Abstract
Purpose
The immature rat brain is highly susceptible to seizures, but has a resistance to pathological changes induced by seizures as compared to adult rats. However, prolonged seizures during early-life enhance cellular injury and hyperexcitability induced by convulsive insults later in adulthood. The mechanisms underlying these phenomena are not understood. In adult models, the CA1 axons reorganize their projections to subiculum. Seizure induced plasticity in this pathway has not been investigated in immature seizure models, and may contribute to the vulnerability to later seizures.
Methods
On postnatal day 15, rats experienced convulsive status epilepticus with kainic acid (KA). Seizure induced plasticity was examined with Timm histochemistry and iontophoretic injections of sodium selenite, a retrograde tracer. Cellular injury was evaluated with Fluoro-Jade B histochemistry.
Results
Retrograde tracing experiments determined a 67% larger dorsoventral extent of retrograde labeling in the CA1 pyramidal region after tracer injections in subiculum. The synaptic reorganization of the CA1 projection to subiculum was noted in the absence of overt neuronal injury in subiculum or CA1. In contrast, mossy fiber sprouting was detected into the stratum oriens of CA3 with limited neuronal injury to CA3 pyramidal neurons. No mossy fiber sprouting into the inner molecular layer of the dentate gyrus, or CA1 sprouting into the stratum moleculare of CA1 were noted.
Conclusions
The results indicate that the developing brain has distinct mechanisms of seizure induced reorganization as compared to the adult brain. Our experiments show that the concept of “resistance of the immature brain to excitotoxicity” is considerably more complicated than generally believed. Morphological plasticity in the immature brain appears more extensive in distal, but not proximal, projections of hippocampal pathways, and across hippocampal lamellae. The abnormal connectivity between hippocampal lamellae might play a role in the increased susceptibility to injury and hyperexcitability associated with later convulsive insults.
Additional Key Words: sprouting, seizures, immature, retrograde tracing, Zinc
Introduction
The developing rat brain has an increased susceptibility to seizures due to its developmental neurobiology (Moshe et al., 1983; Sanchez and Jensen, 2001; Jensen, 2002). Several factors during normal brain development promote neuronal hyperexcitability (Plotkin et al., 1997; Ben-Ari, 2002) that manifests as an increased susceptibility to early-life seizures (Baram et. al., 1997; Jensen, 2002). Seizures occurring during early life appear to be detrimental to neurological development (Liu et al., 1999; Sogawa et al., 2001; Brunquell et al., 2002; Miller et al., 2002), and increase the risk for epileptogenesis later in life (Cowan, 2002).
Experimental evidence indicates that most structures of the developing brain are resistant to cellular injury and long term synaptic reorganization in the hippocampus that is typically associated with convulsive insults in adult animals (Sperber et al., 1991; Haas et al., 2001). The notion of resistance suggests that seizures during development are less significant than during adulthood and not susceptible to long-term pathological changes. However, recent studies demonstrate that some CA3 pyramidal neurons are vulnerable to cellular injury and thus synaptic reorganization. Four models of early life seizures demonstrate granule cells in the dentate gyrus sprouting new mossy fiber synaptic terminals onto the basal dendrites in the CA3 region, the stratum oriens (Holmes et al., 1998, 1999; de Rogalski et al, 2001; Cilio et al., 2003).
Kainic acid induced status epilepticus (KA-SE) on postnatal day 15 (P15) induces minimal or no damage to hippocampal structures and the mossy fiber pathway does not exhibit the prominent reorganization into the inner molecular layer (IML) of the dentate gyrus (DG) that is typically observed in adult rats. This maturational resistance to sprouting in the DG has also been observed in other immature seizure models such as the fluorothyl model and the lithium-pilocarpine model on postnatal day 16 (Sperber et al., 1999; Cilio et al., 2003).
Studies investigating sprouting and synaptic reorganization in the hippocampus beyond the mossy fiber pathway have been focused to the CA1 axonal projections (Cavazos et al., 1989, 1999, 2004; Esclapez et al., 1999; Lehmann et al., 2000, 2001), and none of these studies have examined early-life seizure models. In models of adult partial onset epilepsy, sprouting of the proximal axons of CA1 pyramidal neurons has been observed in the basal and apical dendrites of CA1 pyramidal neurons using in-vitro hippocampal slices (Esclapez et al., 1999; Lehmann et al., 2000, 2001) and cannot depict the complexity of the distal CA1 terminal projections. Recurrent collateral sprouting of the CA1 pyramidal neurons after neuronal injury may underlie the persistent cellular hyperexcitability of CA1 region (Meier et al., 1992; Meier and Dudek, 1996; Smith and Dudek, 2001, 2002). The main projection of CA1 axons is to the subiculum, which also demonstrates cellular hyperexcitability in humans and animal model of epilepsy (Cohen et. al., 2002, Wozny et al., 2003; Knopp et. al., 2005; Stafstrom 2005) and thus, plasticity of CA1 synaptic connections might have a major role underlying hyperexcitability in subiculum physiology. The present study examined whether synaptic reorganization of the CA1 pyramidal axons develops after KA-SE on P15. Preliminary observations have been published in abstract form (Cross et al., 2003).
Methods
We examined the permanent morphological alterations and neuronal degeneration of the projection field of the mossy fiber and CA1 pyramidal axons three to four weeks (P36-P43) after an episode of status epilepticus induced with kainic acid (KA) on postnatal age day 15 (P15). This postnatal age was chosen to provide a comparison to similar studies that examine synaptic reorganization, neuronal excitability, neuronal cell death, spatial learning, and an enhanced susceptibility to seizures as an adult (Yang et. al., 1998; Koh et al., 1999; Lynch 2000; Haas et al., 2001). However, it is also important to note that at the ages (P38 and P74) used during the assessment of synaptic reorganization, the extent of the lamellar organization of the hippocampus has been studied extensively with neurophysiological techniques (in-vivo; Andersen et al., 1973, 2000; Anderson et al., 1971) as well as with histological techniques (in-vitro; Amaral and Witter, 1989, Cavazos et al., 2004).
Chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or Fisher-Scientific (Hampton, NH) unless otherwise specified. The procedures and protocols discussed in this paper were approved by the institutional animal care and use committee of our institution, and conform to local and international guidelines on the ethical use of animals. Attempts were made to minimize the number of animals used in the present studies and their suffering.
Systemic kainic acid model
Sprague-Dawley rats (Harlan, Madison, WI) of both sexes received repeated subcutaneous injections of 2.5 mg/kg of kainic acid (OPIKA-1, Ocean Produce, Inc., Shelburne, Nova Scotia) in 0.9 % saline (pH 7.4) on postnatal day 15. Three to four injections of 2.5 mg/kg of KA were given every 45 minutes based on the severity of seizures. A fourth injection of KA was only given if the rat had not shown evidence of convulsions. Rats were monitored for behavioral signs seizures for at least 5 hours after the first injection. The seizure severity was scored every 15 minutes using a previously validated scale (Hu et al. 1998; Koh et al., 1999). A score of 0 was given for normal behavior; score of 1 was given for repetitive scratching or “wet-dog” shakes; score of 2 for falling over and immobility; score of 3 for unilateral tonic or clonic jerking; score of 4 for bilateral tonic or clonic jerking; and a score of 5 for continuous clonus. The maximum score during the 15 minute epoch was considered the seizure score for that period. This protocol was devised to reduce the mortality induced by KA to less than 30% while also maintaining convulsive status epilepticus in all KA treated rats. Matched control rats were littermate rats that received three injections of vehicle (0.9% saline) every 45 minutes on P15. Paired epileptic and control rats were used for either Timm histochemistry or sodium selenite retrograde tracing experiments three to four weeks after the injections on P15.
Timm histochemistry
At the appropriate time, rats from experimental and control groups were deeply anesthetized with lethal dose of Beuthanasia-D (1 ml i.p., Schering-Plough, Kenilworth, NJ) and perfused transcardially with an aqueous solution of 500 ml of 0.4% (w/v) sodium sulphide, followed by 500 ml of 1.0% (w/v) paraformaldehyde/1.25% (w/v) glutaraldehyde solution according to previously published procedures (Cavazos et al., 1991, 2003, 2004). In brief, the brains were removed and left overnight in a 30% (w/v) solution of sucrose in fixative. Horizontal 40μm frozen sections were developed in the dark for 30–45 minutes in a 12:6:2 mixture of gum arabic (20% w/v), hydroquinone (5.6% w/v), and citric acid-sodium citrate buffer with 1.5 ml of a silver nitrate solution (17% w/v). Alternate sections were stained with cresyl violet to assess overt neuronal injury (Cavazos et al., 1990, 1994).
The laminar pattern of Timm histochemistry in the IML of the DG, stratum oriens of CA3, and stratum moleculare of CA1 were examined in rats that experienced KA-SE on age P15 and compared to the pattern in control rats. Ordinal scoring scales that have been previously validated (Cavazos et al, 1991, 1992, 2004; Holmes et al., 1999) were used to quantify the intensity the alterations of the laminar pattern in the areas of interest by two independent observers that were blinded to the identity of the rat. The ordinal scales for mossy fiber sprouting consist of a six-step ordinal ranking from 0–5 (see Fig. 2 of Cavazos et al., 1991 for IML; see Fig. 1 of Holmes et al., 1999 for CA3). A representative horizontal section of the mid-portion along the dorsal-ventral axis was selected for each rat. The scoring scale for CA1 sprouting into the stratum moleculare of CA1 consisted of a two-step ordinal ranking depicting the presence or absence of Timm granules in the stratum moleculare at a 10x magnification (Cavazos et al., 2004). A representative horizontal section at the level of the anterior commissure was selected for scoring. For each rat, the scores were averaged at each area of interest and the differences between the scores of KA-SE and control rats were analyzed using the nonparametric Kruskal-Wallis test.
Figure 2.
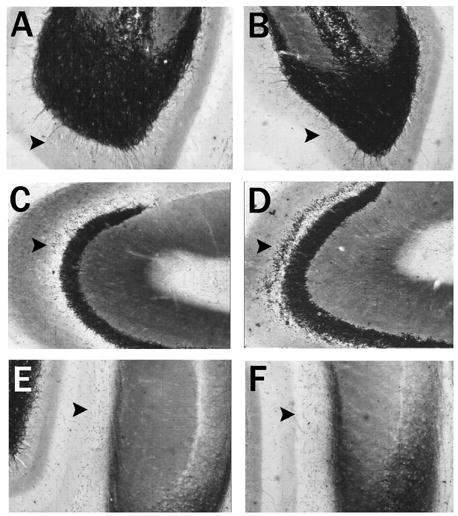
Hippocampus horizontal brain sections stained with Timm histochemistry after saline and kainic acid on postnatal day 15. Timm’s histochemistry depicts alterations in the laminar pattern of staining three weeks after rats experience an episode of convulsive status epilepticus induced by kainic acid (KA-SE) on postnatal day 15 as compared to controls. The inner molecular layer of the dentate gyrus (arrow) shows few or no punctate granules three weeks after saline (A) injections on postnatal day 15 (P15) or KA-SE on P15 (B). As compared to A, there are no alterations in the inner molecular layer of the dentate gyrus. The stratum oriens of CA3 in a normal control rat (C) sacrificed three weeks after saline injections on P15 shows few punctate granules (arrow). However, the stratum oriens of CA3 three weeks after a rat experienced KA-SE on P15 (D) developed a band of Timm granules in the stratum oriens of CA3 indicating sprouting of the mossy fiber projection to this region. These differences were quantified in Table 1. The stratum moleculare of the ventral CA1 region in a normal rat (E) sacrificed three weeks after saline injections on P15 shows few or no granules in this region (arrow); similar to rats that experienced KA-SE on P15 (F). As compared to E, there were no alterations in the pattern of Timm staining in this region.
Fig. 1.
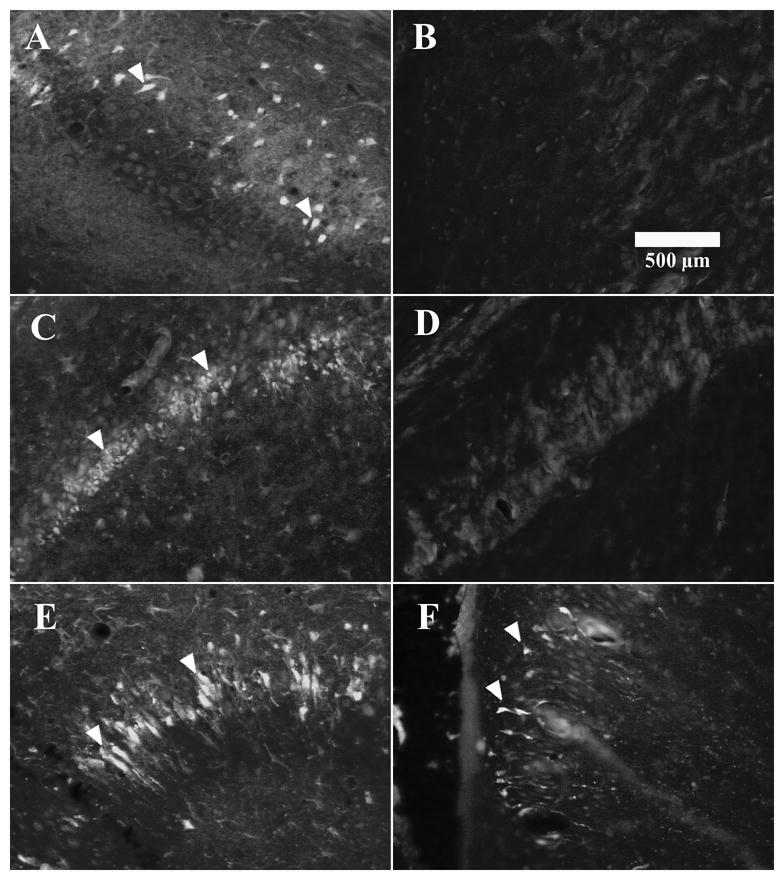
Fluoro-Jade B histochemistry indicating neuronal injury in the rat hippocampus. Fluoro-Jade B positive neurons in the (A) subiculum, (C) CA1, and (E) CA3, two days after adult rats were treated with kainic acid (KA). The (B) subiculum, and (D) CA1 region were absent of Fluoro-Jade B positive neurons two days after kainic acid on postnatal day 15. (F) Fluoro-Jade B positive pyramidal neurons in the CA3 region two days after KA on P15.
Sodium selenite retrograde tracing
Zinc containing synaptic terminals are present in excitatory glutamatergic neurons and also serve an important modulatory role. A retrograde neuroanatomical tracing method using iontophoretic injection of sodium selenite (Na2SeO3; Sigma-Aldrich) was selected to assess the location of the cell bodies of origin of the zinc containing terminals of the CA1 projection to the subiculum because of the high specificity of this technique to selectively label this subset of synaptic terminals (Howell and Frederickson, 1990). Selenite anions are taken up by a heavy metal transporter that is present in zinc-containing pre-synaptic terminals to re-uptake released zinc (Danscher, 1984). The selenite anions in the presynaptic terminal precipitate with ionic zinc to form a molecule that is retrograde transported to the cell soma (to lysosome-like organelles) and later visualized using an auto-metallographic silver enhancement stain (Howell et al., 1989; Mandava et al., 1993, Cavazos et al., 2004). The histochemical reaction is quite similar to the developing process of Timm histochemistry. One-day survival after iontophoretic injection in the subiculum is sufficient to local retrograde transport into the CA1 pyramidal cell body. Although this histochemical method is highly specific and clearly identifies the cells of origin of the zinc containing terminals (Slomianka, 1992; Long et. al., 1995), it only faintly stains the axonal arbor not showing their trajectory as exquisitely as other neuronal tracers. In a lamellar structure, examining the ratio between the dorsoventral extent of labeled cell bodies and the extent of the injection site can be used to assess the projection field of a given pathway (Cavazos et al., 2004).
Rats were anesthetized with sodium pentobarbital (50–60 mg/kg, i.p.; Nembutal, Abbott Labs, Chicago, IL), and placed in a Kopf stereotactic apparatus for iontophoretic infusion with micropipettes filled with sodium selenite (2% in deionized water) aimed to the subiculum (5.90 mm posterior, 4.65 mm lateral and 3.50 mm ventral from bregma). The fire polished micropipettes tips had a 1–2 μm outside diameter. Iontophoresis was performed using a Midgard constant current source (Stoelting, Wood Dale, IL) of 2 mA negative current for 0.2–1 min with a 7 sec duty cycle, and according to previously published protocols (Howell and Frederickson, 1990; Mandava et al, 1993; Cavazos et al., 2004). The rats were allowed to survive for 24 hours, and then were deeply anesthetized with Beuthanasia-D (1 ml i.p.). They were perfused with a 1.0% (w/v) paraformaldehyde/1.25% (w/v) glutaraldehyde solution in buffered Sorenson’s solution. The brains were removed and left overnight in a 30% (w/v) solution of sucrose in fixative. Horizontal 40μm frozen sections were obtained, mounted in gelatin-coated slides, and developed in the dark using the Timm histochemistry protocol described above. Every fifth section was stained with Timm developer. A consecutive section to the Timm stained section was used to stain with Cresyl violet (Cavazos et al., 1991), and a third consecutive section was used for Fluoro-Jade B staining (see below).
The extent of the injection site of sodium selenite and the retrograde-labeled cell bodies were mapped using an image analysis system (Neurolucida, Microbrightfield, Inc., Williston, VT, USA). A tri-dimensional reconstruction of injection site and retrograde labeling was obtained for each rat. As the adult hippocampal formation is organized in a lamellar distribution, the lamellar organization of mature rats was examined in rats that had experienced an episode of status epilepticus during the maturational process on P15. The ratios of the dorsal-ventral extent of the retrograde labeling / injection site in juvenile rats that experienced KA-SE on P15 were examined statistically using previously validated techniques (Cavazos et al., 2004). The extent of retrograde labeling was defined by the most dorsal and most ventral slice with retrograde labeling in at least 5 CA1 pyramidal neurons. Injection sites that were 1400 μm or larger along the dorsoventral axis were excluded from the analysis. The ratios obtained from the KA-SE and saline treated controls were statistically compared using standard two-tailed Student’s t test with unequal variance. The absolute dorsoventral extent in-vivo for the injection sites and retrograde labeling are at least 50% larger than values described in this manuscript because the aldehyde fixed brains were cryoprotected overnight with fixative in saturated sucrose. The cryoprotection induces a considerable shrinkage of the measurements in histological brain sections as compared to measurements obtained during physiological experiments in the in-vivo brain (Cavazos et al., 1991, 1994).
Fluoro-Jade B histochemistry
Histological frozen 40μm sections consecutive to cresyl violet (see above) were prepared for fluorescence FITC microscopy of Fluoro-Jade B (Histo-Chem, Inc., Jefferson, AR) labeled neurons in the hippocampus according to the protocol described by Schmued et al (1997). In brief, two hours after sectioning, the mounted sections were placed in graded alcohols, oxidized in 0.06% potassium permanganate solution for 15 min, and then bathed in a 0.001% Fluoro-Jade B solution in the dark for 30 min. After water rinses, the slides were dried overnight, dipped into xylene, and protected with cover slips glued with DPX (Electron Microscopy Sciences, Inc., Fort Washington, PA). Microphotographs of Fluoro-Jade B fluorescence were saved at 12.5X magnification from the entorhinal cortex, subiculum, CA3 and CA1 pyramidal regions, and hilar polymorphic region. Degenerating Fluoro-Jade B positive neurons are depicted by bright fluorescence over a lightly stained background. The distribution of Fluoro-Jade positive neurons in the hippocampal formation was examined 2 days after the exposure to KA or saline. A positive control consisted of adult rats treated with KA, which is known to induce prominent hippocampal degeneration.
Photography
Micrographs were photographed using a Zeiss Axioskop microscope and DC-330 CCD digital camera (Dage-MTI, Inc., Michigan City, IN) at native resolution of 752 x 582. The micrographs were saved as BMP files, imported into Adobe Photoshop, converted into grayscale from RBG color format, and subsequently labeled for illustration. The files were then converted into TIFF format.
Results
Sprague-Dawley rats experienced convulsive status epilepticus on postnatal day 15 (P15) after they were given either two hourly doses of 5mg/kg of kainic acid (KA) subcutaneously or four doses of 2.5mg/kg of KA every 45 minutes. The mortality rate for first protocol was 45% while the second protocol had 26% mortality. For the rest of this manuscript, we used the second protocol inducing status epilepticus with three to four injections of 2.5 mg/kg of KA subcutaneously. The average total dose of KA was 9.53 ± 1.68 mg/kg (mean ± SEM). Electroencephalography confirmed an excellent agreement between the video-EEG classification of seizures and the behavioral scoring scale (data not shown). Only rats that experienced KA induced convulsive status epilepticus (KA-SE) were included in this report.
2.1 Examination for cellular degeneration
Fluoro-Jade B histochemistry from all adult rats exposed to KA-SE demonstrated neuronal injury in neurons from the hilar polymorphic region, CA3 and CA1 pyramidal region, and in the subiculum. In the pyramidal region, Fluoro-Jade B positive labeling was observed in pyramidal shaped neurons with faint apical and basal dendrites. Two days after KA-SE, adult rats (n=5/5) demonstrated Fluoro-Jade B positive pyramidal neurons in the subiculum (Fig. 1A) CA1 (Fig 1C), and CA3 region (Fig 1E). In contrast, rats exposed to KA-SE on P15 (n=5/5) were absent of any labeled pyramidal neurons in the subiculum (Fig. 1B) or CA1 (Fig 1D) region two days after KA-SE, which was similar to saline treated controls. However, neuronal injury occurred after KA-SE on P15, but was limited to the CA3 pyramidal neurons (Fig. 1F). The CA3 region had sparse Fluoro-Jade B positive degenerating pyramidal neurons (n=3/5) two days after KA-SE on P15 (Fig. 1E and F). Fluorescent labeling was never observed in (n=5/5) saline injected rat controls (data not shown).
2.2 Mossy fiber sprouting – IML of DG and Str. Oriens of CA3
The average age of sacrifice for all Timm’s histochemistry experiments was postnatal day 73.5 ± 0.6 (mean ± SEM) after experiencing convulsive KA-SE at P15. In agreement with prior observations, there was no significant development of mossy fiber sprouting into the IML of the DG in (n=5/5) KA-SE rats as compared to age-matched saline injected controls (Figs. 2A, B). Despite the absence of mossy fiber sprouting into the IML after KA-SE on P15, mossy fiber sprouting into the stratum oriens of CA3B was observed (n=3/5) in the same rats that also did not have mossy fiber sprouting into the more proximal axon projection to the IML (Fig. 2C, D). The presence of mossy fiber sprouting into the stratum oriens of CA3 is a previously unrecognized finding in the immature KA seizure model. The differences in Timm scores between the saline injected control group and the KA-SE on P15 group were quantified using previously validated ordinal scales (Table 1; Cavazos et. al., 1991; Holmes et. al., 1999).
TABLE 1.
Analysis of scores reflecting alterations in Timm histochemistry observed in rats that experienced convulsive status epilepticus induced by repeated subcutaneous injections of kainic acid (KA-SE) on postnatal day 15 as compared to control rats injected with isotonic saline.
| P15 Saline | P15 KA-SE | |||||
|---|---|---|---|---|---|---|
| IML-DG | CA3B- St. Or. | CA1- St. Mol | IML-DG | CA3B- St. Or. | CA1- St. Mol | |
| Mean* | 0.600 | 0.600 | 0.714 | 0.556 | 2.700 | 0.750 |
| St. Dev. | 0.843 | 0.516 | 0.518 | 0.527 | 0.949 | 0.463 |
| S.E.M. | 0.377 | 0.231 | 0.231 | 0.236 | 0.424 | 0.207 |
| p-value | 0.754 | 0.009 | 0.835 | |||
referes to an ordinal scale from 0–5 (Cavazos et al., 1991; Liu et al., 1999).
2.3 CA1 synaptic reorganization – Str. moleculare of CA1
In contrast to adult limbic models (Cavazos et al., 2004), there was no difference in the laminar pattern of punctate granules in the stratum moleculare of CA1 between the saline injected control group (n=5/5) and the KA-SE on P15 group (n=5/5) (Fig. 2E, F). The results were quantified using a previously validated ordinal scale (Table 1; Cavazos et al., 2004).
2.4 CA1 synaptic reorganization – Subiculum
Iontophoretic injections of sodium selenite were performed at least 3 weeks after P15 KA-SE (or saline) to allow for the development of sprouting. The average age at perfusion was postnatal day 37.7 ± 0.6 (mean ± SEM). Most of the injection sites in the subiculum were at the mid-portion of the dorsoventral axis of the hippocampus at the level of anterior commissure. CA1 pyramidal neurons were consistently labeled ipsilaterally in all injections that included the subiculum. Neurolucida tracings of the anatomical distribution of the sodium selenite injection site and retrograde labeled CA1 pyramidal neurons were obtained (Fig. 3A–B). The tracings were then reconstructed into unilateral flat maps of the CA1 pyramidal region and subiculum (Fig. 3C–D). Photomicrographs of the serial sections used for Neurolucida tracing were submitted as supplementary material (Fig I, control; Fig. II, P15 KA-SE).
Fig. 3.
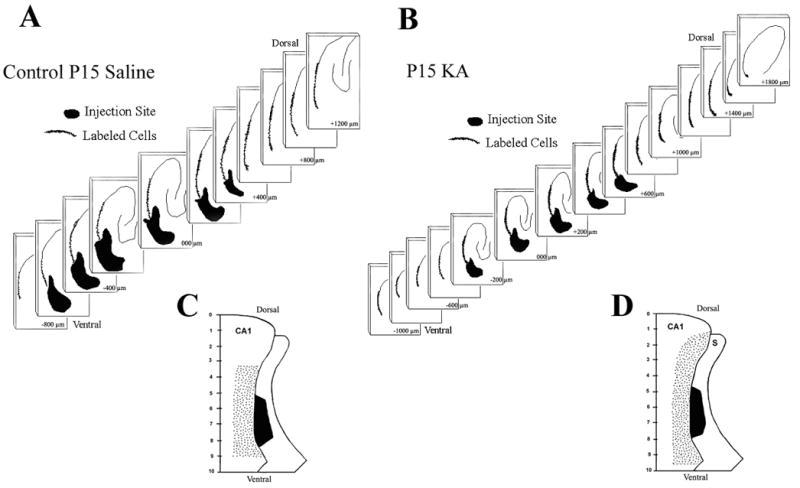
A. & B. Serial Neurolucida reconstructions of the rat hippocampus in horizontal brain sections of sodium selenite retrograde tracing in a rat that received saline injections (A) on postnatal day 15 (P15) and a rat that experienced convulsive status epilepticus induced by kainic acid (KA-SE) (B) on P15. The series of two-dimensional reconstructions demonstrate the dorsoventral extent of the injection site in the subiculum (dark filled area), and the larger dorsoventral extent of the retrograde labeling in the CA1 pyramidal region (dots depict labeled cells). Sections are arranged serially from the most dorsal section with labeling at the upper right corner to the most ventral section demonstrating labeling in the lower left corner. C. & D. Uni-dimensional flat maps of the dorsoventral extent of retrograde sodium selenite labeling in the CA1 pyramidal region and injection site in the subiculum. Saline treated controls (C) demonstrate a smaller dorsoventral extent of retrograde labeling in the CA1 pyramidal region as compared to a rat that experienced an KA-SE on P15 (D). The ratios of CA1 retrograde labeling / subiculum injection site for both groups of rats are shown in Table 2.
In the control rats (n=8/8), the dorsoventral extent of the injection site was 975 ± 88 μm (mean ± SEM) while the dorsoventral extent of the retrograde labeled pyramidal neurons was 2063 ± 150 μm. The ratio of dorsoventral extent of the retrograde labeled neurons to the injection site was 2.213 ± 0.20 (retrograde transport extent / injection site). In the group of rats that had experienced convulsive status epilepticus induced by KA on P15 (n=12/12), the dorsoventral extent of the injection site was 817 ± 87 μm while the dorsoventral extent of the retrograde labeled pyramidal neurons was 2667 ± 235 μm. The ratio of dorsoventral extent of the retrograde labeled neurons to the injection site was 3.686 ± 0.53. The statistical analysis revealed a significant difference between the lamellar ratios (p = 0.02) and the dorsoventral extent of retrograde labeling (p=0.04) of control and KA-SE on P15 rats using a two-tailed Student’s “t” test with unequal variance. There were no significant differences between the sizes of injection sites (p=0.22). The results are shown in Table 2. This experiment showed that subicular neurons in the P15 KA-SE group receive projections from a larger number of CA1 lamellae than normal controls.
TABLE 2.
Retrograde tracing of the CA1 projection to subiculum using sodium selenite in rats that experienced convulsive status epilepticus induced by repeated subcutaneous injections of kainic acid (KA-SE) on postnatal day 15 as compared to control rats injected with isotonic saline.
| P15 Saline | P15 KA-SE | |||||
|---|---|---|---|---|---|---|
| Ratio | Retrograde Labeling (μm) | Injection Site (μm) | Ratio | Retrograde Labeling (μm) | Injection Site (μm) | |
| Mean | 2.213 | 2062.500 | 975.000 | 3.686 | 2666.667 | 816.667 |
| St. Dev | 0.560 | 424.054 | 249.285 | 1.822 | 815.011 | 301.008 |
| S.E.M. | 0.198 | 149.926 | 88.135 | 0.526 | 235.273 | 86.894 |
| p-value | 0.0202 | 0.0446 | 0.2180 | |||
Discussion
Seizure-induced morphological plasticity in the immature brain is demonstrated in multiple limbic pathways in a KA-SE early-life seizure model, but with significant differences as compared to the adult brain. Rats exposed to KA-SE on P15 demonstrated synaptic reorganization of distal mossy fiber axons into the stratum oriens of CA3 and the distal CA1 projection to the subiculum. However, reorganization was absent in the proximal mossy fiber pathway (IML of the DG) and the proximal CA1 axons (CA1 stratum moleculare) which reorganize in the adult KA model (Cavazos et. al., 2004). Furthermore, synaptic reorganization in these hippocampal pathways was observed in the presence of limited neuronal injury to the CA3 pyramidal neurons, but also in the absence of overt neuronal injury in the subiculum, CA1, or polymorphic regions. Although neuronal injury in these areas is easily detected and quite prominent using Fluoro-Jade B histochemistry in the adult KA model, neuronal counts of these regions need to be performed to demonstrate the absence of neuronal injury. Several of our observations have not been previously described.
Synaptic reorganization induced by excitotoxic injury in the immature brain has been shown in the distal projection of the mossy fibers, to the stratus oriens of the CA3 pyramidal region, in four experimental models (Holmes et al.,1998; 1999; de Rogalski et al, 2001; Cilio et al., 2003) and in models of adult seizures (Represa et al., 1989a). Our study describes similar reorganization of the mossy fiber projection to the CA3 region in an early-life KA seizure model.
Synaptic reorganization induced by seizures has been studied extensively in the mossy fiber pathway due to the ease of detecting changes in the laminar pattern of this pathway using Timm histochemistry or dynorphin-A immunocytochemistry (Sutula et al., 1988, 1989; Represa et al., 1989a, 1989b; Houser et al., 1990; Cavazos et al., 1991, 1992, 2003; Golarai et al., 1992). Most studies in experimental models of partial-onset epilepsy have described sprouting of the mossy fiber axons into the IML of the DG. However, after early-life seizures, mossy fiber sprouting in the DG has not been observed (Sperber et al., 1999; Holmes et al., 1999; Haas et al., 2001; Cilio et al., 2003). Our study confirms these previous observations in the IML of the DG in an early-life KA seizure model.
A novel approach in our study was the examination of synaptic reorganization and sprouting of proximal and distal axonal projections from CA1 pyramidal neurons after early-life KA-SE. No previous studies investigating early-life seizures have examined morphological plasticity in this region. We utilized a retrograde tracing method to study the axonal projection of CA1 pyramidal neurons to the subiculum which is topographically organized from a hippocampal lamellar organization to a columnar organization in the subiculum. Using the retrograde tracer sodium selenite, we have been able to demonstrate that injection sites in subiculum in adult epileptic models received projections from a greater number of hippocampal lamellae (Cavazos et al., 2004). In the present study, we discover a similar type of morphological reorganization after early-life KA-SE induced on P15. Our experiments showed retrograde CA1 pyramidal labeling extending 67% beyond the normal topographic organization to include additional CA1 axonal projections from lamellae above and below the normal distribution. Visualization of sodium selenite utilizes the Timm’s histochemical method. Ultrastructural analysis of punctate Timm granules in the ventral CA1 corresponded to pre-synaptic terminals that contained spheroid presynaptic vesicles associated with asymmetric synapses (Gray type I synapse) with dendritic spines and shaft profiles suggestive of the excitatory neurotransmitter glutamate (Cavazos et al., 2004) onto subicular pyramidal neurons. This evidence suggests synaptic reorganization of the CA1 axonal projection to the subiculum, with greater interconnectivity between CA1 pyramidal lamella and the pyramidal neurons of the subiculum. Therefore, the results of this study do not support the widely held belief that developing brain is resistant to synaptic reorganization. As compared to the adult brain, the immature brain’s response to excitotoxicity seems to involve more prominent hippocampal neurogenesis but less widespread neuronal loss. Synaptic reorganization in hippocampal pathways proximal to the site of axon origin (i.e.: mossy fiber sprouting into the inner molecular layer; CA1 sprouting into the CA1 dendrites) does not appear to occur in the immature brain in response to excitotoxicity, while our study demonstrates that plasticity in the hippocampal pathways distal to the site of axon origin (i.e.: mossy fiber projection to CA3; CA1 projection to subiculum) displays a similar type of reorganization in the immature brain as it is seen in the adult brain in response to excitotoxicity. Therefore, the concept that the immature brain is resistant to excitotoxicity is considerably more complex than is generally quoted in the literature, and the belief that seizures in the immature brain are of little consequence is incorrect.
To validate the hypothesis that synaptic reorganization occurs in the hippocampus after early life seizures, anterograde tracing studies would be required to clarify the extent of the CA1 projection pathway, because the retrograde tracer sodium selenite only labels zinc containing synapses. Histological studies using fluorescent neuronal tracers, and excitatory and inhibitory synaptic markers simultaneously would be helpful to determine if the number of excitatory synapses has increased actually increased, or our results only indicate a greater interconnectivity between hippocampal lamella. Seizure induced anatomical reorganization of excitatory synapses in the hippocampus is usually associated with alterations in the functional neurophysiological properties of the circuitry. The functional consequences of CA1 synaptic reorganization should also be directly tested using in-vivo electrophysiological recordings to record evoked field excitatory postsynaptic potentials (fEPSPs) and plot the anatomical locations with abnormal current sources (current source density analysis) in the subiculum. These experiments would indicate if changes in the topographical organization of the CA1 projection to the subiculum are truly physiologically functional.
The finding of increased lamellar ratio and dorsoventral extent of CA1 pyramidal neurons might be better understood in the context of the lamella hypothesis of hippocampal organization. The hypothesis of functional lamella organization of the hippocampal formation was derived from extracellular field evoked potential mapping of the hippocampus (Andersen et al., 1973; Andersen, 1979; Anderson et al., 1971) in young adult rodents. The CA1 projection to subiculum is topographically organized in a columnar manner across the transverse plane (Amaral et al., 1991); however, there is some preservation of dorsoventral lamellar organization (Cavazos et al., 2004). The additional CA1 projections to the subiculum in the KA model of early-life seizures might provide pathways for further activation of hippocampal circuits that do not normally receive these projections. The substantial plasticity along the dorsoventral axis of the hippocampus allows for increased transverse connectivity among hippocampal lamellae above and below the normal circuitry. This might permit epileptiform discharges in a given hippocampal lamella to recruit additional lamellae, amplifying the epileptic response, and playing a role in establishing persistent hyperexcitability that characterizes intractable partial onset epilepsy (Bartesaghi, 1994; Bartesaghi and Gessi, 1986, 1990; Bartesaghi et al., 1989, 1995; Shao and Dudek, 2004). Activation of additional lamellae perhaps might also increase neuronal synchrony. Although rats that experienced kainic acid on postnatal 15 do not develop spontaneous seizures (data not shown), they are more susceptible to later seizures. Perhaps, synaptic reorganization in the distal CA3 and subiculum contribute to prime their hippocampal circuitry, enhancing their vulnerability to a convulsive insult later in life (Koh et al., 1999, 2004).
Supplementary Material
Acknowledgments
We would like to thank Yen Diep, Laura Moreno, and Kun Zhang for their technical assistance. This study was supported by a NINDS grant (NS02078-J.E.C.).
Abbreviations
- DG
dentate gyrus
- IML
inner molecular layer
- KA
Kainic acid
- KA-SE
Kainic acid induced status epilepticus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projection to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–436. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter M. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;3:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P. Factors influencing functional connectivity during hippocampal development. Progress in Brain Research. 1979;51:139–147. doi: 10.1016/S0079-6123(08)61302-3. [DOI] [PubMed] [Google Scholar]
- Andersen P, Bland BH, Dudar JD. Organization of the hippocampal output. Experimental Brain Research. 1973;17:152–168. doi: 10.1007/BF00235025. [DOI] [PubMed] [Google Scholar]
- Andersen P, Soleng AF, Raastad M. The hippocampal lamella hypothesis revisited. Brain Research. 2000;886:165–171. doi: 10.1016/s0006-8993(00)02991-7. [DOI] [PubMed] [Google Scholar]
- Anderson P, Bliss TV, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Experimental Brain Research. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Baram TZ, Gerth A, Schultz L. Brain Research Developmental Brain Research. 2. Vol. 98. 1997. Feb 20, Febrile seizures: an appropriate-aged model suitable for long-term studies; pp. 265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartesaghi R. Hippocampal-entorhinal relationships: electrophysiological analysis of the ventral hippocampal projections to the ventral entorhinal cortex. Neuroscience. 1994;61:457–466. doi: 10.1016/0306-4522(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T. Electrophysiological analysis of the hippocampal output to the presubiculum. Neuroscience. 1990;37:335–345. doi: 10.1016/0306-4522(90)90404-r. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T. Hippocampal output to the subicular cortex: an electrophysiological study. Experimental Neurology. 1986;92:114–133. doi: 10.1016/0014-4886(86)90129-9. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T, Migliore M. Input-output relations in the entorhinalhippocampal-entorhinal loop: entorhinal cortex and dentate gyrus. Hippocampus. 1995;5:440–451. doi: 10.1002/hipo.450050506. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T, Sperti L. Electrophysiological analysis of the hippocampal projections to the entorhinal area. Neuroscience. 1989;30:51–62. doi: 10.1016/0306-4522(89)90352-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nature Reviews Neuroscience. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Brunquell PJ, Glennon CM, DiMario FJ, Jr, Lerer T, Eisenfeld L. Prediction of outcome based on clinical seizure type in newborn infants. Journal of Pediatrics. 2002;140:707–712. doi: 10.1067/mpd.2002.124773. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula T. Kindling Induces a Pattern of Neuronal Loss in the Hippocampus that Resembles Human Hippocampal Sclerosis. Journal of Neuroscience. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Sutula TP. Morphological evidence for synaptic reorganization induced by kindling in the stratum moleculare of the CA1/subiculum transitional region of the rat hippocampal formation. Society for Neuroscience Abstracts. 1989;15:454. [Google Scholar]
- Cavazos JE, Sutula TP. Progressive neuronal loss induced by kindling: A possible mechanism for mossy fiber synaptic reorganization and hippocampal sclerosis. Brain Research. 1990;527:1–6. doi: 10.1016/0006-8993(90)91054-k. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: Time course of development, progression, and permanence. Journal of Neuroscience. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos JE, Golarai G, Sutula TP. Septotemporal Variation of the Supragranular Projection of the Mossy Fiber Pathway in the Dentate Gyrus of Normal and Kindled Rats. Hippocampus. 1992;2:363–372. doi: 10.1002/hipo.450020404. [DOI] [PubMed] [Google Scholar]
- Cavazos J, Levine S, Jones S. Recurrent Excitatory Projection of CA1 Pyramidal Neurons to the Stratum Lacunosum of CA1 in Normal and Epileptic Rats. Epilepsia. 1999;40 (Suppl 7):21. [Google Scholar]
- Cavazos JE, Zhang P, Qazi R, Sutula TP. Ultrastructural Features of Sprouted Mossy Fiber Synapses in Kindled and Kainic Acid-Treated Rats. Journal of Comparative Neurology. 2003;458:272–292. doi: 10.1002/cne.10581. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Jones SM, Cross DJ. Sprouting and Synaptic Reorganization in the Subiculum and CA1 Region the Hippocampus in Acute and Chronic Models of Partial-Onset Epilepsy. Neuroscience. 2004;126:675–686. doi: 10.1016/j.neuroscience.2004.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilio MR, Sogawa Y, Cha BH, Liu X, Huang LT, Holmes GL. Long term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia. 2003;44:518–528. doi: 10.1046/j.1528-1157.2003.48802.x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002 Nov 15;298(5597):1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Cowan LD. The epidemiology of the epilepsies in children. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8:171–181. doi: 10.1002/mrdd.10035. [DOI] [PubMed] [Google Scholar]
- Cross D, Diep Y, Zhang K, Cavazos J. Neonatal Status Epilepticus induces Sprouting and Synaptic Reorganization of the CA1 to Subiculum pathway in the Hippocampus. Society for Neuroscience Abstracts. 2003;29:211.2. [Google Scholar]
- Danscher G. Dynamic changes in the stainability of rat hippocampal mossy fiber boutons after local injections of sodium sulphide, sodium selenite, and sodium diethyldithiocarbamate. In: Frederickson CJ, Howell GA, Karsarskis EJ, editors. The neurobiology of zinc. New York: Alan R. Liss; 1984. p. 177. [Google Scholar]
- de Rogalski Landrot I, Minokoshi M, Silveira DC, Cha BH, Holmes GL. Recurrent neonatal seizures: relationship of pathology to the electroencephalogram and cognition. Developmental Brain Research. 2001;129:27–38. doi: 10.1016/s0165-3806(01)00177-8. [DOI] [PubMed] [Google Scholar]
- Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. Journal of Comparative Neurology. 1999;408:449–460. doi: 10.1002/(sici)1096-9861(19990614)408:4<449::aid-cne1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Research. 1983;271:201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Golarai G, Cavazos JE, Sutula TP. Activation of the dentate gyrus by pentylenetetrazol evoked seizures induces mossy fiber synaptic reorganization. Brain Research. 1992;593:257–264. doi: 10.1016/0006-8993(92)91316-7. [DOI] [PubMed] [Google Scholar]
- Haas KZ, Sperber EF, Opanashuk LA, Stanton PK, Moshe SL. Resistance of immature hippocampus to morphologic and physiologic alterations following status epilepticus or kindling. Hippocampus. 2001;11:615–625. doi: 10.1002/hipo.1076. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Annals of Neurology. 1998;44:845–857. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Sarkisian M, Ben-Ari Y, Chevassus-Au-Louis N. Mossy fiber sprouting after recurrent seizures during early development in rats. Journal of Comparative Neurology. 1999;404:537–553. doi: 10.1002/(sici)1096-9861(19990222)404:4<537::aid-cne9>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Houser C, Miyashiro J, Swartz B, Walsh G, Rich J, Delgado-Escueta A. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. Journal of Neuroscience. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell GA, Frederickson CJ. A retrograde transport method for mapping zinccontaining fiber systems in the brain. Brain Research. 1990;515:277–286. doi: 10.1016/0006-8993(90)90607-d. [DOI] [PubMed] [Google Scholar]
- Howell GA, Frederickson CJ, Danscher G. Evidence from dithizone and selenium zinc histochemistry that perivascular mossy fiber boutons stain preferentially “in vivo”. Histochemistry. 1989;92:121–125. doi: 10.1007/BF00490230. [DOI] [PubMed] [Google Scholar]
- Hu RQ, Koh S, Torgerson T, Cole AJ. Neuronal stress and injury in C57/BL mice after systemic kainic acid administration. Brain Research. 1998;810:229–240. doi: 10.1016/s0006-8993(98)00863-4. [DOI] [PubMed] [Google Scholar]
- Jensen FE. The role of glutamate receptor maturation in perinatal seizures and brain injury. International Journal of Developmental Neuroscience. 2002;20:339–347. doi: 10.1016/s0736-5748(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Koh S, Storey TW, Santos TC, Mian AY, Cole AJ. Early-life seizures in rats increase susceptibility to seizure induced brain injury in adulthood. Neurology. 1999;53:915–921. doi: 10.1212/wnl.53.5.915. [DOI] [PubMed] [Google Scholar]
- Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005 Mar 21;483(4):476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- Lehmann TN, Gabriel S, Kovacs R, Eilers A, Kivi A, Schulze K, Lanksch WR, Meencke HJ, Heinemann U. Alterations of neuronal connectivity in area CA1 of hippocampal slices from temporal lobe epilepsy patients and from pilocarpinetreated epileptic rats. Epilepsia. 2000;41 (Suppl 6):S190–S194. doi: 10.1111/j.1528-1157.2000.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Lehmann TN, Gabriel S, Eilers A, Njunting M, Kovacs R, Schulze K, Lanksch WR, Heinemann U. Fluorescent Tracer in Pilocarpine-Treated Rats Shows Widespread Aberrant Hippocampal Neuronal Connectivity. European Journal of Neuroscience. 2001;14:83–95. doi: 10.1046/j.0953-816x.2001.01632.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang LT, Stafstrom CE, Holmes GL. Consequences recurrent seizures during early brain development. Neuroscience. 1999;92:1443–1454. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Long Y, Hardwick AL, Frederickson CJ. Zinc-containing innervation of the subicular region in the rat. Neurochemistry International. 1995;27:95–103. doi: 10.1016/0197-0186(94)00171-p. [DOI] [PubMed] [Google Scholar]
- Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. European Journal of Neuroscience. 2000 Jul;12(7):2252–64. doi: 10.1046/j.1460-9568.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Mandava P, Howell GA, Frederickson CJ. Zinc-containing neuronal innervation of the septal nuclei. Brain Research. 1993;608:115–122. doi: 10.1016/0006-8993(93)90781-h. [DOI] [PubMed] [Google Scholar]
- Meier CL, Dudek FE. Spontaneous and Stimulation-Induced Synchronized Bursts Afterdischarges in the Isolated CA1 of Kainate-Treated Rats. Journal of Neurophysiology. 1996;76:2231–2239. doi: 10.1152/jn.1996.76.4.2231. [DOI] [PubMed] [Google Scholar]
- Meier CL, Obenaus A, Dudek FE. Persistent Hyperexcitability in Isolated Hippocampal CA1 of Kainate-Lesioned Rats. Journal of Neurophysiology. 1992;68:2120–2127. doi: 10.1152/jn.1992.68.6.2120. [DOI] [PubMed] [Google Scholar]
- Miller SP, Wiess J, Barnwell A, Ferriero DM, Latal-Hajnal B, Ferrer-Rogers A, Newton N, Partridge JC, Glidden DV, Vigneron DB, Barkovich AJ. Seizure associated brain injury in term newborns with perinatal asphyxia. Neurology. 2002;58:542–548. doi: 10.1212/wnl.58.4.542. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Albala BJ, Ackermann RF, Engel J. Increased Seizure Susceptibility of the Immature Brain. Developmental Brain Research. 1983;7:81–85. doi: 10.1016/0165-3806(83)90083-4. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: a possible mechanism underlying GABA’s excitatory role in immature brain. Journal of Neurobiology. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rawlins JN, Green KF. Lamellar organisation in the rat hippocampus. Experimental Brain Research. 1977;28:335–44. doi: 10.1007/BF00235715. [DOI] [PubMed] [Google Scholar]
- Represa A, Le Gal La Salle G, Ben-Ari Y. Hippocampal plasticity in the kindling model of epilepsy in rats. Neuroscience Letters. 1989a;99:345–350. doi: 10.1016/0304-3940(89)90471-0. [DOI] [PubMed] [Google Scholar]
- Represa A, Robain O, Tremblay E, Ben-Ari Y. Hippocampal plasticity in childhood epilepsy. Neuroscience Letters. 1989b;99:351–355. doi: 10.1016/0304-3940(89)90472-2. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Jensen FE. Maturational Aspects of Epilepsy Mechanisms and Consequences for the Immature Brain. Epilepsia. 2001;42:577–585. doi: 10.1046/j.1528-1157.2001.12000.x. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Research. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Shao LR, Dudek FE. Increased excitatory synaptic activity and local connectivity of hippocampal CA1 pyramidal cells in rats with kainate-induced epilepsy. Journal of Neurophysiology. 2004;92:1366–1373. doi: 10.1152/jn.00131.2004. [DOI] [PubMed] [Google Scholar]
- Slomianka L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience. 1992;48:325–352. doi: 10.1016/0306-4522(92)90494-m. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Short- and long-term changes in CA1 network excitability after kainate treatment in rats. Journal of Neurophysiology. 2001;85:1–9. doi: 10.1152/jn.2001.85.1.1. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dudek FE. Network Interactions mediated by new excitatory connections between ca1 pyramidal cells in rats with kainate-induce epilepsy. Journal of Neurophysiology. 2002;87:1655–1658. doi: 10.1152/jn.00581.2001. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Stanton PK, Moshe SL. Resistance of the immature hippocampus to seizure-induced synaptic reorganization. Developmental Brain Research. 1991;60:88–93. doi: 10.1016/0165-3806(91)90158-f. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Haas KZ, Romero MT, Stanton PK. Flurothyl status epilepticus in developing rats: behavioral, electrographic histological and electrophysiological studies. Developmental Brain Research. 1999;116:59–68. doi: 10.1016/s0165-3806(99)00075-9. [DOI] [PubMed] [Google Scholar]
- Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Developmental Brain Research. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Epilepsy Currents. 4. Vol. 5. 2005. The Role of the Subiculum in Epilepsy and Epileptogenesis; pp. 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic Reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Annals of Neurology. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, Nojyo Y. Columnar organization in the subiculum formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. Brain Research. 1987;412:156–160. doi: 10.1016/0006-8993(87)91452-1. [DOI] [PubMed] [Google Scholar]
- Wozny C, Kivi A, Lehmann TN, Dehnicke C, Heinemann U, Behr J. Comment on “On the origin of interictal activity in human temporal lobe epilepsy in vitro”. Science. 2003 Jul 25;301:5632–463. doi: 10.1126/science.1084237. [DOI] [PubMed] [Google Scholar]
- Yang Y, Tandon P, Liu Z, Sarkisian MR, Stafstrom CE, Holmes GL. Synaptic reorganization following kainic acid-induced seizures during development. Developmental Brain Research. 1998;107:169–177. doi: 10.1016/s0165-3806(97)00211-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


