Abstract
The goal of this editorial is to revisit soluble human leukocyte antigens (sHLA) and to highlight the findings reported by Albitar et al. in this issue on the relation between sHLA levels in Non-Hodgkin’s Lymphoma (NHL) and Hodgkin’s Disease (HD). We will review key aspects of sHLA including soluble HLA-G, which has received a lot of attention in recent publications. We will then address the role of sHLA in lymphoproliferative diseases and in solid organ tumors. Lastly, we will comment on the results of Albitar et al. and their relevance to clinical application in NHL.
Keywords: soluble human leukocyte antigen, non-Hodgkin’s Lymphoma, graft monitoring, immune modulation
Soluble HLA
In humans, the major histocompatability complex (MHC) consists of a cluster of genes located on the short arm of chromosome 6. These genes encode for protein products known as human leukocyte antigens (HLA). The MHC genes, and consequently the HLA proteins, are extremely polymorphic and can be grouped into two classes, class I (HLA-I) and class II (HLA-II). HLA-I molecules are heterodimers composed of a 44 kD α heavy chain non-covalently bound to a 12 kD β2-microglobulin light chain. In contrast, HLA-II molecules are heterodimers composed of α and β subunits not associated with β2-microglobulin1,2.
Additionally, HLA-I molecules can be further divided into classical and non-classical subgroups. The classical HLA-I molecules exhibit a high degree of polymorphism and include HLA-A, HLA-B, and HLA-C. The non-classical HLA-I molecules, on the other hand, are non-polymorphic. These non-classical HLA-I molecules include HLA-G, HLA-E, HLA-F and HLA-H; and they are also composed of a non-polymorphic α heavy chain non-covalently associated with β 2-microglobulin3.
HLA molecules are predominantly expressed as cell surface proteins anchored in the cell membrane. While HLA-I molecules are expressed on the surface of almost all nucleated cells, expression of HLA-II molecules is restricted to professional antigen presenting cells, endothelial cells, and activated T cells1. The cell surface expression of HLA molecules emphasizes their important role in presenting peptide antigen to T cells and initiating T cell mediated immune responses. HLA-I molecules typically bind intracellular antigens for presentation to cytotoxic CD8+ T cells whereas HLA-II molecules typically bind extracellular antigens for presentation to helper CD4+ T cells.
The existence of non-cell bound soluble HLA (sHLA) molecules in the serum of healthy individuals was first reported in 1970. van Rood et al. identified soluble HLA-A2 in the serum of normal individuals that was capable of inhibiting anti-HLA-A2 alloantibodies4. Charlton et al. also reported the presence of what they termed sHLA-A7 in the low-density β-lipoprotein fraction of human serum5. The structure of these sHLA molecules is similar to their cell-bound counterparts. sHLA-I molecules consist of an α chain non-covalently bound to β2-microglobulin, while some sHLA-II molecules have been identified circulating as intact heterodimers2.
Soluble forms of the non-classical HLA-I molecules have also recently been identified. In 2000, Paul et al. reported the discovery of a new splice variant of HLA-G that lacked the transmembrane and cytoplasmic regions of the α chain6. Consequently, this HLA-G splice variant named HLA-G7 was found to be soluble. In fact, it is now known that there are seven different isoforms of HLA-G and that these isoforms are alternative splice variants of the primary HLA-G mRNA transcript6. Four of the HLA-G isoforms (HLA-G1-G4) are membrane-bound proteins containing both the transmembrane domain and cytoplasmic tail, while the remaining three isoforms (HLA-G5-G7) lack both of these regions and are soluble3.
Further biochemical analysis has revealed heterogeneity in the molecular masses of sHLA-I products detected in serum and cell supernatants, with major molecular sizes of 44-46, 35–37, and 39-41 kD as identified by Western Blot analysis. This heterogeneity is most likely the result of multiple mechanisms including: 1) shedding from the cell membrane; 2) proteolytic cleavage of membrane bound molecules; and 3) alternative splice variants that yield truncated, secreted sHLA-I1. The larger 44-46 kD isoforms are likely the result of shed membrane-bound molecules as they contain both a transmembrane domain and a cytoplasmic domain. The smaller 35-37 kD forms are presumably products of proteolytic cleavage because these molecules are composed of neither a transmembrane nor a cytoplasmic domain. The intermediate 39-41 kD forms appear to be products of alternate mRNA splicing that generates truncated HLA-I lacking the transmembrane domain2 (Figure 1).
Figure 1. Soluble HLA.
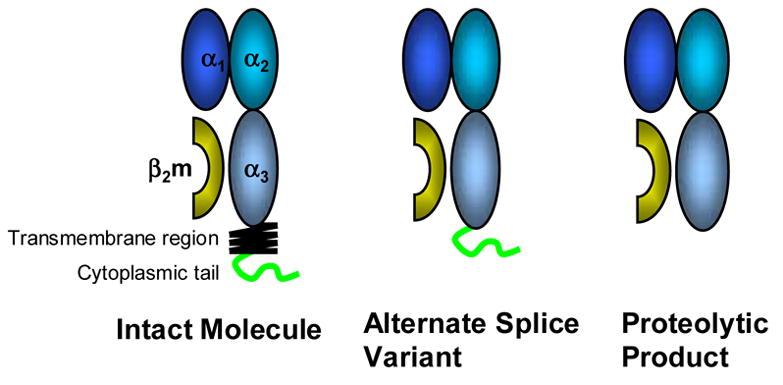
HLA molecules are transmembrane glycoproteins that belong to the immunoglobulin superfamily. In human serum, several products have been detected: namely the intact molecule, likely a product of shedding; the products of alternative splicing which lack the transmembrane region; and proteolytic products lacking both the transmembrane and cytoplasmic regions.
Much less is known about the biochemistry of sHLA-II molecules. One study reported the molecular mass of sHLA-II molecules as a 60 kD molecule7, while another study identified both a 28 kD and a 43 kD fragment8. Here, the remainder of this editorial will focus on sHLA-I molecules.
sHLA-I Molecules in Healthy and Diseased Patients
Stable amounts of sHLA-I molecules circulate in all individuals, although some individuals have substantially higher concentrations than others dependent on their HLA haplotypes. In general, healthy individuals appear to express about 1 μg/ml of sHLA9. The general population can be divided into high and low sHLA concentration groups, and the major determinant between these groups appears to be their MHC allotype. It has been reported that individuals with the HLA-A9 allotype have higher serum levels of sHLA-I molecules. For example, Billing et al. in 1977 observed that serum of HLA-A9 individuals expressed high levels of sHLA10. This observation has been substantiated in numerous findings by others, including in a workshop on soluble HLA11. Race also appears to play a role in determining serum levels of sHLA-I molecules in normal individuals. For example, HLA-A23 and HLA-A24 allotypes are correlated with high serum sHLA-I molecules in both Caucasians and African Americans. In contrast, HLA-A29 is associated with high sHLA-I levels in Caucasians but not in African Americans, while HLA-A33 correlates with high sHLA-I levels in African Americans but not in Caucasians2.
Many investigators have reported the relationship between sHLA and several disease states including autoimmunity, transplant rejection, infection, and cancer. sHLA-I has been detected at significantly elevated levels in the serum of patients with active rheumatoid arthritis and active systemic lupus erythematosus12,13. Elevated levels of both donor- and recipient-derived sHLA-I molecules have been reported during acute rejection of cardiac, renal, and liver allografts9,14. The level of serum HLA-I molecules also markedly increases during the course of viral infections such as cytomegalovirus, hepatitis B, hepatitis C, human immunodeficiency virus, and varicellazoster virus15. This upregulation of sHLA-I molecules during infection appears to be caused by the increased production of cytokines, interferon-γ in particular, that are known to be associated with viral infections. In fact, administration of interferon-γ for therapeutic purposes has been shown to increase the levels of serum sHLA-I molecules17.
In contrast to autoimmune diseases, acute transplant rejection, and viral infections, the relationship between sHLA-I molecules and cancer is not quite as clear. A study by Shimura et al. demonstrated that patients with Stage IV advanced gastric cancer had significantly lower values of sHLA-I compared to normal healthy volunteers and also compared to patients with less advanced Stage I and Stage II gastric cancers17. This study also showed that sHLA-I levels were significantly lower in all gastric cancer patients with the HLA-A24 allotype, regardless of stage. In a study by Westhoff et al., patients with metastatic malignant melanoma showed no significant difference in sHLA-I levels compared to healthy controls; however, patients with less advanced primary malignant melanoma demonstrated significantly lower levels of serum sHLA-I18. In contrast, another study by Shimura et al. demonstrated significantly elevated levels of sHLA-I molecules in Japanese patients with pancreatic cancer19. These data suggest that there is no true trend in the relationship between serum sHLA-I and cancer progression. Moreover, the diagnostic or prognostic value of sHLA-I molecules in patients with solid tumors may depend on the particular cancer involved.
In addition to solid tumors, levels of sHLA-I have also been studied in blood borne cancers including non-Hodgkin’s lymphoma (NHL) and Hodgkin’s disease (HD). Nocito et al. reported that prior to treatment, patients with either NHL or HD had significantly elevated levels of sHLA-I compared to healthy controls20. Post-treatment sHLA-I levels in NHL and HD patients in complete remission normalized. Interestingly, two NHL patients experienced a relapse, and during the relapse their sHLA-I levels were elevated. In this study, no significant correlation between sHLA levels at diagnosis, stage of disease, and survival was identified20.
Soluble forms of the non-classical HLA-I molecules such as sHLA-G have also been detected in several malignancies and lymphoproliferative disorders. Clinical studies performed by Rebmann et al. demonstrated that sHLA-G levels were significantly elevated in patients with malignant melanoma, breast, and ovarian cancer3. For patients suffering from glioblastoma multiforme, a significant negative correlation was found between patient survival after diagnosis and sHLA-G levels3. In other studies, Sebti et al. have reported elevated levels of sHLA-G in patients with chronic lymphocytic leukemia, B cell NHL, and T cell NHL21.
Clinical Relevance of sHLA-I in NHL
In this issue, Albitar et al. report significantly higher pre-therapy sHLA-I levels in NHL and HD patients than in control subjects22. These results are in agreement with the results of Nocito et al.20 discussed above. In the Albitar et al. study, serum samples from patients with NHL or HD were collected prior to initiation of therapy and were analyzed for both levels of sHLA-I and β2-microglobulin. The positive correlation between β2-microglobulin and disease aggressiveness in various solid organ tumors and hematologic malignancies has been well established, with elevated serum β2-microglobulin levels serving as a negative prognostic factor for overall survival23,24,25. Interestingly, for NHL patients the serum levels of sHLA-I and β2-microglobulin showed significant correlation in both pre-therapy and post-therapy samples22. This data suggests that sHLA-I levels may serve as another prognostic tool in the clinical management of NHL. In contrast, no statistically significant correlation was found between sHLA-I and β2-microglobulin in pre-therapy or post-therapy samples from HD patients22.
Albitar et al. then went on to further characterize the clinical relevance of sHLA and β2-microglobulin in predicting survival in patients with NHL22. Interestingly, pre- and post-therapy levels of sHLA-I molecules were both significantly associated with survival, and threshold values for both pre- and post-therapy sHLA-I levels were determined. NHL patients with poorer prognosis could then be identified based on their sHLA-I levels because those patients with sHLA-I levels higher than the determined threshold showed significantly shorter survival than those with levels lower than threshold. These results are in direct disagreement with the study by Nocito et al., who reported no significant correlation between sHLA-I levels and survival20. Albitar et al. also report that levels of β2-microglobulin higher than pre- and post-therapy threshold values are significantly associated with decreased survival22. This result agrees with their previous finding that levels of sHLA-I and β2-microglobulin are correlated in NHL patients. Interestingly, sHLA and β2-microglobulin were found to be non-redundant markers in NHL. Post-therapy sHLA-I levels were highest in patients with no-response and lowest in those with complete response. In comparison, post-therapy β2-microglobulin levels did not differ significantly between the response groups. Overall, these findings suggest that pre-therapy, both sHLA-I and β2-microglobulin can be used as prognostic indicators in NHL, while post-therapy only sHLA has any prognostic value.
Although the use of sHLA as a prognostic indicator for NHL shows promise, caution must be applied before its widespread implementation in clinical practice. Perhaps the greatest limitation to the clinical use of sHLA is the lack of standardized, commercially available sHLA assays. Serum sHLA levels are currently measured by the enzyme-linked immunosorbent assay (ELISA). In the studies cited previously, the ELISAs were performed using differing reagents available in the respective labs. This may, in part, explain some of the differences reported between sHLA-I and certain disease states. These ELISAs also did not include measurement of sHLA-G. Here, although the results reported by Albitar et al.22 have been statistically well evaluated, they still need verification by others, especially since their findings regarding the negative correlation between elevated sHLA-I and survival disagree with those of Nocito et al20. Another significant caveat with the studies in this issue is the lack of information on infections. As discussed above, cytokines, in particular IFN-γ induce upregulation of MHC antigens. The authors discuss B symptoms, but these are not specific enough to rule out a viral or bacterial infection leading to a possible bias on the results and conclusions.
It is interesting to speculate on the role of elevated sHLA-I molecules in NHL and decreased survival. Previous studies have reported that normal T and B lymphocytes secrete sHLA-I molecules, and that this secretion is considerably increased after T or B cell activation20. In this regard, elevated sHLA in NHL patients may simply be the by-product of lymphoma cell proliferation. It is unlikely, however, that the increase in serum sHLA in NHL has no biological significance. The elevated sHLA-I molecules may provide the lymphoma cells a means of anti-tumor immune-evasion allowing them continual survival and growth until the patient is overwhelmed. In fact, an immunomodulatory role for sHLA-I has been proposed15. Previous studies have demonstrated the ability of sHLA-I to bind to cytotoxic T cells and induce apoptosis in both alloreactive cytotoxic T cells26 and EBV-specific cytotoxic T cells27. In addition, it has been reported that sHLA-I engagement with either the C-type lectin inhibitory receptor (CLIR) or killer Ig-like receptor (KIR) on natural killer (NK) cells induced NK cell apoptosis28. With respect to these reports, it is not difficult to imagine that in NHL, lymphoma cells secrete sHLA-I molecules loaded with tumor-generated peptides (Figure 2). The peptide-sHLA-I complex can then bind to the TCR of tumor specific CTLs causing either apoptosis or anergy, or to the CLIR and KIR of NK cells inducing NK cell apoptosis. Further studies are needed to evaluate these possibilities. In conclusion, the results by Albitar et al. in this issue are interesting but require verification by others and some mechanistic studies.
Figure 2. Immunomodulatory role of sHLA in Non-Hodgkin’s Lymphoma.

Non-Hodgkin’s Lymphoma (NHL) tumor cells produce elevated levels of sHLA-I molecules bearing tumor derived peptide antigens. The sHLA can then bind to the TCR of anti-tumor specific T cells and induce either apoptosis or anergy. sHLA can also bind to the CLIR or KIR of NK cells inducing NK cell apoptosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zavazava N. Soluble HLA class I molecules: biological significance and clinical implications. Molecular Medicine Today. 1998;4(3):116–121. doi: 10.1016/s1357-4310(97)01185-4. [DOI] [PubMed] [Google Scholar]
- 2.Adamashvili IM, Kelley RE, Pressly T, McDonald JC. Soluble HLA: patterns of expression in normal subjects, autoimmune diseases, and transplant recipients. Rheumatology International. 2005;25:491–500. doi: 10.1007/s00296-005-0585-y. [DOI] [PubMed] [Google Scholar]
- 3.Rebman V, Regel J, Stolke D, Grosse-Wilde H. Secretion of sHLA-G molecules in malignancies. Seminars in Cancer Biology. 2003;13:371–377. doi: 10.1016/s1044-579x(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 4.van Rood JJ, van Leeuwen A, van Santen M. Anti HL-A2 inhibitor in normal human serum. Nature. 1970;226:366–367. doi: 10.1038/226366a0. [DOI] [PubMed] [Google Scholar]
- 5.Charlton RK, Zmijewski CM. Soluble HL-A7 antigen: Localization in the β-lipoprotein fraction of human serum. Science. 1970;170:636–637. doi: 10.1126/science.170.3958.636. [DOI] [PubMed] [Google Scholar]
- 6.Paul P, Cabestre FA, Ibrahim EC, Lefebvre S, Khalil-Daher I, Vazeux G, Quiles RM, Bermond F, Dausset J, Carosella ED. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Human Immunology. 2000;61(11):1138–1149. doi: 10.1016/s0198-8859(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 7.Jendro M, Goronzy JJ, Weyand CM. Structural and functional characterization of HLA-DR molecules circulating in the serum. Autoimmunity. 1991;8(4):289–296. doi: 10.3109/08916939109007636. [DOI] [PubMed] [Google Scholar]
- 8.Aultman D, Adamashvili I, Yaturu K, Langford M, Gelder F, Gautreaux M, Ghali GE, McDonald J. Soluble HLA in human body fluids. Human Immunology. 1999;60(3):239–244. doi: 10.1016/s0198-8859(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 9.Zavazava N, Bottcher H, Ruchholtz WM. Soluble MHC class I antigens (sHLA) and anti-HLA antibodies in heart and kidney allograft recipients. Tissue Antigens. 1993;42(1):20–26. doi: 10.1111/j.1399-0039.1993.tb02161.x. [DOI] [PubMed] [Google Scholar]
- 10.Billing RJ, Safani M, Peterson P. Soluble HLA antigens present in normal human serum. Tissue Antigens. 1977;10(2):75–82. doi: 10.1111/j.1399-0039.1977.tb01122.x. [DOI] [PubMed] [Google Scholar]
- 11.Pouletty P, Ferrone S, Amesland F, Cohen N, Westhoff U, Charron D, Shimizu RM, Grosse-Wilde H. Summary report from the first international workshop on soluble HLA antigens. Paris, August 1992 Tissue Antigens. 1993;42(1):45–54. doi: 10.1111/j.1399-0039.1993.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 12.Adamashvili IM, McDonald JC, Fraser PA, Milford EL, Pressly TA, Gelder FB. Soluble class I HLA antigens in patients with rheumatoid arthritis and their families. Journal of Rheumatology. 1995;22:1025–1031. [PubMed] [Google Scholar]
- 13.Tsuchiya N, Shiota M, Yamaguchi A, Ito K. Elevated serum level of soluble HLA class I antigens in patients with systemic lupus erythematosus. Arthritis and Rheumatism. 1996;39(5):792–796. doi: 10.1002/art.1780390511. [DOI] [PubMed] [Google Scholar]
- 14.Puppo F, Pellicci R, Brenci S, Nocera A, Morelli N, Dardano G, Bertocchi M, Antonucci A, Ghio M, Scudeletti M, Barocci S, Valente U, Indiveri F. HLA class-I-soluble antigen serum levels in liver transplantation. A predictor marker of acute rejection. Human Immunology. 1994;40(3):166–170. doi: 10.1016/0198-8859(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 15.Puppo F, Scudeletti M, Indiveri F, Ferrone S. Serum HLA class I antigens: markers and modulators of an immune response? Immunology Today. 1995;16:124–127. doi: 10.1016/0167-5699(95)80127-8. [DOI] [PubMed] [Google Scholar]
- 16.Aulitzky WE, Grosse-Wilde H, Westhoff LT, Tilg H, Aulitzky W, Gastl G, Herold M, Huber C. Enhanced serum levels of soluble HLA class I molecules are induced by treatment with recombinant interferon-gamma. Clinical and Experimental Immunology. 1994;86:236–239. doi: 10.1111/j.1365-2249.1991.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimura T, Hagihara M, Yamamoto K, Takebe K, Munkhbat B, Ogoshi K, Mitomi T, Nagamachi Y, Tsuji K. Quantification of serum-soluble HLA class I antigens in patients with gastric cancer. Human Immunology. 1994;40:183–186. doi: 10.1016/0198-8859(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 18.Westhoff U, Fox C, Otto FJ. Soluble HLA class I antigens in plasma of patients with malignant melanoma. Anticancer Research. 1998;18(5B):3789–3792. [PubMed] [Google Scholar]
- 19.Shimura T, Tsutsumi S, Hosouchi Y, Kojima T, Kon Y, Yonezu M, Kuwano H. Clinical significance of soluble form of HLA class I molecule in Japanese patients with pancreatic cancer. Human Immunology. 2001;62:615–619. doi: 10.1016/s0198-8859(01)00246-4. [DOI] [PubMed] [Google Scholar]
- 20.Nocito M, Montalban C, Gonzalez-Porque P, Villar LM. Increased soluble serum HLA class I antigens in patients with lymphoma. Human Immunology. 1997;58:106–111. doi: 10.1016/s0198-8859(97)00227-9. [DOI] [PubMed] [Google Scholar]
- 21.Sebti Y, Le Friec G, Pangault C, Gros F, Drenou B, Guilloux V, Bernard M, Lamy T, Fauchet R, Amiot L. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Human Immunology. 2003;64:1093–1101. doi: 10.1016/j.humimm.2003.08.345. [DOI] [PubMed] [Google Scholar]
- 22.Albitar M, Vose JM, Johnson MM, Do K, Day A, Jilani I, Kantarjian H, Keating M, O’Brien SM, Verstovsek S, Armitage JO, Giles FJ. Clinical relevance of soluble HLA-I and β2-microglobulin levels in non-Hodgkin’s lymphoma and Hodgkin’s disease. Leukemia Research. doi: 10.1016/j.leukres.2006.02.013. In Press. [DOI] [PubMed] [Google Scholar]
- 23.Bataille R, Durie BG, Grenier J. Serum beta 2 microglobulin and survival duration in multiple myeloma: a simple reliable marker for staging. British Journal of Haematology. 1983;55:439–447. doi: 10.1111/j.1365-2141.1983.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 24.Chronowski GM, Wilder RB, Tucker SL, Ha CS, Sarris AH, Hagemeister FB, Barista I, Hess MA, Cabanillas F, Cox JD. An elevated serum beta-2-microglobulin level is an adverse prognostic factor for overall survival in patients with early-stage Hodgkin disease. Cancer. 2002;95:2534–2538. doi: 10.1002/cncr.10998. [DOI] [PubMed] [Google Scholar]
- 25.Simonsson B, Wibell L, Nilsson K. Beta 2-microglobulin in chronic lymphocytic leukaemia. Scandinavian Journal of Haematology. 1980;24:174–180. doi: 10.1111/j.1600-0609.1980.tb02364.x. [DOI] [PubMed] [Google Scholar]
- 26.Zavazava N, Kronke M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nature Medicine. 1996;2(9):1005–1010. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- 27.Contini P, Ghio M, Merlo A, Poggi A, Indiveri F, Puppo F. Apoptosis of antigen-specific T lymphocytes upon the engagement of CD8 by Soluble HLA Class I Molecules is FasLigand/Fas mediated: Evidence for the involvement of p56lck, calcium calmodulin kinase II, and calcium-independent protein kinase C signaling pathways and for NF-κB and NF-AT nuclear translocation. The Journal of Immunology. 2005;175:7244–7254. doi: 10.4049/jimmunol.175.11.7244. [DOI] [PubMed] [Google Scholar]
- 28.Spaggiari GM, Contini P, Dondero A, Carosio R, Puppo F, Indiveri F, Zocchi MR, Poggi A. Soluble HLA class I induces NK cell apoptosis upon the engagement of killer-activating HLA class I receptors through FasL-Fas interaction. Blood. 2002;100:4098–4107. doi: 10.1182/blood-2002-04-1284. [DOI] [PubMed] [Google Scholar]


