude under the conditions of experiments in Fig. 1.
While the most prominent effect of changing [Mg2+]i, in its physiological concentration range, is suppression of current amplitude [1-4], acceleration of current decay is also notable, as shown by the reduction of r200 with increased [Mg2+]i. Hartzell & White [2] also observed similar effects of increased [Mg2+]i on the decay of Ca2+ current. The mechanism explaining this acceleration of current decay was not explored thoroughly in the present experiments. Nonetheless, it seems possible that permeation block by cytosolic Mg2+ might play a role in this phenomenon. The basis for this conclusion also comes from the tail current experiments where decay rate was accelerated at higher [Mg2+]i only at VM positive to -20 mV (Fig. 3E), where the ratio of tail currents was also decreasing and we observe VM-dependent effects on current ratio that we attribute to permeation effects of cytosolic Mg2+. Currents in Fig. 1 were measured at 0 mV. Consequently, the acceleration of current decay in these experiments might be due, at least in part, to Mg2+ block of ion permeation.
Cytosolic Mg2+ effects on ion permeation are unlikely to fully explain the acceleration of current decay. Increasing [Mg2+]i changed the kinetics of decay from a bi-exponential to a single exponential process where the decay time constant in the presence of 1.8 mM [Mg2+]p was slower than the fast component, but faster than the slow component, of current decay observed with 0.2 mM [Mg2+]p . This effect of [Mg2+]i was observed under a wide variety of conditions including the presence of BayK8644, high phosphorylation conditions induced by protein kinase A, and Ba2+ as the permeating divalent cation. These changes in current kinetics were also not due to a simple shift in the VM dependence of current inactivation. The mechanism underlying the observed changes in current decay kinetics will require further investigation.
Finally, a -20 mV shift in the VM dependence of steady state current inactivation was observed on increasing [Mg2+] . Previously, we have reported a 5-10 mV shift of Ca2+p current inactivation in the negative direction with shorter conditioning pulses (up to 3 sec in duration), and a similar shift has been observed by others [2]. This smaller shift has been attributed to screening of membrane surface charges. We have only observed the larger 20 mV shift with the longer prepulses necessary to achieve true steady state inactivation and these results suggest that cytosolic Mg2+ may have a greater effect on some channel closed states, consistent with its ability to decrease channel availability. This point, however, also requires further investigation.
In summary, the reduction of Ca2+ current amplitude produced by increasing [Mg2+]i across its physiologic range appears to depend predominantly on a decrease in channel availability rather than block of ion permeation. Once the channel opens, it is possible that cytosolic Mg2+ has the opportunity to block ion permeation, especially at those membrane potentials encountered during physiologic processes such as the plateau of a cardiac ventricular action potential.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- [1].White RE, Hartzell HC. Science. 1988;239:778–780. doi: 10.1126/science.2448878. [DOI] [PubMed] [Google Scholar]
- [2].Hartzell HC, White RE, Gen J. Physiol. 1989;94:745–767. doi: 10.1085/jgp.94.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang M, Tashiro M, Berlin JR. J Physiol. 2004;555:383–396. doi: 10.1113/jphysiol.2003.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang M, Berlin JR. Am J Physiol - Cell Physiol. 2006;291:C83–C92. doi: 10.1152/ajpcell.00579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yamaoka K, Seyama I. Pflügers Arch. 1996;431:305–317. doi: 10.1007/BF02207267. [DOI] [PubMed] [Google Scholar]
- [6].Kuo CC, Hess P. J Physiol. 1993;466:629–655. [PMC free article] [PubMed] [Google Scholar]
- [7].Carbone E, Lux HD, Carabelli V, Aicardi G, Zucker H. J Physiol. 1997;504:1–15. doi: 10.1111/j.1469-7793.1997.001bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bara M, Cuiet-Bara A, Durlach J. Magnesium Res. 1993;6:167–177. [PubMed] [Google Scholar]
- [9].Harris RE, Isacoff EY. Biophys J. 1996;71:209–219. doi: 10.1016/S0006-3495(96)79217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mitra R, Morad M. A, Am J Physiol. 1985;249:H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- [11].McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- [12].Sun H, Leblanc N, Nattel S. Am J Physiol. 1997;272:H1625–H1635. doi: 10.1152/ajpheart.1997.272.4.H1625. [DOI] [PubMed] [Google Scholar]
- [13].Sipido KR, Callewaert G, Carmeliet E. Circ Res. 1995;76:102–109. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- [14].Adachi-Akahane S, Cleemann L, Morad M. J Gen Physiol. 1996;108:435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sham JS. J Physiol. 1997;500:285–295. doi: 10.1113/jphysiol.1997.sp022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Delgado C, Artiles A, Gomez AM, Vassort G. J Mol Cell Cardiol. 1999;31:1783–1793. doi: 10.1006/jmcc.1999.1023. [DOI] [PubMed] [Google Scholar]
- [17].Linz KW, Meyer R. Pfluegers Arch. 2000;439:588–599. doi: 10.1007/s004249900212. [DOI] [PubMed] [Google Scholar]
- [18].Findlay I. J Neurosci. 2003;19:1912–1921. [Google Scholar]
- [19].Kass RS, Sanguinetti MC. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hille B, editor. Ion Channels of Excitable Membranes. Sinauer Associates, Inc.; Sunderland, MA: 2001. p. 445. [Google Scholar]
- [21].Hagiwara S, Byerly L. Ann Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- [22].Yamaoka K, Seyama I. Pflügers Arch. 1996;432:433–438. doi: 10.1007/s004240050155. [DOI] [PubMed] [Google Scholar]
- [23].Yamaoka K, Seyama I. Pflügers Arch. 1998;435:329–337. doi: 10.1007/s004240050519. [DOI] [PubMed] [Google Scholar]
- [24].Hess P, Lansman JB, Tsien RW. J Gen Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pelzer S, La CC, Pelzer KL. Am J Physiol. 2001;281:H1532–H1544. doi: 10.1152/ajpheart.2001.281.4.H1532. [DOI] [PubMed] [Google Scholar]


