Abstract
Depression is the most common psychiatric comorbidity in epilepsy. To better understand the contribution of seizures versus environment to depression in epilepsy, we investigated differential gene expression using microarray and quantitative RT-PCR, and depressive behavior, in the Porsolt forced swim test in juvenile rats reared in different environments after kainic acid (KA)-induced seizures. We selected for genes significantly down-regulated by KA seizures and upregulated by environmental enrichment. This common gene selection process yielded one known gene involved in mood and affect: serotonin receptor 5B. The changes in serotonin receptor gene expression were paralleled by decreased mobility in the forced swim tests; depressive behavior exhibited after seizures was no longer evident in rats reared in environmental enrichment. Our results suggest that seizures lead to increased susceptibility to depression through transcriptional regulation while environment, in turn, can interact with gene expression to influence the behavioral outcome of epilepsy.
Keywords: depression, childhood epilepsy, kainic acid, status epilepticus, 5-HT, Porsolt forced swim test, microarray
1. Introduction
Depression is the most frequent psychiatric comorbidity in patients with epilepsy and contributes significantly to increased morbidity and mortality [1,2]. Its prevalence ranges from 20 to 55% in patients with poorly controlled seizures [1,2], significantly higher than prevalence of 13–21% reported for the general public [3]. Depression can severely affect quality of life [4–6]; suicide is nearly ten times more frequent among epilepsy patients than in the general population [1,2,7]. In children with epilepsy, the rate of depression, as measured by self-reporting instruments, ranges from 23 to 26%, while the availability of mental health services remains inadequate and reported suicide is on the rise [2,8].
It was once accepted that depression in persons with epilepsy developed as a result of the psychosocial burden of living with a chronic disabling neurological condition. Recent studies, however, have begun to demonstrate that the genesis of depression and epilepsy may share some common etiological features [9–11]. Mutual features of both epilepsy and depression include the link to serotonergic pathways and the involvement of hippocampal and limbic circuitry. In patients with temporal lobe epilepsy, serotonergic receptor binding in the limbic area is decreased [12], while in patients with affective disorder, the efficacy of selective serotonergic reuptake inhibitors (SSRIs) is associated with the activation of regional metabolic rate in the limbic area [13,14]. Increased risk of depression is highly correlated with the cerebral structural and metabolic abnormalities in epilepsy [15,16], while significant reduction in hippocampal volume is observed in major depression [11,17]. Furthermore, not only do seizures and epilepsy increase the risk of major depressive episodes, but the reverse also holds true - depression and suicide attempt significantly increase the risk for future incidence of unprovoked seizures and development of epilepsy [10,18]. Available evidence thus supports the emerging notion that epilepsy and depression share common biological pathways.
Using high-density oligonucleotide microarrays, we have previously shown that complex social and sensory-motor stimulations, provided by exposing developing rats to an enriched environment after kainic acid (KA)-induced seizures, improve exploratory behavior and increase expression of genes involved in synaptic plasticity and memory consolidation such as Arc, Homer1a and Egr1 [19]. In a separate microarray analysis, one gene was noted to be significantly decreased by KA-seizures, and subsequently increased by environmental enrichment - Htr5b, a serotonin receptor gene. This led us to question whether an enriched environment, which has been reported to attenuate the cell death and behavioral deficits associated with seizures [19–21], may also reverse the effects of seizures on depressive behavior. To further investigate the link between epilepsy and depression, in the present study we examined: (1) whether seizure-induced down-regulation of serotonin receptor (5-Htr) gene expression correlates with behavioral changes and confirm the involvement of serotonergic pathways in seizure-induced depressive behavior, (2) whether Htr5b expression change detected by microarray analysis is validated by real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR), and (3) whether an enriched environment ameliorates the depressive behavior and decrease in Htr5b transcript that result from early life seizures.
We exposed postnatal day (P) 20–25 rats to an isolated or enriched environment for 7–14 days after KA-induced seizures. Depressive behavior, or learned despair, was then assessed by measuring immobility in the Porsolt forced swim test. We used high-density oligonucleotide microarrays and real-time qRT-PCR to analyze gene expression changes in the hippocampus, one of terminal fields of serotonergic innervation and a site of seizure initiation after systemic KA injection [22,23].
2. Methods
2.1. Experimental Design
A total of 18 litters of P 20– 21 Long Evans male rats (10 pups per litter, Charles River Laboratories) were injected with KA (10mg/kg, intraperitoneal) or phosphate-buffered saline (PBS). Only the animals with grade III or IV seizures (see below) were included in the KA group. Within 24 hours of injection, the animals were placed either singly in a standard vivarium cage (24cm x 45cm x 31cm; Control Isolated (C-Iso) and Kainate Isolated (KA-Iso)), or as a group of 8 in an enriched environment (Control Enriched (C-Enr) and Kainate Enriched (KA-Enr)). An enriched environment consists of a larger cage made of two 40cm x 58cm x 38cm clear plastic boxes connected by two tunnels, as well as various moving objects, wooden chew toys, balls and bells, a running wheel, a mirror and a plastic igloo. Ten days after exposure to different environments, two third of the animals (n=96) were anesthetized with isoflourane inhalation and decapitated. To optimize detection of transient gene expression changes after KA-induced seizures that may persist in animals reared under the conditions of isolation while normalized under the conditions of enriched environment, we have chosen a 10 day time point of environmental exposure. The hippocampi were rapidly dissected from the brain and frozen on isopentane over dry ice for total RNA preparation to be used for microarray analysis and for qRT-PCR. Fourteen days after the exposure to different environments, the remaining animals (n=48) underwent the Porsolt forced swim test.
2.2. KA induced Seizures
After KA administration, seizure activity was recorded over a 3-hour period. A seizure severity grade was assigned based on maximal response achieved on a scale from 0 to V as follows: 0, no response; I, wet dog shake (WDS) and/or behavioral arrest; II, WDS, staring, pawing, and clonic jerks; III, WDS, staring, pawing, clonic jerks, rearing and falling; IV, continuous grade III seizures for longer than 30 minutes (status epilepticus (SE)); and V, death. Approximately 60–70 % of P20–P25 rats treated with KA reached grade III–IV seizures and were included in the study. Thus, from each litter of 10, one or two animals died of status epilepticus while two or three animals had only wet dog shake or intermittent forelimb clonus (grade II) seizures and were excluded from the study.
2.3. Microarray analysis
We used the rat RAE230A Affymetrix Genechip® arrays that contain 26,800 probe sets. Total RNA was isolated (TRIzol Reagent, Invitrogen) individually from hippocampi and an equal amount of RNA was pooled from 4 animals to hybridize to each chip. RNA concentration was determined spectrophotometrically, and RNA integrity was confirmed by agarose gel electrophoresis. Three independent hybridizations were performed per condition: C-Iso, C-Enr, KA-Iso and KA-Enr, for a total of 12 RAE230A profiles. Preparation of cRNA, array hybridization and scanning were performed by Microarray Consortium (NINDS/NIMH) at the Translational Genomics Research Institute (Phoenix, Arizona) as described previously [19]. In probe-level analysis Affymetrix GDAS v. 3.0 was used to produce signal intensities and detection call (Present, Marginal and Absent). We included in our data analysis only those probe sets that were “Present” for detection call in all 12 chips. This stringent threshold effectively eliminated genes with low precision (about 40%); over 9,300 probe sets were selected from a total 15,923 hybridized genes. We then used Significance Analysis of Microarrays (SAM) to screen for genes with q value <0.5 to generate our target list to 428 genes. Further pair-wise statistical comparison using SAM (two class, unpaired data, 720 permutations) were performed between C-Iso and KA-Iso; KA-Iso and KA-Enr to select for common genes significantly down-regulated (q<0.05) by KA, then up-regulated by environmental enrichment.
2.4. Real time Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
Based on microarray results, we chose Htr5b for validation through qRT-PCR. Htr5b primers and probe sequences were as follows: forward primer: 5’-ACCGTAGGGCCTGGAATGTT-3’; reverse primer: 5’-GACGCGTAGAAGGCATGG AT-3’; probe: 5’-TCACGAAGGTAGACAACGGTTTGGCA-3’. Primers were purchased from Qiagen (Germantown, MD), and the TaqMan probe, from Megabases, Inc. (Chicago, IL).
Individual RNA samples were prepared for qRT-PCR and were treated with RNase-free DNase I (Roche Diagnostics) for 20 min at room temperature followed by inactivation for 10 min at 75°C. All of the RNA samples were further purified using RNA Easy Kit (Qiagen Inc. CA) to remove any remaining genomic DNA and salts and the PCR reaction was run as described in detail previously [19].
Relative quantification of the expression of the 5-Htr5b mRNA was performed with the ABI Prism 7700 System (Perkin-Elmer Applied Biosystems, Inc., Foster City, CA). Quantification is based on the threshold cycle (Ct) which is the PCR cycle when the amplification signal is first detected to be above the baseline signal. The Ct is inversely proportional to the copy number of the target template; the higher the template concentration, the lower the threshold cycle measured. The comparative Ct method of relative quantification was utilized to analyze the real-time qRT-PCR data. With this method, the data is normalized by comparison to an endogenous reference gene such as β-actin (ΔCt = Ctsample − Ctβ-actin). This normalized Ct is then used to compare control versus KA-treated samples (ΔΔCt = ΔCtcontrol − ΔCtKA). Due to the exponential nature of PCR, the ΔΔCt is converted to a linear form (2 ΔΔCt) and expressed as a fold change.
2.5. Porsolt forced swim test
Depressive behavior was tested in the Porsolt forced swim test, a standard animal test of depression often used to show the efficacy of anti-depressants; immobility during forced swim can be reduced by a range of clinically active antidepressant drugs [24]. The animal is placed in a narrow cylinder of water from which there is no escape. After an initial period of vigorous activity, it adopts a characteristic immobile posture, which has come to be known as behavioral despair or learned helplessness.
After seven days of environmental conditions, the rats in our study were habituated to the forced swim cylinder. Each animal was placed for 15 minutes in a cylindrical tank of water (40 cm height, 25 cm diameter). No scoring of immobility was performed during habituation. They were returned to their home cage (enriched or isolated) and seven days after habituation, rats were then placed in the cylinder for 5 minutes, during which mobility was scored. Mobility consisted of upward-directed movements of the forepaws along the side of the cylinder, as well as swimming behavior throughout the cylinder. Immobility consisted of no additional activity other than that required for keeping the head above water.
2.6. Statistics
One-way analysis of variance (ANOVA) with a post hoc t-test and Tukey corrections was used to compare duration of mobility in Porsolt forced swim test, and fold changes in quantitative RT-PCR between four experimental groups. Values are expressed as mean ± SEM (standard error of the mean), and significance was defined as p<0.05. Significance Analysis of Microarrays (SAM) v. 1.21 (Stanford University) was used to select genes that are significantly changed by KA-induced seizures or by environmental enrichment. SAM generates q values that reflect false discovery rate.
3. Results
3.1. Microarray analysis
We performed pair-wise comparisons (KA-Iso vs. C-Iso; KA-Iso vs. KA-Enr) to search for common genes that are significantly down-regulated by KA and up-regulated by environmental enrichment in the hippocampus. We identified three known genes that were significantly down-regulated in KA-Iso compared to C-Iso. They were Htr5b (5-hydroxytryptamin (serotonin) receptor 5B), Hrh3 (histamine receptor H3) and Anp32a (acid nuclear phosphoprotein). Found among 75 known genes significantly up-regulated in KA-Enr compared to KA-Iso [19] was Htr5b. Thus serotonin receptor 5B gene expression was uniquely decreased after KA-induced seizures and significantly increased by environmental enrichment (Figure 1).
Figure 1. Htr5b microarray data.
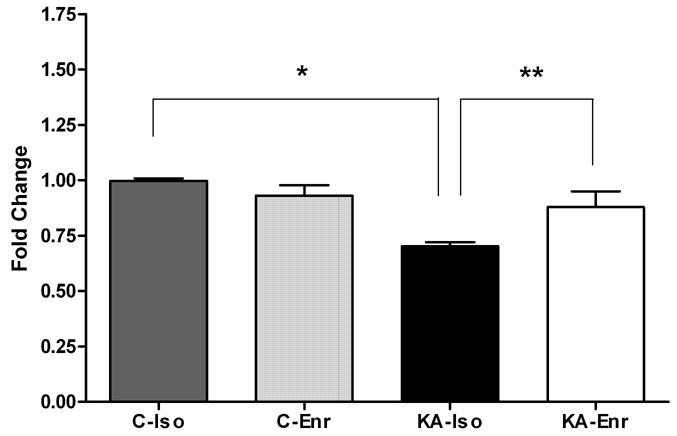
Signal intensities of each probe sets from the four groups were normalized against the median value in the C-Iso to calculate the fold changes. Htr5b expression in the hippocampus was significantly lower in KA-Iso compared to C-Iso (* q<0.02) and to KA-Enr (**q<0.01).
3.2. qRT-PCR
qRT-PCR data for Htr5b emulated the expression trends seen in microarray results (Figure 2). Similar to the microarray data, qRT-PCR results showed a decrease (0.6-fold) in Htr5b expression after KA that was reversed by environmental enrichment. The qRT-PCR results presented here include both technical validation of microarray data using the same hippocampal RNA samples (n = 24) as well as biological validation by utilizing independent hippocampal RNA samples for qRT-PCR (n = 24). The independent RNA samples for qRT-PCR were not pooled, which may account for the larger standard error bars seen in qRT-PCR due to the biological variability of gene expression in individual hippocampal samples.
Figure 2. Validation of microarray data by qRT-PCR.
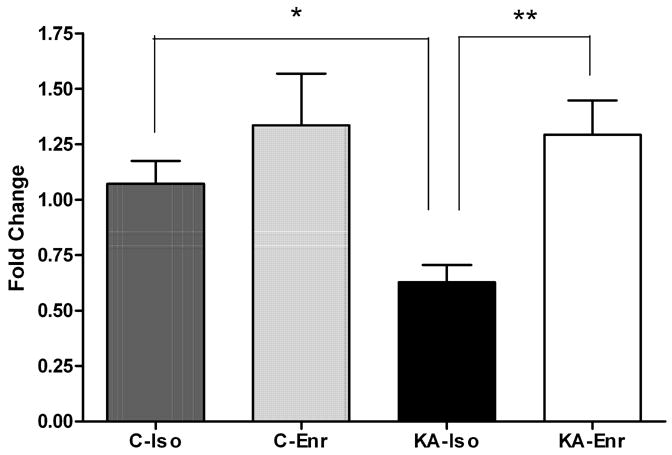
Real time qRT-PCR results confirms the expression level trends seen in the microarray analysis for Htr5b. Htr5b expression in the hippocampus was significantly lower in KA-Iso compared to C-Iso (*p<0.05) and to KA-Enr (**p<0.05), one-way ANOVA Tukey’s Multiple Comparison Test, n=48.
3.3. Porsolt forced swim test
Mobility in the Porsolt swim test was quantified in control and KA groups after two weeks of rearing in different environments. During the 5 minute testing period, KA-Iso rats were significantly less mobile than combined controls (p<0.02). While control animals spent 37%, KA-Iso animals spent only 25 % of their time in the cylinder attempting to escape. These animals were observed to adopt a horizontal and immobile posture almost immediately after being placed in the water. This posture was punctuated by brief attempts at escape that were notably less vigorous than the attempts made by control animals. In contrast, KA-Enr animals showed significantly increased mobility compared to KA-Iso (p<0.001). They spent 54% of the test time actively attempting to escape (Figure 3). While decrease in motor strength or endurance may affect the forced swimming test, no apparent motor deficit was noted during habituation which required 15 minutes of swimming in the cylinder one week after KA or saline injections, or at the time of mobility timing during the forced swim test a week later.
Figure 3. Mobility in the Porsolt forced swim test.
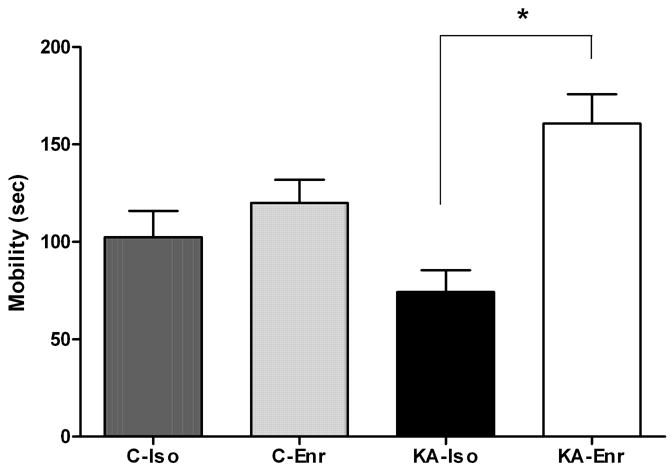
KA-Iso rats tended to be less mobile than C-Iso (p=0.11). The mobility of KA-Iso was significantly less than combined controls (C-Iso + C-Enr) (p<0.02, 111.2 sec. ± 9.0 vs. 74.2 sec. ± 11.3). Depressive behavior observed in KA-Iso animals was alleviated by environmental enrichment (KA-Enr 160.7 ± 15.2). One-way ANOVA, n=48, p=0.0003. Tukey’s Multiple Comparison Test * KA-Iso vs. KA-Enr: p<0.001.
4. Discussion
We found that early-life seizures caused a decrease in serotonin receptor gene expression and decreased mobility in the Porsolt forced swim test. Depressive behavior after kainic acid-induced status epilepticus in juvenile rats is correlated with selective down-regulation of serotonin receptor in the hippocampus. Isolation appeared to exacerbate these seizure-induced changes in behavior and gene expression, while environmental enrichment mitigated these effects. Our results indicate that seizures may lead to increased susceptibility to depressive behavior through transcriptional regulation; environment, in turn, also interacts with gene expression to counteract the disease process. Reversal of depressive behavior in the Porsolt forced swim test by an enriched environment thus demonstrates the therapeutic power of environment to influence the behavioral outcome of prolonged seizures.
It will be interesting to know how long it takes for the rats to develop decreased mobility in the forced swim test following status epilepticus and whether the time course of this depressive behavior correlates with the serotonin receptor gene down-regulation. We observed significant increase in depressive behavior 14 days after KA-induced seizures in 21 day old rats reared in isolation compared to rats reared in enriched environment. In a time course study of gene expression after KA-induced seizure at P15, significant decreases in serotonin receptor gene (5-Htr5b) were seen as early as 6 hours (h) after KA (49 % of control, p<0.0003) and remained low at 24 h (63% of control, p<0.01) and 72 h (71 % of control, p<0.02) after seizures in the hippocampal tissues. The decrease was no longer significant by 10 days in these group-housed rats (unpublished observation). Thus, it is possible that environmental enrichment augmented, while social isolation prevented the homeostatic response of animals to normalize serotonin receptor expression and to increase mobility in the forced swim test, and thus caused a persistent depressive behavior that otherwise could have been a short-lived post-ictal phenomenon along with a transient serotonin receptor down-regulation.
Our finding that a member of the 5-HT family is significantly and uniquely affected by the experience of early-life seizures has important implications regarding the cause of depression in childhood epilepsy. It has been debated whether the elevated risk of depression is due to the biology of epilepsy specifically or is attributable to the psychosocial burden of having a chronic neurological condition. Observation of down-regulation of serotonin receptors, concurrent with exhibition of depressed behavior in the Porsolt forced swim test, suggests that a prolonged seizure itself contributes to the depressed state via impaired serotonergic transmission resulting from down-regulation of Htr5b. The etiology of depression has long been known to involve serotonergic pathways [25], and selective serotonergic reuptake inhibitors (SSRIs) are currently the recommended first-line medication for depression [26]. Furthermore, a recent epidemiological study has shown that people with less efficient serotonin transporter gene (5-HTT, by functional polymorphism in the promoter region - short allele) were twice as likely as those with long allele to develop major depression given similar adverse life events. Among individuals carrying a short allele, childhood maltreatment predicted adult depression and they carried 63% risk of a major depressive episode [27]. In addition to giving further credence to involvement of serotonergic pathways in depression, this serotonin transporter gene polymorphism study underscores the role of early-life experience in later development of disease and also provides an evidence of a gene-by-environment interaction in a disease expression.
While the comorbidity of epilepsy and depression has been documented empirically over the past several decades, a comprehensive understanding of a causal relationship has only recently emerged due, at least in part, to advances in neuroimaging tools [14,28]. PET measurement of 5-HT1A receptor binding in patients with mesial temporal lobe epilepsy has shown a significant reduction in the epileptogenic hippocampus and its limbic connections [12,29]. Serotonergic hypofunction demonstrated in human temporal lobe epilepsy is consistent with data from present study and from other labs using the KA seizure animal model [30]. Together these data suggest a potential pathogenetic mechanism for depression in this patient population with high risk for affective disorder [12,31].
Further investigation of the relationship between depression and epilepsy is critical to the understanding of etiology and development of appropriate treatments. There is little doubt that depression in epilepsy is an important variable and a significant predictor of health related quality of life [4,6,32,33]. Yet treatment of depression in patients with epilepsy remains controversial and inadequate, particularly with respect to children. This shortfall is at least partly due to the limited understanding of the precise causes as well as the apparent lack of efficacy of traditional antidepressants in this patient sub-population. The present study shows seizure-induced down-regulation of serotonin receptor expression and depressive behavior in young rats reared in isolation are no longer observed in rats reared in enriched environment. Social isolation, which appears to occur frequently in people with epilepsy [34], can prevent innate homeostatic response of the brain after seizures, thus rendering the individuals more susceptible to future behavioral problems. Rarely, however, is social support considered a treatment modality to improve neurological outcome. Therapeutic efficacy of environmental enrichment indicates that intense educational and social intervention early in life may allow compensation for vulnerability of children with epilepsy and may afford improved long-term psychosocial outcome in young persons with epilepsy. Children and adolescent with epilepsy need to be informed of risk of depression and of therapeutic role of social engagement and support to combat this important comorbidity in epilepsy.
Acknowledgments
We thank Jose Hernandez for his help in construction and maintenance of enriched environment cages. This work was supported by a CURE (Citizens United for Research in Epilepsy), NIH grant (K02 NS48237) and NIMH/NINDS Microarray Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gilliam FG, Santos J, Vahle V, Carter J, Brown K, Hecimovic H. Depression in epilepsy: ignoring clinical expression of neuronal network dysfunction? Epilepsia. 2004;45 (Suppl 2):28–33. doi: 10.1111/j.0013-9580.2004.452005.x. [DOI] [PubMed] [Google Scholar]
- 2.Pellock JM. Understanding co-morbidities affecting children with epilepsy. Neurology. 2004;62:S17–23. doi: 10.1212/wnl.62.5_suppl_2.s17. [DOI] [PubMed] [Google Scholar]
- 3.Blazer DG, Kessler RC, McGonagle KA, Swartz MS. The prevalence and distribution of major depression in a national community sample: the National Comorbidity Survey. Am J Psychiatry. 1994;151:979–986. doi: 10.1176/ajp.151.7.979. [DOI] [PubMed] [Google Scholar]
- 4.Boylan LS, Flint LA, Labovitz DL, Jackson SC, Starner K, Devinsky O. Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology. 2004;62:258–261. doi: 10.1212/01.wnl.0000103282.62353.85. [DOI] [PubMed] [Google Scholar]
- 5.Perrine K, Hermann BP, Meador KJ, et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol. 1995;52:997–1003. doi: 10.1001/archneur.1995.00540340089017. [DOI] [PubMed] [Google Scholar]
- 6.Gilliam F. Optimizing health outcomes in active epilepsy. Neurology. 2002;58:S9–20. doi: 10.1212/wnl.58.8_suppl_5.s9. [DOI] [PubMed] [Google Scholar]
- 7.Jones JE, Hermann BP, Barry JJ, Gilliam FG, Kanner AM, Meador KJ. Rates and risk factors for suicide, suicidal ideation, and suicide attempts in chronic epilepsy. Epilepsy Behav. 2003;4 (Suppl 3):S31–38. doi: 10.1016/j.yebeh.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Plioplys S. Depression in children and adolescents with epilepsy. Epilepsy Behav. 2003;4 (Suppl 3):S39–45. doi: 10.1016/j.yebeh.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Gilliam FG. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol. 2005;18:129–133. doi: 10.1097/01.wco.0000162853.29650.ec. [DOI] [PubMed] [Google Scholar]
- 10.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- 11.Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- 12.Savic I, Lindstrom P, Gulyas B, Halldin C, Andree B, Farde L. Limbic reductions of 5-HT1A receptor binding in human temporal lobe epilepsy. Neurology. 2004;62:1343–1351. doi: 10.1212/01.wnl.0000123696.98166.af. [DOI] [PubMed] [Google Scholar]
- 13.Buchsbaum MS, Wu J, Siegel BV, et al. Effect of sertraline on regional metabolic rate in patients with affective disorder. Biol Psychiatry. 1997;41:15–22. doi: 10.1016/s0006-3223(96)00097-2. [DOI] [PubMed] [Google Scholar]
- 14.Carey PD, Warwick J, Niehaus DJ, et al. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry. 2004;4:30. doi: 10.1186/1471-244X-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quiske A, Helmstaedter C, Lux S, Elger CE. Depression in patients with temporal lobe epilepsy is related to mesial temporal sclerosis. Epilepsy Res. 2000;39:121–125. doi: 10.1016/s0920-1211(99)00117-5. [DOI] [PubMed] [Google Scholar]
- 16.Bromfield EB, Altshuler L, Leiderman DB, et al. Cerebral metabolism and depression in patients with complex partial seizures. Arch Neurol. 1992;49:617–623. doi: 10.1001/archneur.1992.00530300049010. [DOI] [PubMed] [Google Scholar]
- 17.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–249. [PubMed] [Google Scholar]
- 19.Koh S, Chung H, Xia H, Mahadevia A, Song Y. Environmental enrichment reverses the impaired exploratory behavior and altered gene expression induced by early-life seizures. J Child Neurol. 2005;20:796–802. doi: 10.1177/08830738050200100301. [DOI] [PubMed] [Google Scholar]
- 20.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 21.Faverjon S, Silveira DC, Fu DD, et al. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology. 2002;59:1356–1364. doi: 10.1212/01.wnl.0000033588.59005.55. [DOI] [PubMed] [Google Scholar]
- 22.Collins RC, McLean M, Olney J. Cerebral metabolic response to systemic kainic acid: 14-C-deoxyglucose studies. Life Sci. 1980;27:855–862. doi: 10.1016/0024-3205(80)90080-6. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 25.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 26.Mann JJ. The medical management of depression. N Engl J Med. 2005;353:1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 27.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 28.Drevets WC, Frank E, Price JC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 29.Toczek MT, Carson RE, Lang L, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 30.Van Bogaert P, De Tiege X, Vanderwinden JM, Damhaut P, Schiffmann SN, Goldman S. Comparative study of hippocampal neuronal loss and in vivo binding of 5-HT1a receptors in the KA model of limbic epilepsy in the rat. Epilepsy Res. 2001;47:127–139. doi: 10.1016/s0920-1211(01)00301-1. [DOI] [PubMed] [Google Scholar]
- 31.Theodore WH. Does Serotonin Play a Role in Epilepsy? Epilepsy Curr. 2003;3:173–177. doi: 10.1046/j.1535-7597.2003.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson EK, Jones JE, Seidenberg M, Hermann BP. The relative impact of anxiety, depression, and clinical seizure features on health-related quality of life in epilepsy. Epilepsia. 2004;45:544–550. doi: 10.1111/j.0013-9580.2004.47003.x. [DOI] [PubMed] [Google Scholar]
- 33.Suurmeijer TP, Reuvekamp MF, Aldenkamp BP. Social functioning, psychological functioning, and quality of life in epilepsy. Epilepsia. 2001;42:1160–1168. doi: 10.1046/j.1528-1157.2001.37000.x. [DOI] [PubMed] [Google Scholar]
- 34.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–1722. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]


