Abstract
Menkes Disease (MD) is a neurodegenerative disorder caused by mutations in the copper transporter, ATP7A, a P-type ATPase. We previously used the olfactory system to demonstrate that ATP7A expression is developmentally, not constitutive, regulated, peaking during synaptogenesis when it is highly expressed in extending axons in a copper-independent manner. Although not known to be associated with axonal functions, we explored the possibility that the inability of mutant ATP7A to support axon outgrowth contributes to the neurodegeneration seen in MD. In vivo analysis of the olfactory system in mottled brindled (Atp7aMobr) mice, a rodent model for MD, demonstrates that ATP7A deficiency affects olfactory sensory neuron (OSN) maturation. Disrupted OSN axonal projections and mitral/tufted cell dendritic growth lead to altered synapse integrity and glomerular disorganization in the olfactory bulbs of Atp7aMobr mice. Our data indicate that the neuronal abnormalities observed in MD are a result of specific age-dependent developmental defects. This study demonstrates a role for ATP7A and/or copper in axon outgrowth and synaptogenesis, and will further help identify the cause of the neuropathology that characterizes MD.
Keywords: ATP7A, Menkes Disease, olfactory system, olfactory sensory neurons, neurodevelopment, axonal outgrowth, synapse
Introduction
Menkes Disease (MD) is an X-linked recessive disorder associated with mutation of ATP7A, the Menkes protein, a P-type ATPase involved in cellular copper transport (Mercer, 2001; Mercer et al., 2003; Mercer and Llanos, 2003). MD causes systemic problems as well as severe mental retardation, neurodegeneration, autonomic dysfunction, and death in early childhood. CNS findings include abnormal myelination, gliosis (Leventer et al., 1997), cerebral and cerebellar atrophy, abnormal dendritic arborization and focal axonal swelling along with prominent neuronal loss (Geller et al., 1997; Waggoner et al., 1999). The molecular basis of the profound neurodegeneration is not known (Waggoner et al., 1999; Schlief et al., 2005).
At the cellular level, ATP7A is required for the transport of copper from intracellular stores into the trans-Golgi network (TGN) for incorporation into cuproenzymes or for cellular efflux (Petris et al., 1996; Harrison and Dameron, 1999; Mercer, 2001; Mercer and Llanos, 2003). In non-neuronal cells, ATP7A trafficking is responsive to intracellular copper levels. ATP7A is localized to the TGN when cytosolic copper levels are normal or low, while ATP7A shifts to a cytoplasmic vesicular compartment for copper excretion in the presence of excess copper. In fibroblasts, ATP7A mutation causes ATP7A to remain in the TGN as it is unable to sense increased copper levels and translocate to efflux copper (La Fontaine et al., 1999).
Loss of functional ATP7A in MD causes failure of copper transfer across the placenta, gastrointestinal tract and kidney tubules, as well as impaired transport of copper across the blood brain barrier, resulting in lowered serum and tissue copper levels (Mercer, 1998; Harrison and Dameron, 1999). Cuproenzymes whose activities may be compromised include dopamine-β-monooxygenase, peptidylglycine α-amidating monooxygenase, cytochrome c oxidase, superoxide dismutase, lysyl oxidase, and tyrosinase (Kaler, 1994; Kodama and Murata, 1999; Mercer, 2001; Steveson et al., 2003; Qin et al., 2006). The neurodegeneration characteristic of MD is not correlated to the impaired function of any specific copper-dependent enzyme (Waggoner et al., 1999).
Little is known about the functions of ATP7A in neurons. In olfactory sensory neurons (OSNs) within the olfactory epithelium, ATP7A expression is developmentally regulated, not constitutive (El Meskini et al., 2005). While initially expressed in the cell bodies of developing neurons, ATP7A shifts to extending axons, where its levels of expression peaked prior to synaptogenesis. After injury-stimulated neurogenesis, ATP7A expression again increases in neurons and axons prior to synaptogenesis (El Meskini et al., 2005). These findings suggest that ATP7A functions in neurons at a critical time during neuronal development. Unexpectedly, both wild type and mutant copper-transport-deficient ATP7A localize to the axonal compartment as well as the TGN in mouse olfactory neurons. Schlief et al. (2005) showed that NMDA receptor activation of primary hippocampal neurons induces copper-independent rapid and reversible ATP7A trafficking to neuronal processes (Schlief et al., 2005). Both studies suggest that, in contrast to other cell types, ATP7A localization, trafficking and function in neurons are not exclusively copper dependent (El Meskini et al., 2005; Schlief et al., 2005).
We hypothesized that the neuronal abnormalities observed in MD are the result of a specific developmental defect incurred by ATP7A mutation/copper deficiency. The peripheral olfactory system is an advantageous model for exploring this hypothesis (Halasz and Greer, 1993). Ongoing neurogenesis and developmental stage-specific markers, coupled with the favorable cellular organization of the peripheral olfactory system, life cycle, and straightforward axonal projections of the OSNs, make this an ideal model for identifying the specific phases in neuronal development that are affected by loss of ATP7A function (see Fig. 9A for developmental stage-specific marker distribution) (Ronnett and Snyder, 1992; Ronnett and Payne, 1995; Buck, 1996; Roskams et al., 1998).
Fig. 9. Model of olfactory cell population homeostasis and olfactory bulb connection defects in Atp7aMobr mouse.

Olfactory epithelium schematic illustrating OSN cell cycle. The olfactory system displays a simple lamellar organization and ongoing functional neurogenesis throughout life (Graziadei and Monti-Graziadei, 1979). As OSNs mature, they move apically through the epithelium such that neuronal age is indicated by position within the epithelium and expression of stage-specific markers. OE-1 identifies all cells of the neuronal lineage; GAP-43, marker for late immature neuron; OMP, marker for mature neurons. BrdU labeling of precursor cells is used to determine cell proliferation rate; programmed cell death is assessed by TUNEL staining. The functionality of olfactory glomerulus synapses can be assessed indirectly by determining TH expression in periglomerular cells. OE: olfactory epithelium, OB: olfactory bulb, OSN: olfactory sensory neuron. A, in WT mice the precursor neuron population gives rise to immature neurons that differentiate into mature neurons that establish connections with dendrites in compact glomerular structures. The mature neuronal population is ultimately renewed by a steady feedback signal to the immature neurons to signal maturation. B, In Atp7aMobr mice, an increase in mature neuron cell death signals an increase in immature neuron number for renewal. However, the maturation process is delayed, since the transition from the immature to mature neuronal stage is defective. The failure of young neurons to mature may be secondary to the failure of mature neurons to provide retrograde signaling for replacement. In the mutant olfactory bulb, mature neuron connectivity is altered as reflected by disorganized overgrown OSN axonal projections and defects in the dendritic arborization of mitral/tufted cells. Finally, OSN-mitral cell synaptic defect result in mature neuron cell death. Dashed lines indicate defective progression in neuronal development (straight arrows) or defective retrograde signaling (curved arrows).
In the present study, we sought to determine whether previously unrecognized neurodevelopmental functions of ATP7A contribute to the defects observed in the Atp7aMobr mouse and thus the neuropathology seen in MD. We investigated the consequences of the presence of functionally deficient ATP7A to olfactory epithelium and olfactory bulb development using the mottled brindled mouse (Atp7aMobr), a model of MD. We assessed the distribution of cells within the neuronal lineage, cell turnover, neuronal maturation, and the process of dendritic and axonal targeting at different postnatal ages. Our results demonstrate a previously unrecognized role for ATP7A in neurodevelopment, with therapeutic implications.
Materials and Methods
Animals and tissue preparation
All experimental protocols were approved by the Johns Hopkins University Institutional Animal Care and Use Committee, and all applicable guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals were followed. The mottled brindled (Atp7aMobr) used in these experiments has mutations of the X-linked mottled locus (Mo) in mice, which produces a model of human MD (Cecchi et al., 1997; Grimes et al., 1997; Reed and Boyd, 1997). The Atp7aMobr mouse has a 6 bp deletion in the Atp7a gene, which eliminates two amino acids (Ala799 and Leu800) in frame (based on Genbank sequence Q64430), in addition to a reported substitution of Ala514 for Thr (Reed and Boyd, 1997). Affected male hemizygotes, Atp7aMobr/y, are hypopigmented, have severe neurological abnormalities, and die by 15 days after birth. Mottled brindled male (Atp7aMobr) and wild type male (WT) mice were obtained by mating heterozygous mottled-brindled (C57BL/6, Atp7aMobr/+) female mice with wild type (C57BL/6, Atp7a+/y) male mice (Jackson Laboratory, Bar Harbor, ME). Compared to the WT males (black), 2 week-old hemizygous brindled males (Atp7aMobr) are easily identified due to their lack of pigmentation. Their genotype was confirmed by extracting tail DNA for PCR using primers specific to the deleted region in the ATP7A gene (Grimes et al., 1997; El Meskini et al., 2005). Mice were anesthetized with Xylaket (100 mg/ml/25% Ketamine-HCl, 100 mg/ml/2.5% Xylazine, 14.2% ethanol) and perfused with phosphate–buffered saline (PBS, pH 7.4), followed by Bouin’s or 4% paraformaldehyde (PFA) fixative (Sigma, St Louis, MO). Tissue was dissected, post-fixed in Bouin’s or PFA at 4°C, washed and cryoprotected overnight in 30% sucrose in PBS, and embedded in Tissue-Tek O.C.T. (VWR, Baltimore, MD). Coronal sections (18 μm or 50 μm) of olfactory epithelium and bulb were cut on a cryostat (HM500M, Zeiss, Waldorf, Germany), mounted on Superfrost Plus™ slides (Fisher Scientific, Pittsburgh, PA), dried and stored at −80°C until processed for immunohistochemistry.
Immunohistochemistry
Immunohistochemical processing was conducted on regionally-matched sections from both genotypes. According to the number of turbinates present in the sections from anterior to posterior the number of turbinates varies. Using the turbinate system innate of the OE, we consistently used sections with 5–6 turbinates visible. Cryosections were rinsed in PBS (pH 7.4), permeabilized with 0.2 % or 0.3 % Triton-X100 in PBS for 30 min, blocked and processed as described (El Meskini et al., 2005). Sections were incubated overnight at 4°C with the following antibodies: olfactory epithelium 1 transcription factor (OE-1; 1:200; gift from Randall Reed, Johns Hopkins University School of Medicine, Baltimore, MD), olfactory marker protein (OMP; 1:1000; gift from F. Margolis, University of Maryland), growth-associated protein-43 (GAP-43; 1:500; Chemicon), synaptophysin (1:100; DAKO), microtubule-associated protein 2 (MAP-2; 1:200; Chemicon), postsynaptic density protein of 95 kDa (PSD95; 1:200; abcam), tyrosine hydroxylase (TH; 1:1000; Chemicon) and neural cell adhesion molecule (NCAM, 1:1000; Chemicon). Antigen-antibody complexes were visualized using cyanine (Cy3) -conjugated donkey anti-rabbit IgG, Cy3-conjugated donkey anti-goat IgG or anti-mouse IgG, or fluorescein (FITC)-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA). Antibodies were used in immunohistochemistry according to manufacture specification. For each immunostaining experiment controls were conducted; a positive primary antibody signal is considered positive only when a negative staining signal was obtained with a pre-immune serum (specific for each antibody used) or when the primary antibody against a particular protein was omitted. In addition, when used at the same concentration no positive immunostaining was obtained with all antibodies used in this study in other tissues that do not express those particular proteins.
Detection of proliferative cells using Bromodeoxyuridine (BrdU) labeling in vivo
Mice received a single intraperitoneal dose of 5-bromo-2′-deoxyuridine (BrdU, 50 mg/kg). After 6h for postnatal day 1 (P1) or 24 h for postnatal day 7 (P7) or 14 (P14), mice were perfusion-fixed and tissue was processed for immunohistochemistry. Sections were treated with 2N HCl, 0.2% Triton X-100 and neutralized with 0.1 M borate buffer pH 8.5 before they were incubated with antibody to BrdU (1:4000; Caltag Laboratory) and visualized with a Cy3-tagged donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc, West Grove, PA).
BrdU pulse
BrdU pulse labeling was performed as previously described (Kimura et al., 1990; Huard and Schwob, 1995; Takahashi et al., 1996). Three day old mice (P3) were injected with BrdU (50 mg/kg) twice, 5 h apart, to label a considerable number of cells in S-phase. At postnatal day 7 (P7) mice were perfusion-fixed and olfactory epithelium tissue was processed for BrdU and GAP-43 immunohistochemistry as described above using Cy3-conjugated donkey anti-mouse secondary for visualization of BrdU and FITC-conjugated donkey anti-rabbit for visualization of GAP-43.
Detection of cell death using Terminal Uridine deoxynucleotidyl transferase-mediated biotinylated UTP Nick End Labeling (TUNEL)
TUNEL staining was performed sequentially after GAP-43 or OMP immunostaining. After a 10 min wash with 0.1 M Tris-HCl (pH 8.0), tissue sections were incubated in 5 μg/ml proteinase K for 15 min. Washed sections were equilibrated in 30 mM Tris-HCl, 140 mM sodium cacodylate, 1 mM CoCl2, pH 7.2 before end labeling fragmented DNA with biotinylated dUTP (40 μM) and terminal transferase (0.3 U/ml) for 1h at 37°C. Biotinylated DNA was detected by amino-methyl coumarin acetic acid (AMCA) -conjugated streptavidin (Jackson Immunoresearch Laboratories, Inc, West Grove, PA).
Image processing and data analysis
Images were viewed on a Zeiss Axiovert microscope and captured using an Axiocam digital camera (Zeiss, Germany). Cells in matched sections from WT and Atp7aMobr mice and stained for either neuronal specific marker (OE-1, GAP-43 and OMP), BrdU or TUNEL were counted. Three fields per section were evaluated and averaged, with 6 to 9 sections taken from 3 to 4 animals from each experimental group. Openlab software (Improvision Inc., Lexington, MA) was used to measure the olfactory epithelium area (mm2) or length (mm). Statistical analysis was performed using Student’s two-tailed t test with samples of unequal variance; (**) and (***) were assigned, respectively, to p<10−6 and p<10−9 respectively.
Confocal microscopy
Regionally-matched coronal sections (50 μm) taken midway along the rostral-caudal axis were compared for different experimental groups. Sections were processed for immunohistohemistry as described above. Z-stack digital images (1 μm slice, 12 μM to 25 μM total stack size) were captured from a single optical plane using a LSM 5 Pascal Axioskop 2 scanning confocal microscope (Zeiss, Germany). Images were merged for reconstruction of the glomeruli.
Tissue extraction and Western blot
Olfactory bulbs from P1, P7 and P14 mice were harvested in ice-cold TMT [20 mM Na N-tris[hydroxymethyl]methyl-2-aminoethanesulfonic acid] (TES), 10 mM mannitol, 1% Triton X-100, pH 7.4] buffer containing protease inhibitors (El Meskini et al., 2000; El Meskini et al., 2001; El Meskini et al., 2005). Extracts were frozen and thawed three times and centrifuged at 13,000 rpm to remove cell debris. Protein concentrations were determined using bicinchoninic acid (Pierce, Rockford, IL). Aliquots of olfactory tissue were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4–15% gradient gels) (Laemmli, 1970) and subjected to Western blot analysis. Proteins transferred to Immobilon-P membranes (Millipore, Bedfore, MA). Blots were blocked with 5% nonfat dry milk in Tween 20 and Tris-buffered saline buffer and were visualized with synaptophysin (1:1000; DAKO), PSD95 (1:5000; Upstate) or MAP-2 antiserum (1:5000; Chemicon) and Super Signal West Pico Chemiluminescence Substrate Kit (Pierce, Rockford, IL). As a protein loading control, antibody to actin (JLA 20; 1:1000; Developmental Studies Hybridoma Bank, Iowa, IA) was used to probe the same blot.
Results
Effect of ATP7A mutation on OSN development in the olfactory epithelium
OSN distribution and development were assessed using neuronal-specific markers (Hartman and Margolis, 1975; Calof and Chikaraishi, 1989; Verhaagen et al., 1990a; Roskams et al., 1998) (see Fig. 9A) to compare the olfactory epithelium of WT and Atp7aMobr mice.
Atp7aMobr mice display an age-specific decrease in cell number in the neuronal lineage in the olfactory epithelium
As neuronal precursors leave the cell cycle, they express OE-1, identifying cells in the neuronal lineage (Davis and Reed, 1996; Simpson et al., 2002). Using antisera to OE-1, we determined the consequences of expression of a mutant ATP7A protein on the overall organization and distribution of neurons and their precursors within the olfactory epithelium (Fig. 1A–F). At postnatal days 1 (P1), 7 (P7) and 14 (P14), OE-1-positive cells were evenly distributed throughout the olfactory epithelium of WT mice, showing tightly packed neurons and their precursors (Fig. 1A, C and E). In the P1 and P7 epithelium of Atp7aMobr mice, OE-1 positive cells appeared reduced in number and disorganized (Fig. 1B and D). This difference in OE-1 positive cell organization was less apparent at P14 (Fig. 1E and F). These data suggested that ATP7A mutation affects the organization of cells in the neuronal lineage at P1 and P7, when neurons are maturing and forming synapses. This supported the hypothesis that functional ATP7A is important at specific stages of development for normal neuronal differentiation.
Fig. 1. ATP7A mutation affects number of cells of the olfactory neuronal lineage.
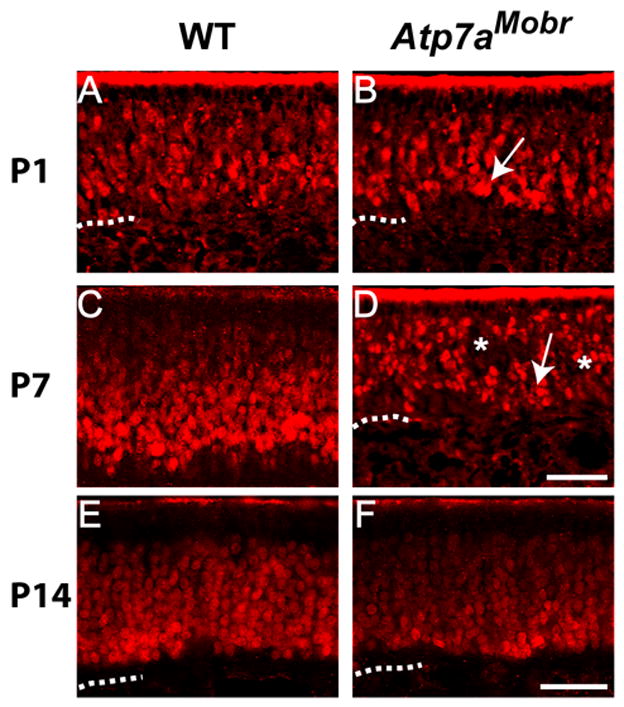
A–F, OE-1 immunofluorescence staining was performed to visualize cells of the neuronal lineage in WT (A, C and E) and Atp7aMobr mice (B, D and F). At P1 (B) and P7 (D) the olfactory epithelium was thinner in Atp7aMobr compared to WT mice (A and C). In WT mouse tissue, OE-1 staining was compact and dense throughout the epithelium. In Atp7aMobr tissue, OE-1 positive cells were disorganized, forming a dispersed zone at P1 and P7 (B and D, arrows). Patches void of OE-1 staining were noted throughout the Atp7aMobr epithelium at P1, P7 and P14 (B, D and F, asterisks). Scale bar: 40 μm (A–D) and 100 μm (E–F). Dashed line: Lamina propria.
ATP7A deficiency disrupts homeostasis among the neuronal populations in the olfactory epithelium
As a renewing system, a dynamic balance is maintained between cell birth and cell death to preserve populations of immature and mature neurons within the olfactory epithelium (Constanzo and Graziadei, 1983; Schwartz Levey et al., 1991; Carr and Farbman, 1992; Simpson et al., 2003). If ATP7A plays a role in OSN maturation, disruption of ATP7A function might alter directly or indirectly this balance. GAP-43 (growth associated protein-43) is expressed in immature OSNs that are actively extending axons prior to their contact with the olfactory bulb (Verhaagen et al., 1990b). OMP (olfactory mature protein) is expressed in mature OSNs that have established synaptic contact in the olfactory bulb (Keller and Margolis, 1975). There is normally very little overlap of GAP-43 and OMP expression during the transition of OSNs from the immature to the mature stage. We performed GAP-43 and OMP immunohistochemistry on Atp7aMobr and WT olfactory epithelium at P1, P7, and P14 (Fig. 2A–F) to determine whether ATP7A deficiency affected immature or mature OSNs, or the transition between these two populations.
Fig. 2. ATP7A dysfunction alters olfactory epithelium neuronal populations.
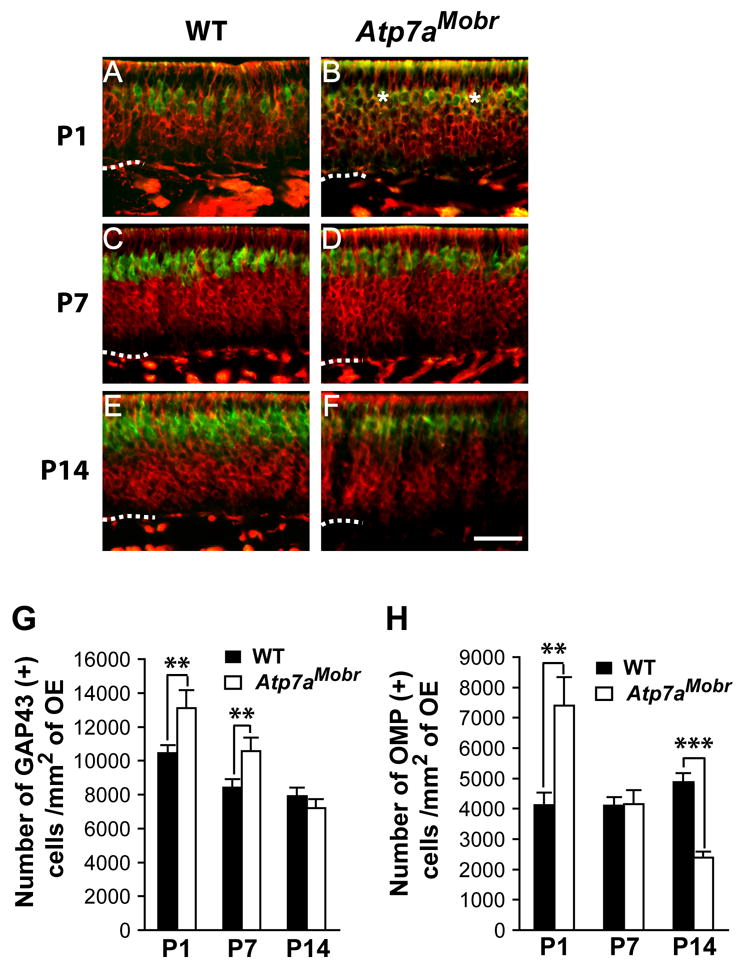
A–F, Olfactory epithelia from Atp7aMobr and WT mice were stained for GAP-43 (red) and OMP (in green) immunohistochemistry on P1, P7 and P14 to identify immature and mature OSNs, respectively. Scale bar: 40 μm. Dashed line: Lamina propria. G and H, Immature (GAP-43 positive) and mature (OMP positive) cells counted. More immature OSNs were present in Atp7aMobr mouse epithelium at P1 and P7 (Student’s t test; **, p<10−6) (G). At P14, the number of immature OSNs did not differ in the Atp7aMobr and WT epithelium. GAP-43 positive cells increased at P1 (**, p<10−6) and decreased at P14 (***, p<10−9) (H).
A dramatic difference in cell composition between WT and Atp7aMobr olfactory epithelium was apparent at P1. Mice lacking functional ATP7A had more GAP-43- and OMP-positive neurons. In addition, there was a population of neurons expressing both of these markers in the Atp7aMobr mice not present in WT mice (Fig. 2B, asterisk). Both immature (GAP-43-positive) and mature (OMP-positive) layers of the Atp7aMobr olfactory epithelium were larger than the corresponding regions in WT epithelium. These immunohistochemical observations were confirmed by cell counting (Fig. 2G and H). GAP-43- and OMP-positive cell numbers were increased significantly in P1 Atp7aMobr mice compared to their littermate WT controls. In addition, in the mutant epithelium, cells expressing both GAP-43 and OMP markers represented 12 ± 2% of the total cells expressing GAP-43 marker and/or OMP marker. By P7, a time of axon extension and increased synaptogenesis (Hinds and Hinds, 1976b), the situation changes (Fig. 2C and D). Cell count analyses showed that the number of GAP-43-positive cells remained significantly higher in Atp7aMobr mice than in WT mice (Fig. 2G) even though the number of mature OSNs in Atp7aMobr mice had normalized (Fig. 2H). At P14, there was no difference in the number of GAP-43 positive cells (Fig. 2E, F and G). However, there was a sharp decrease in the mature neuron population (OMP-positive) in the Atp7aMobr olfactory epithelium compared to WT (Fig. 2E, F, and H). Thus, the cellular population/homeostasis from immature to mature neurons in the olfactory epithelium is perturbed as a result of ATP7A dysfunction, suggesting a role for ATP7A function in terminal differentiation and/or neuronal turnover.
ATP7A function is crucial to neuronal turnover in the olfactory epithelium
Increased neuronal apoptosis has been observed in MD (Rossi et al., 2001; Ohno et al., 2002), suggesting that ATP7A mutation may affect neuronal survival, and perhaps account for the disruption of the balance between immature and mature OSNs. To determine whether ATP7A is essential for mature OSN survival, we assessed cell death by TUNEL labeling, which serves as a marker of programmed cell death (apoptosis) in many cell types, including OSNs (Holcomb et al., 1995). The numbers of TUNEL-positive cells were determined at P1, P7 and P14 in Atp7aMobr and WT olfactory epithelia (Fig. 3A). Indeed, ATP7A mutation resulted in an increase of total TUNEL positive cells in the P1 and P14 olfactory epithelium (Fig. 3A); no difference in TUNEL labeled cells between Atp7aMobr mutant mice and their littermate controls was found at P7 (Fig. 3A).
Fig. 3. Functional ATP7A is crucial to OSN survival.
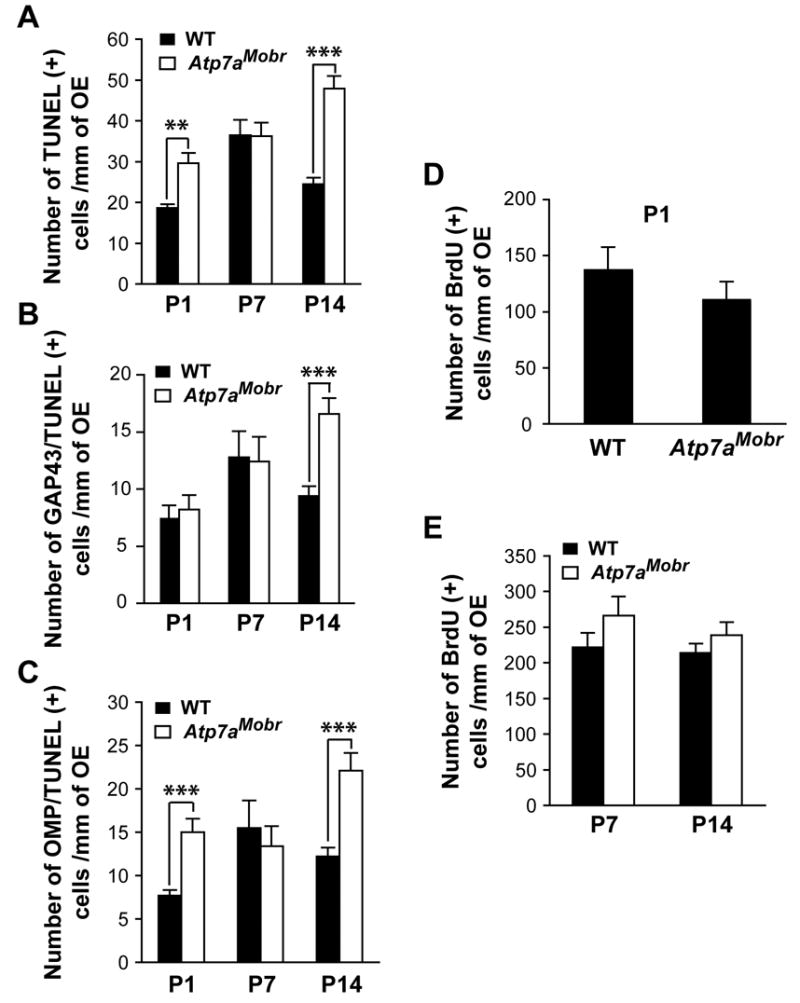
Atp7aMobr and WT olfactory epithelium was processed sequentially for GAP-43 and OMP immunofluorescent staining and TUNEL labeling. A–C, Total TUNEL positive cells, double labeled GAP-43/TUNEL cells and cells positive for OMP/TUNEL were counted at P1, P7 and P14. A, Total OSN cell death increased in ATP7A mutant mouse specifically at P1 and P14 (**, p<10−6 and ***, p<10−9 respectively). B and C, At P14, an increased of both immature (GAP-43-positive) and mature (OMP-positive) cell death (***, p<10−9) counts into the increase of the total TUNEL positive cells in Atp7aMobr mice. D and E, the number of proliferating cells in the olfactory epithelium was determined by BrdU labeling. Animals were analyzed after 6h (D, for P1) or 24h (E, for P7 and P14) following BrdU injection. At any analyzed age, the rate of precursor cell proliferation was not affected in Atp7aMobr mutant compared to WT mice.
To determine whether the survival of a specific population of OSNs (immature vs. mature) was affected in the Atp7aMobr mutant mice at P1 and P14 (Fig. 3A), GAP43/TUNEL and OMP/TUNEL double staining was performed. Surprisingly, while there was no increase in TUNEL staining in GAP-43-positive neurons at P1 (Fig. 3B), more TUNEL-positive cells were found in the mature cell (OMP-positive) population at P1 in Atp7aMobr compared to WT epithelium (Fig. 3C). Consistent with the lack of effect of ATP7A mutation on total TUNEL-positive cell numbers at P7 (Fig. 3A), no changes were noted in the numbers of TUNEL-labeled immature or mature cells at P7 (Fig. 3B and C). At P14, both GAP-43-positive (immature) and OMP-positive (mature) neurons contribute to the increase in cell death observed in the mutant mice (Fig. 3A–C). Thus, there appear to be two phases of development during which ATP7A supports neuronal survival: ATP7A plays a role in mature OSN survival in the early post-natal period and later on for the survival of both immature and mature neurons.
Changes in proliferation could contribute to the increased number of GAP-43-positive cells observed. We performed BrdU labeling to quantify the number of proliferating OSN precursor cells. WT and Atp7aMobr mice were injected with BrdU at P1, 7 and 14, and BrdU incorporation was detected immunohistochemically (Fig. 3D and E). There was no difference in the number of proliferating precursor cells in the olfactory epithelia of WT and Atp7aMobr mice at any age analyzed. Thus, ATP7A does not play a role in neuronal precursor proliferation. Collectively, these results suggest that ATP7A mutation disrupts neuronal differentiation, resulting in the accumulation of immature cells at P7.
ATP7A mutation delays OSN maturation at the time of peak synaptogenesis
The accumulation of immature GAP-43-positive OSNs with no increase in the number of mature neurons and no increase in proliferation of neuronal precursors in Atp7aMobr mutant mice at P7 suggests that ATP7A mutation results in a delay in OSN terminal maturation, which here is defined as the establishment of synapses in the olfactory bulb. To test this hypothesis, we performed a BrdU pulse experiment to label a cohort of OSN precursor cells at P3, in order to determine the fate of these cells at P7 (Fig. 4). To assess the fate of BrdU-labeled cells, we immunostained mutant and WT tissues for GAP-43 (Fig. 4A–C). BrdU labeling was predominantly found in the immature GAP-43 cell population in the Atp7aMobr mouse epithelium compared to the WT epithelium (Fig. 4A and B). The number of BrdU/GAP-43 double-immunolabeled neurons was significantly higher in the ATP7A mutant epithelium than in the WT epithelium (Fig. 4C). Since both the rate of precursor cell proliferation and the survival of immature neurons are not affected by ATP7A mutation at P7 (Fig. 3E and B, respectively), this increase in the number of GAP-43 positive cells is due to a block in neuronal maturation. These results demonstrate that ATP7A is required for OSNs to make a normal transition from immature to terminally differentiated neurons at a specific developmental time point when synaptogenesis occurs.
Fig. 4. OSN maturation is altered by ATP7A deficiency.
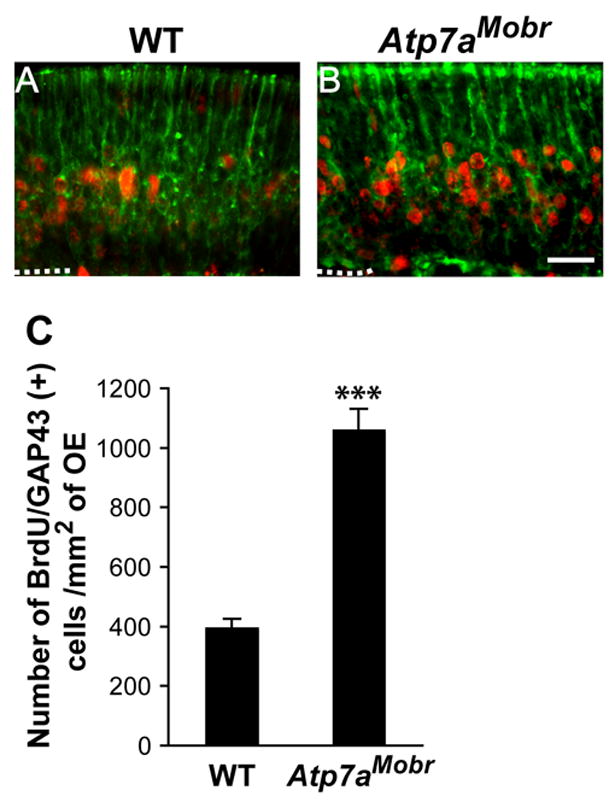
A and B, BrdU labeling (red) and GAP-43 immunoreactivity (green) were visualized by BrdU pulse double-labeling of olfactory epithelium at P7 after BrdU injection at P3. Scale bar: 20 μM. C, Number of BrdU/GAP-43 double-labeled cells in the epithelium of Atp7aMobr compared to WT mice. A significant increase in this population was observed in the mutant mouse epithelium (***, p<10−9).
Consequences of ATP7A mutation to OSN synaptogenesis in the olfactory bulb
Neuronal terminal differentiation is associated with axonal targeting and synaptogenesis, causing us to hypothesize that ATP7A mutation may disrupt synaptogenesis. We therefore investigated a role for ATP7A in synapse formation and in synapse maintenance as a mechanism accounting for the maturational delay seen in Atp7aMobr mice.
ATP7A mutation affects glomerular organization in the olfactory bulb of the Atp7aMobr mouse
We examined glomerular maturation and olfactory bulb organization at P1, P7, and P14. The glomerulus is organized into regions in which OSN axonal subcompartments interdigitate with dendritic subcompartments (Shepherd, 1972; Kasowski et al., 1999; Mori et al., 1999; Treloar et al., 1999). OSN axon targeting and connection with mitral and tufted cells were evaluated using OMP expression (Verhaagen et al., 1990b). In parallel, we used representative presynaptic and postsynaptic markers, synaptophysin (Fig. 5A–F) and PSD95 (Fig. 5G and H) respectively, as a means to define synaptogenesis and synapse maintenance (Bergmann et al., 1993; Morys et al., 1998; Chetkovich et al., 2002). Synaptophysin, a synaptic vesicle protein, is widely used as a marker of presynaptic endings (Bergmann et al., 1993; Ovtscharoff et al., 1993). PSD95 is one of a family of membrane-associated proteins found in neuronal postsynaptic densities (Chetkovich et al., 2002).
Fig. 5. Glomerulus morphology and subcompartmentalization are altered in the olfactory bulb of Atp7aMobr.
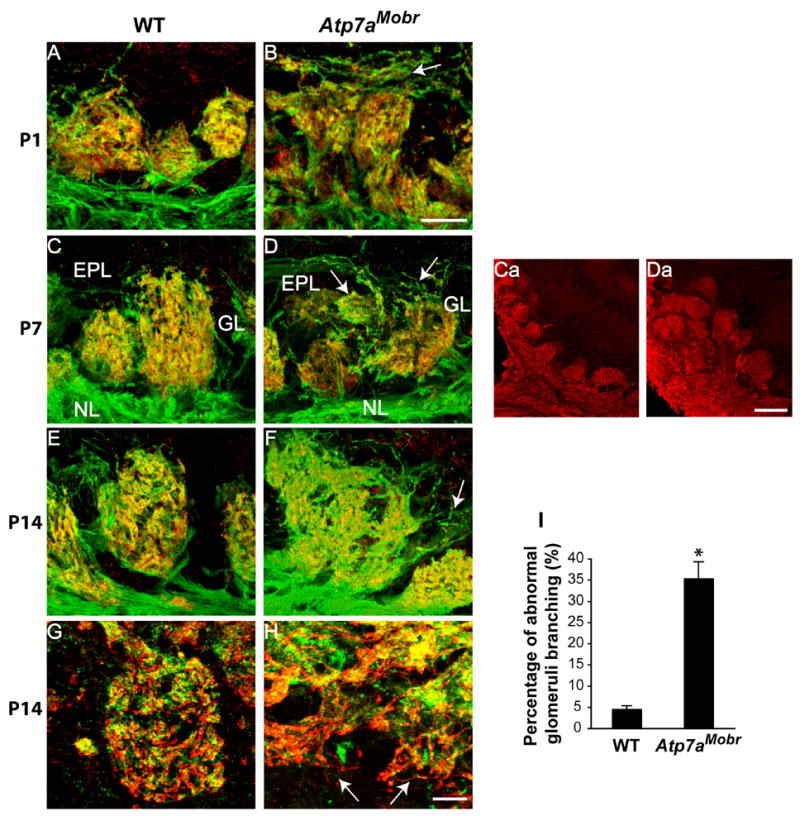
Confocal images: glomeruli were visualized by OMP (green) and synaptophysin (red) immunostaining, or by OMP (red) and PSD95 (in green) immunostaining in the olfactory bulb of WT (A, C, E and G) and ATP7A mutant mice (B, D, F and H) at P1, P7 and P14. Images represent a serial optical plane reconstruction of 1 mm sections (12 to 20 sections per stacks). At all ages analyzed, ATP7A mutant mouse glomerulus morphology was dramatically disorganized and significantly larger in Atp7aMobr compared to WT. Very diffuse and dispersed OMP positive islands were characteristic of mutant bulb glomeruli (B, D and F arrows). Synaptophysin staining indicated undefined synaptic regions in the olfactory bulb of Atp7aMobr compared to WT. A decrease in OMP and synaptophysin colocalization was also noted in the mutant mice. In P14 WT glomerulus, clear juxtaposition of OMP-positive axon terminals with post-synaptic PSD95 staining (G), while in the glomeruli of mutant mice, OMP positive axonal projections often grow right past the PSD95-positive post-synaptic targets (H, arrows). NCAM immunostaining showing overall glomeruli distribution in the mutant (Da) and WT (Ca) at P7. Percentage of abnormal glomerular branching observed at P14 in WT and Atp7aMob is represented (I) (*, p<0.0001). Scale bars: 20 μm (A and B; C–H), 10 μm (Ca and Da). EPL, external plexiform layer; GL, glomerular layer; NL, nerve layer.
Confocal microscopy was used to visualize OMP and synaptophysin simultaneously in the olfactory bulb of Atp7aMobr and WT mice (Fig. 5). At all ages analyzed, glomeruli in the olfactory bulbs of Atp7aMobr mice were loosely structured, misshapen, and disorganized compared to those of WT mice. In WT mice, OMP-positive fibers (green) enter the glomeruli and terminate near synaptophysin-positive endings (red), resulting a yellow color; OMP-positive fibers enter from the olfactory epithelium and generally do not continue past the glomeruli. In contrast, in the Atp7aMobr mice, OMP positive axons extend beyond the glomerular layer and reach the external plexiform layer (Fig. 5B, D and F, arrows) and granule cell layer (Fig. 5H, arrows). The overall glomeruli distribution in the mutant and WT is illustrated by NCAM immunostaining in the olfactory bulb of Atp7aMobr compared to WT mice (Fig. 5 Ca and 5Da). Aberrant projections count of OMP-positive OSN axon in the Atp7aMobr compared to WT mice is represented in Fig. 5I. These abnormal projections are found in the WT and are usually resolved by P14 (Kim and Greer, 2000). However in Atp7aMobr mice, aberrant projections reaching the glomerular layer and extending into the external plexiform layer were present in 35 ± 4% of the glomeruli analyzed (Fig. 5I). This result agrees with a delay of OSN axons convergence in glomerular regions. Based on synaptophysin immunoreactivity (which is found in OSN endings and endings from periglomerular cells), synaptic regions are poorly defined in Atp7aMobr mice. In addition, ATP7A deficiency results in decreased OMP and synaptophysin co-localization in Atp7aMobr mice compared to WT (Fig. 5D and F). In the P14 WT glomerulus, there is clear juxtaposition of OMP-positive axon terminals with post-synaptic PSD95 staining (Fig. 5G), while in the glomeruli of mutant mice, OMP positive axonal projections often grow right past the PSD95-positive post-synaptic targets and (Fig. 5H, arrows). This suggests that many of the OMP-positive OSN axons fail to form normal synapses with mitral/tufted cells in the mutant.
To better analyze glomerular organization, we performed double label immunostaining for OMP and MAP-2, a neuronal somato-dendritic marker. This allowed us to analyze dendritic processes originating from mitral and tufted cells as they enter the glomerular layer to meet the OSN fibers projecting from the olfactory epithelium (Fig. 6). In the WT bulb, an overlap in the immunostaining of axonal (OMP positive) and dendritic (MAP-2) subcompartments is observed; in contrast, Atp7aMobr mice display diffuse and disorganized MAP-2 immunostaining at P1, P7 (Fig. 6A–D) and P14 (Fig. 6E and F). The arrows indicate the abnormal external plexiform layer region in the mutant bulb compared to the WT at P14 (Fig. 6E and F). The abnormal glomerular morphology in the Atp7aMobr mouse suggests that ATP7A deficiency affects both OSN axonal targeting and the dendritic growth of the mitral and tufted cells, altering glomerular organization and synapse integrity in the olfactory bulb.
Fig. 6. ATP7A deficiency altered dendritic arborization of mitral and tufted cell layers in the olfactory bulb.
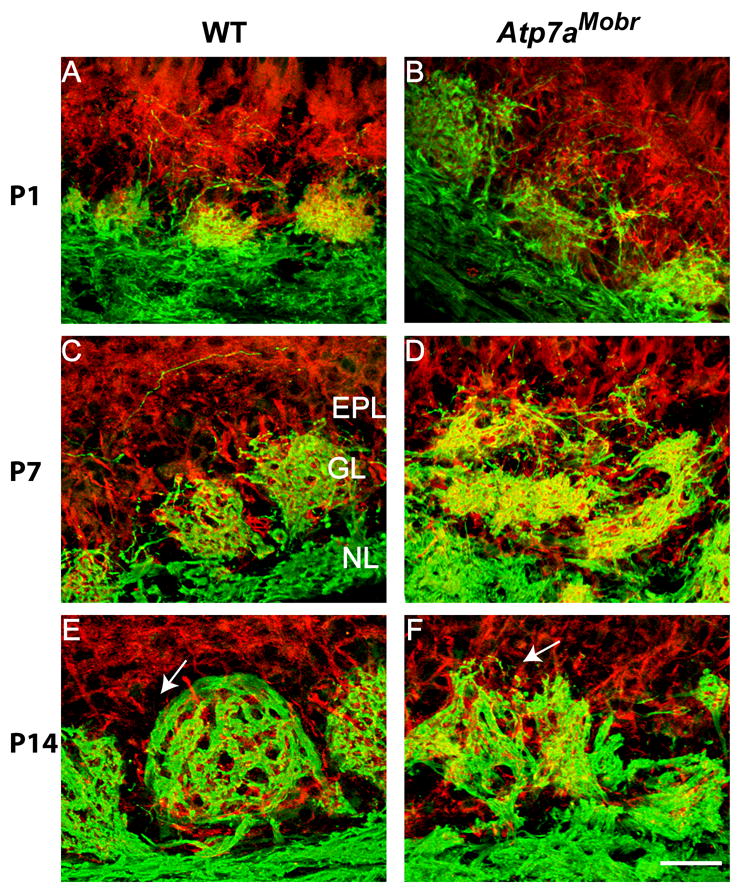
A–F, Present in nerve fibers, OMP immunostaining (green) was used to identify glomeruli in olfactory bulb of WT and Atp7aMobr mice. MAP-2 immunostaining (red) visualized dendritic processes originating from mitral and tufted cells as they entered the glomerular layer. At all postnatal ages analyzed, MAP-2 processes penetrated the glomerular layer, extending to both the external plexiform and olfactory nerve layers and establishing aberrant connections with OMP-positive axons in oversized, malformed glomeruli. Arrows indicate the abnormal EPL (external plexiform layer) region in the mutant bulb compared to the same region in the WT at P14 (E and F). Scale bar: 40 μm
If ATP7A mutation caused a global disruption of cellular function, we might expect that the levels of synaptic proteins would be compromised and thus that the disruption of glomerular organization might just reflect cellular failure. Western blot analysis was used to determine whether levels of presynaptic (synaptophysin), postsynaptic (PSD95), or dendritic (MAP-2) markers were altered. Mutant and WT bulbs were analyzed at P1, P7 and P14 (Fig. 7A–C). No differences in levels of synaptophysin, PSD95, or MAP-2 were detected in the olfactory bulbs of Atp7aMobr and WT mice (Fig. 7). Thus, the dramatic differences in fiber patterns and glomerular integrity (Fig. 5 and 6) are due to aberrant targeting of axons and dendrites, not due to a direct general failure of neuronal function or protein expression of tested markers (Fig. 7).
Fig. 7. Presynaptic, postsynaptic and dendritic protein prevalence is not altered in Atp7aMobr olfactory bulb.
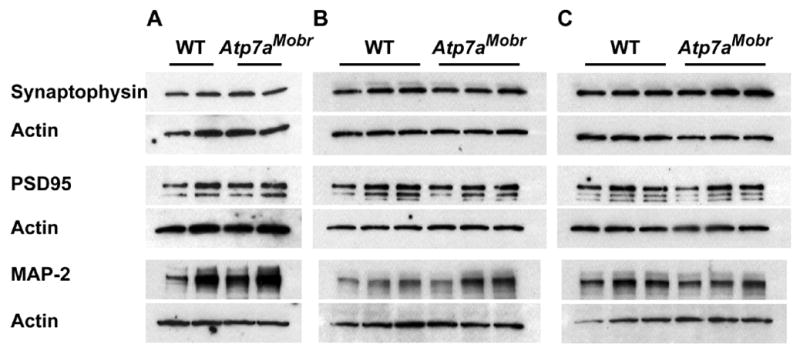
A–C, Protein level comparisons in Atp7aMobr and WT bulb for presynaptic (synaptophysin), post-synaptic protein (PSD-95) and dendritic marker (MAP-2) proteins by Western-blot. No significant changes were observed in expression levels between WT and ATP7A mutant mice at P1 (A), P7 (B) or P14 (C). Protein levels were normalized by re-probing the blots for actin. Representative blots show 2 pools of 2 animals per experimental group for P1 (A), olfactory bulb of 3 animals per experimental group (WT and Atp7aMobr respectively) for P7 (B) and P14 (C).
Synaptic connectivity is affected in the olfactory bulb by ATP7A deficiency
The functionality of olfactory glomerulus synapses, where OSN axons connect with the dendrites of mitral or tufted neurons (Kasowski et al., 1999), can be assessed indirectly by determining the expression of tyrosine hydroxylase (TH) in a subset of periglomerular cells (Smith et al., 1991). Present in the dopaminergic set of periglomerular neurons, TH expression is subject to activity dependent regulation in the olfactory bulb (Baker et al., 1983; Baker et al., 1993; Cho et al., 1996). We therefore investigated the integrity of the OSN-mitral cell synapses by simultaneously visualizing OMP and TH (Fig. 8).
Fig. 8. Developmental expression of TH in olfactory bulb of ATP7A mutant and WT mice.
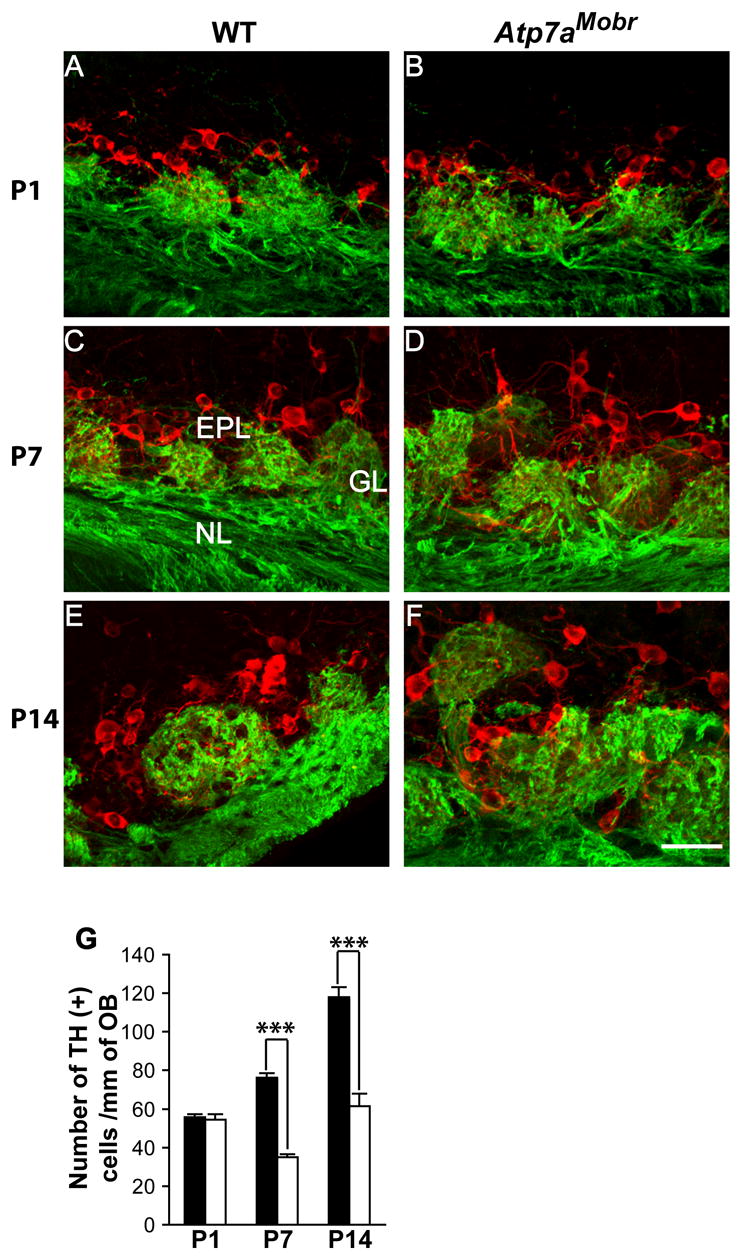
Double immunofluorescence labeling for OMP (green) and TH (red). TH expression is detected in both cell bodies and fibers of periglomerular cells in WT (A, C and E) and Atp7aMobr (B, D and F) mice. Scale bar: 40 μm. TH-positive cells were quantified in the WT and Atp7aMobr mouse OB (G) as cell number/mm of the olfactory nerve layer. A significant decrease of TH-positive cells was noted at P7 and P14 in the mutant compared to WT mouse OB (***, p< 10−9).
In WT mice, the number of TH-positive cells increases from P1 to P14, as glomerular development occurs and connectivity is established (Fig. 8A, C and E). At P1, TH immunostaining is present in mutant mouse periglomerular cells (Fig. 8A and B). In addition, in P1 and P7 mutant olfactory bulb, TH positive cells are disorganized and form aggregates (Fig. 8A–D). By P14, TH positive cells are organized around the glomeruli in the WT (Fig. 8E) while in the mutant they remain more aggregated on the mitral/tufted cell layer side or inside the glomeruli (Fig. 8F). The number of TH-positive cells is significantly reduced in P7 and P14 Atp7aMobr vs. WT mouse olfactory bulb (Fig. 8G). ATP7A mutation altered TH-expressing periglomerular cells number in the olfactory bulb. These data suggest that ATP7A function is involved directly or indirectly in OSN-mitral/tufted cell synapse connectivity.
Discussion
In this study, we demonstrate that ATP7A plays an important and unrecognized role in neurodevelopment at the time of axon extension and synaptogenesis, and that disruption of this function may contribute to the severe neurodegeneration seen in MD (Fig. 9). Copper-independent trafficking of ATP7A to axons, which occurs during axon extension and synaptogenesis suggests a novel function for ATP7A in neurons (El Meskini et al., 2005). The neurodegeneration seen in MD was previously attributed to the systemic decrease in copper levels that occurs as a consequence of ATP7A’s role in intestinal copper uptake. ATP7A dysfunction in MD may instead incur neurological symptoms due to ATP7A’s role in axonal targeting and synaptogenesis. It is unlikely that the abnormalities in axon targeting are the result of global cellular impairment, as levels of axonal protein are not reduced in the Atp7aMobr mice (El Meskini et al., 2005), and as the synaptic defect and maturational disturbance are transient and are followed by a period of normalization of maturation prior to demise. These findings have therapeutic implications.
MD is a neurodevelopmental disorder
The first indications that ATP7A might have a specific neurodevelopmental role arose from studies of its expression in the developing olfactory system (El Meskini et al., 2005) and mouse brain (Niciu et al., 2006). An essential role in development was also attributed to DmATP7, the sole Drosophila orthologue of the ATP7A and ATP7B genes (Norgate et al., 2006). Levels of ATP7A protein expression peak during axon extension and synapse formation. ATP7A is localized in the TGN and in the axons of maturing OSNs (El Meskini et al., 2005) and retinal ganglion neurons (Niciu et al., 2006). Its localization to axons is independent of copper, as ATP7A is still found in axons in Atp7aMobr mice, in which mutant ATP7A is copper-insensitive. This pattern of expression suggested that ATP7A was important for OSN maturation, in particular axon extension and synapse formation (El Meskini et al., 2005).
Functional ATP7A is required for OSN terminal differentiation
We used the mottled brindled mouse to test the hypothesis that ATP7A functions in axonal targeting and synaptogenesis. To maintain the appropriate numbers of cells, homeostatic mechanisms regulate OSN development and precursor proliferation in the olfactory epithelium at each developmental stage (Carr and Farbman, 1992; Mackay-Sima and Chuahb, 2000). ATP7A mutation has several discrete effects on the homeostasis of the neuronal population in the olfactory epithelium. At P1, ATP7A mutation does not affect the commitment of cells to the neuronal lineage (OE-1-immunoreactivity), but does affect the transition of GAP-43-positive cells to OMP-positive cells, an event that occurs upon axonal contact with the olfactory bulb (Verhaagen et al., 1990b). As a consequence, neurons in the mutant mouse express both GAP-43 and OMP, which normally does not occur. This delay is accompanied by increased apoptosis of OMP-positive neurons at P1, while GAP-43-positive immature neurons are spared. The lack of an effect of ATP7A deficiency on BrdU incorporation into precursors indicates that the rate of neurogenesis is not affected, and supports the assertion that ATP7A mutation does not result in a global developmental failure.
At P7, the number of GAP-43-positive neurons is still elevated; however, the incidence of cell death in the mature OMP-positive population (OMP/TUNEL-positive cell number) has returned to normal. These results indicate that loss of ATP7A function results in a delay in the transition from the immature to the mature developmental stage, which manifests itself by an accumulation of immature neurons at P7, without affecting the number of mature OMP-positive neurons. A pulse experiment using BrdU was done to label a discrete cohort of neuronal precursors to follow their fate. Atp7aMobr mice displayed a greater number of immature (GAP-43-positive) BrdU-positive cells than WT mice. ATP7A deficiency causes a maturational delay at the time of axonal outgrowth and synaptogenesis and affects the transition of immature neurons to terminal differentiation. Thus, ATP7A is most critical in early development, when neurons actively extend axons and still rely upon endogenous factors for trophic support.
At P14, there was a significant decrease in the number of mature neurons due to an increase in mature neuronal cell death. The number of GAP-43-positive immature neurons in the Atp7aMobr mouse was unaffected at this age, although the number of TUNEL-GAP-43-positive neurons was increased. Interestingly, neurogenesis remained unaffected at this time, suggesting that there was still no global failure of neuronal function. In agreement with our data, an expression profiling study of cerebral cortex and cerebellum of a MD patient and an age matched normal control brain showed that the second highest number of dysregulated genes are genes involved in neuronal growth and structure, notably nerve growth factor and neuronal growth protein, GAP-43 (Liu et al., 2005).
ATP7A mutant mice display defects in axonal targeting and synaptogenesis
Examination of the olfactory bulbs of WT and Atp7aMobr mice confirmed that axonal targeting and synaptogenesis are disrupted by ATP7A dysfunction (Fig. 9B). In the rat, compartmental organization within the olfactory bulb glomeruli emerges around P12 (Kim and Greer, 2000). OSN axons fasciculate, enter the bulb, and form excitatory synapses with the dendrites of mitral and tufted cells in the glomeruli (Shepherd, 1972; Hinds and Hinds, 1976a; Jastreboff et al., 1984; Halasz and Greer, 1993; Kasowski et al., 1999). Each glomerulus is organized into sub-compartments (Pinching and Powell, 1971; Shepherd, 1972): the axonal sub-compartment has dense OSN axons establishing contiguous islands, interspersed by dendritic sub-compartments of dendritic connections (Kosaka et al., 1998; Kasowski et al., 1999; Treloar et al., 1999). This contact between sensory axons and their target is critical for the development and maintenance of neural circuits. Defective afferent input to the olfactory bulb affects bulb organization and cell survival (Leo et al., 2000). ATP7A protein is highly expressed in the outer nerve layer at P1 and is thereafter expressed in the developing mitral and glomerular layers within the first postnatal week; by P14, its expression extends to the mitral/tufted cell layer and the granule cell layer in the olfactory bulb (El Meskini et al., 2005).
In the presence of mutant ATP7A, glomerular structure is disrupted, with disorganized dendritic and axonal termination subcompartments; many axons overshoot the glomerular region and penetrate the granule cell layer. Glomerular regions do not organize and sub-glomerular compartments do not form, even by P14. In the presence of ATP7A deficiency, the abnormal OSN projections may represent a compensatory repair process supported by an over-expression of GAP43 in the immature OSN. Indeed, it was reported that in mature primary olfactory neurons that have established functional synaptic connections, GAP-43 overexpression results in protrusions originating from OSN preterminal axon shaft and from the actual synaptic bouton (Holtmaat et al., 1997). Thus in ATP7A mutant mouse OSN, a neuronal membrane expansion at synaptic boutons may be due to GAP-43 overexpression as a part of an ongoing structural synaptic plasticity in OSN and in neuronal membrane repair after injury to synaptic fields.
OMP/PSD95 double-labeling of olfactory bulbs from ATP7A mutant mice reveals an absence of well-defined regions of juxtaposition of OMP and PSD95. This defect is confirmed by OMP/MAP-2 immunostaining. Failure of glomerular formation is not the result of global cellular failure, as the expression levels of axonal and synaptic markers is normal in Atp7aMobr mice. While impairing synaptic plasticity, ATP7A deficiency may not affect the properties of OSN basal neurotransmission. Indeed, in other neurodevelopmental diseases, an age-dependent impairment in hippocampal excitatory synaptic plasticity is described with no effect on the levels of expression of synaptic and cytoskeletal proteins including synaptophysin, PSD95 and MAP-2 respectively (Asaka et al., 2006). However, a decrease in neuronal-enriched acidic protein (NAP-22) and synaptotagmin gene levels was reported in a MD patient cerebellum; both proteins are involved in synaptic activity (Liu et al., 2005).
ATP7A deficiency also results in aggregation and disorganization of TH-positive periglomerular cells, indicating that synaptic connectivity is compromised at the OSN axon-mitral/tufted cells dendrites (Nadi et al., 1981; Goheen et al., 1996). The significant decrease in the number of TH-positive periglomerular cells in the olfactory bulb of P7 and P14 ATP7A deficient mice suggests that the connectivity and synapse function of OSN-mitral/tufted cells are altered. OSN-target contacts in the olfactory bulb are essential for the development and the maintenance of normal synaptic function within the glomeruli (Leo et al., 2000).
ATP7A function in axonal outgrowth and synaptogenesis: potential molecular mechanisms
The molecular mechanism whereby ATP7A within glomeruli might control axon outgrowth, synapse formation and connectivity remain to be defined. Mitral/tufted cell dendrites containing ATP7A may require copper transport for the function of proteins needed for signaling to cease growth near the periglomerular cells and/or within the glomerular subcompartments. When mutant ATP7A is present, synapse formation or refinement and subcompartmental organization of the olfactory bulb are disturbed. The other possibility is that ATP7A may interact with cytoskeleton proteins to facilitate axonal or dendritic growth (Stephenson et al., 2005). Using a yeast two-hybrid approach, ATP7A was found to interact with PMCA-interacting single-PDZ protein (PISP), which may interact simultaneously with neuronal PMCA2 and 3, P-type ATPases that play a major role in expelling calcium from neurons. These proteins are implicated in neuronal/axonal damage due to delays in calcium clearance in spinal cord neurons (Kurnellas et al., 2005). Defective interaction of mutant ATP7A with PISP might alter its interaction with PMCA, affecting calcium efflux during OSN axonal extension and blocking terminal differentiation and synaptogenesis.
Alternatively, copper transport to growth cones may be one mechanism by which ATP7A controls axon outgrowth. Neuronal differentiation in NGF-treated PC12 cells, hippocampal neurons, and sympathetic neurons depends on copper accumulation in growth cones and the activity of an essential copper enzyme S-adenosylhomocysteine hydrolase (SAHH), which is involved in protein methylation (Birkaya and Aletta, 2005). This mechanism may be activity-dependent. GABA release controls OSN axon outgrowth in vivo (Priest and Puche, 2004). OSNs detect GABA release from juxtaglomerular cells as they enter the glomerular layer and use this as a signal to limit their outgrowth and find synaptic targets during regeneration and neurodevelopment. This process has been attributed to the expression of GABAB receptor in the OSN axon terminals and by the expression of glutamic acid decarboxylase (GAD), the synthetic enzyme for GABA, in the olfactory bulb. Thus, the aberrant axonal overgrowth that characterizes OSN axons in the bulb of Atp7aMobr mice could be due to a lack of target-dependent neurotransmitter release necessary to OSN development. Indeed, copper has been found to promote neurotransmitter release (Wang, 1999). In addition, it has been shown that copper influences excitability of rat olfactory bulb neurons by multiple mechanisms (Horning and Trombley, 2001). Copper reduces the rate of repetitive firing of action potentials and inhibits voltage-gated calcium channels, thereby exerting an inhibitory effect on neurotransmitter release. Moreover, carnosine, a dipeptide highly expressed in the olfactory system, acts to modulate the effects of copper on neuronal excitability and synaptic transmission (Horning et al., 2000; Trombley et al., 2000). Early during neurodevelopment, lack of regulated copper efflux by ATP7A transport in OSN axon terminals and synaptic copper neuro-modulation might be the cause of impaired synaptogenesis and synaptic plasticity (Doreulee et al., 1997). The presence and trafficking of ATP7A in the axons, whether copper-dependent or –independent (El Meskini et al., 2005; Schlief et al., 2005), controlled by neuronal activity, may be necessary for ATP7A delivery to growth cones and for copper efflux. This ATP7A trafficking is implicated directly or indirectly in axon outgrowth and for the neuron’s ability to form synapses. Finally, ATP7A deficiency may have similar outcome in other brain area affecting synaptogenesis during development and synaptic activity resulting in aberrant synaptic plasticity. This attributes an important role to ATP7A protein in maintaining neuronal process growth and/or copper homeostasis for proper synaptic transmission during CNS development.
Growing evidence supports the finding of extracellular detection of a copper-dependent dismutase activity along with cytosolic SOD1 secretion in neuronal cell lines (Mondola et al., 1996; Cimini et al., 2002; Turner et al., 2005) or extracellular SOD3, whose activity depends tightly on ATP7A interaction for copper loading in the secretory pathway (Qin et al., 2006). Potential paracrine antioxidant and neuroprotective dismutase activities may play an essential role during neuronal development. NO exerts myriads of biological effects in the CNS, in particular during neuronal differentiation, neuronal survival, and synaptic plasticity (Lev-Ram et al., 1997; Baranano et al., 2001). Bioavailability of NO can be regulated by extracellular superoxide (O2●−) (Darley-Usmar et al., 1997; O’Donnell and Freeman, 2001); NO and O2●− undergo an extremely rapid diffusion, limited radical/radical reaction, leading to formation of the potent oxidant peroxynitrite anion (Darley-Usmar et al., 1997; O’Donnell and Freeman, 2001). During OSN maturation, an increase of O2●− production due to a decrease in extracellular superoxide dismutase in ATP7A mutant mice may decrease the level of NO essential in regulating neurite outgrowth and refinement of OSN-mitral cell synapses in the olfactory bulb (Roskams et al., 1994; Van Wagenen and Rehder, 2001) subsequently leading to numerous neuropathological processes.
In summary (Fig. 9B), ATP7A deficiency in the Atp7aMobr mouse results in a delay of OSN maturation and impaired survival of mature OSNs. ATP7A’s effect on neuronal differentiation and survival may be intrinsic to the OSN neurons (cell-autonomous), or may reflect the failure to establish a normal pattern of connections in the olfactory bulb (non-cell-autonomous). During neuronal development, ATP7A is involved in the control of neuronal axon outgrowth, synapse formation and maintenance whether via protein-protein interactions and/or copper transport. In turn, this ATP7A function in neurons is most likely to be modulated by neuronal activity and synaptic plasticity during development. These specific roles of ATP7A during development could be the origin of the profound neurodegeneration seen in MD.
Acknowledgments
We want to thank Amy Kleman for her excellent technical assistance. We would also like to thank Dr. Amy Palmer for critically reading the manuscript. This study was supported by NIH grants DC-04736, NS-41079 and NS-39657 to G.V.R and DK-32949 to B.A.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Baker H, Kawano T, Margolis FL, Joh TH. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J Neurosci. 1983;3:69–78. doi: 10.1523/JNEUROSCI.03-01-00069.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. doi: 10.1016/0006-8993(93)91023-l. [DOI] [PubMed] [Google Scholar]
- Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Schuster T, Grabs D, Marqueze-Pouey B, Betz H, Traurig H, Mayerhofer A, Gratzl M. Synaptophysin and synaptoporin expression in the developing rat olfactory system. Brain Res Dev Brain Res. 1993;74:235–244. doi: 10.1016/0165-3806(93)90009-y. [DOI] [PubMed] [Google Scholar]
- Birkaya B, Aletta JM. NGF promotes copper accumulation required for optimum neurite outgrowth and protein methylation. J Neurobiol. 2005;63:49–61. doi: 10.1002/neu.20114. [DOI] [PubMed] [Google Scholar]
- Buck LB. Information coding in the vertebrate olfactory system. Ann Rev Neurosci. 1996;19:517–544. doi: 10.1146/annurev.ne.19.030196.002505. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Carr VM, Farbman AI. Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium. Exp Neurol. 1992;115:55–59. doi: 10.1016/0014-4886(92)90221-b. [DOI] [PubMed] [Google Scholar]
- Cecchi C, Biasotto M, Tosi M, Avner P. The mottled mouse as a model for human Menkes disease: identification of mutations in the Atp7a gene. Hum Mol Genet. 1997;6:425–433. doi: 10.1093/hmg/6.3.425. [DOI] [PubMed] [Google Scholar]
- Chetkovich DM, Bunn RC, Kuo SH, Kawasaki Y, Kohwi M, Bredt DS. Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms. J Neurosci. 2002;22:6415–6425. doi: 10.1523/JNEUROSCI.22-15-06415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JY, Min N, Franzen L, Baker H. Rapid down-regulation of tyrosine hydroxylase expression in the olfactory bulb of naris-occluded adult rats. J Comp Neurol. 1996;369:264–276. doi: 10.1002/(SICI)1096-9861(19960527)369:2<264::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Cimini V, Ruggiero G, Buonomo T, Seru R, Sciorio S, Zanzi C, Santangelo F, Mondola P. CuZn-superoxide dismutase in human thymus: immunocytochemical localisation and secretion in thymus-derived epithelial and fibroblast cell lines. Histochem Cell Biol. 2002;118:163–169. doi: 10.1007/s00418-002-0429-8. [DOI] [PubMed] [Google Scholar]
- Constanzo RM, Graziadei PPC. A qualitative analysis of changes in the olfactory epithelium following bulbectomy in hamster. JCompNeurol. 1983;215:370–381. doi: 10.1002/cne.902150403. [DOI] [PubMed] [Google Scholar]
- Darley-Usmar VM, McAndrew J, Patel R, Moellering D, Lincoln TM, Jo H, Cornwell T, Digerness S, White CR. Nitric oxide, free radicals and cell signalling in cardiovascular disease. Biochem Soc Trans. 1997;25:925–929. doi: 10.1042/bst0250925. [DOI] [PubMed] [Google Scholar]
- Davis JA, Reed RR. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16:5082–5094. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doreulee N, Yanovsky Y, Haas HL. Suppression of long-term potentiation in hippocampal slices by copper. Hippocampus. 1997;7:666–669. doi: 10.1002/(SICI)1098-1063(1997)7:6<666::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- El Meskini R, Mains RE, Eipper BA. Cell type-specific metabolism of peptidylglycine alpha-amidating monooxygenase in anterior pituitary. Endocrinology. 2000;141:3020–3034. doi: 10.1210/endo.141.8.7620. [DOI] [PubMed] [Google Scholar]
- El Meskini R, Cline LB, Eipper BA, Ronnett GV. The developmentally regulated expression of Menkes protein ATP7A suggests a role in axon extension and synaptogenesis. Dev Neurosci. 2005;27:333–348. doi: 10.1159/000086713. [DOI] [PubMed] [Google Scholar]
- El Meskini R, Galano GJ, Marx R, Mains RE, Eipper BA. Targeting of membrane proteins to the regulated secretory pathway in anterior pituitary endocrine cells. J Biol Chem. 2001;276:3384–3393. doi: 10.1074/jbc.M008062200. [DOI] [PubMed] [Google Scholar]
- Geller TJ, Pan Y, Martin DS. Early neuroradiologic evidence of degeneration in Menkes’ disease. Pediatr Neurol. 1997;17:255–258. doi: 10.1016/s0887-8994(97)00092-1. [DOI] [PubMed] [Google Scholar]
- Goheen BL, Kott JN, Westrum LE. Tyrosine hydroxylase expression in rat olfactory bulb transplants: an electron microscope study. Synapse. 1996;23:132–141. doi: 10.1002/(SICI)1098-2396(199607)23:3<132::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti-Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. JNeurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Grimes A, Hearn CJ, Lockhart P, Newgreen DF, Mercer JF. Molecular basis of the brindled mouse mutant (Mo(br)): a murine model of Menkes disease. Hum Mol Genet. 1997;6:1037–1042. doi: 10.1093/hmg/6.7.1037. [DOI] [PubMed] [Google Scholar]
- Halasz N, Greer CA. Terminal arborizations of olfactory nerve fibers in the glomeruli of the olfactory bulb. JCompNeurol. 1993;337:307–316. doi: 10.1002/cne.903370211. [DOI] [PubMed] [Google Scholar]
- Harrison MD, Dameron CT. Molecular mechanisms of copper metabolism and the role of the Menkes disease protein. J Biochem Mol Tox. 1999;13:93–106. doi: 10.1002/(sici)1099-0461(1999)13:2<93::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hartman BK, Margolis FL. Immunofluorescence localization of the olfactory marker protein. Brain Res. 1975;96:176–180. doi: 10.1016/0006-8993(75)90593-4. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Synapse formation in the mouse olfactory bulb. II. Morphogenesis. J Comp Neurol. 1976a;169:41–61. doi: 10.1002/cne.901690104. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Synapse formation in the mouse olfactory bulb. I. Quantitative studies. J Comp Neurol. 1976b;169:15–40. doi: 10.1002/cne.901690103. [DOI] [PubMed] [Google Scholar]
- Holcomb JD, Mumm JS, Calof AL. Apoptosis in the neuronal lineage of the mouse olfactory epithelium: regulation in vivo and in vitro. Dev Biol. 1995;172:307–323. doi: 10.1006/dbio.1995.0025. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJ, Hermens WT, Sonnemans MA, Giger RJ, Van Leeuwen FW, Kaplitt MG, Oestreicher AB, Gispen WH, Verhaagen J. Adenoviral vector-mediated expression of B-50/GAP-43 induces alterations in the membrane organization of olfactory axon terminals in vivo. J Neurosci. 1997;17:6575–6586. doi: 10.1523/JNEUROSCI.17-17-06575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning MS, Trombley PQ. Zinc and copper influence excitability of rat olfactory bulb neurons by multiple mechanisms. J Neurophysiol. 2001;86:1652–1660. doi: 10.1152/jn.2001.86.4.1652. [DOI] [PubMed] [Google Scholar]
- Horning MS, Blakemore LJ, Trombley PQ. Endogenous mechanisms of neuroprotection: role of zinc, copper, and carnosine. Brain Res. 2000;852:56–61. doi: 10.1016/s0006-8993(99)02215-5. [DOI] [PubMed] [Google Scholar]
- Huard JM, Schwob JE. Cell cycle of globose basal cells in rat olfactory epithelium. Dev Dyn. 1995;203:17–26. doi: 10.1002/aja.1002030103. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Pedersen PE, Greer CA, Stewart WB, Kauer JS, Benson TE, Shepherd GM. Specific olfactory receptor populations projecting to identified glomeruli in the rat olfactory bulb. Proc Natl Acad Sci U S A. 1984;81:5250–5254. doi: 10.1073/pnas.81.16.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler SG. Menkes disease. Adv Pediatr. 1994;41:263–304. [PubMed] [Google Scholar]
- Kasowski HJ, Kim H, Greer CA. Compartmental organization of the olfactory bulb glomerulus. J Comp Neurol. 1999;407:261–274. [PubMed] [Google Scholar]
- Keller A, Margolis FL. Immunological studies of the rat olfactory marker protein. J Neurochem. 1975;24:1101–1106. doi: 10.1111/j.1471-4159.1975.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Greer CA. The emergence of compartmental organization in olfactory bulb glomeruli during postnatal development. J Comp Neurol. 2000;422:297–311. [PubMed] [Google Scholar]
- Kimura Y, Kamide M, Furukawa M, Miwa T, Sakumoto M, Umeda R. [Evaluation of turnover of olfactory epithelium in mice by using anti-BrdU monoclonal antibody] Nippon Jibiinkoka Gakkai Kaiho. 1990;93:165–170. doi: 10.3950/jibiinkoka.93.165. [DOI] [PubMed] [Google Scholar]
- Kodama H, Murata Y. Molecular genetics and pathophysiology of Menkes disease. Pediatr Int. 1999;41:430–435. doi: 10.1046/j.1442-200x.1999.01091.x. [DOI] [PubMed] [Google Scholar]
- Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res. 1998;30:101–110. doi: 10.1016/s0168-0102(98)00002-9. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Nicot A, Shull GE, Elkabes S. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. Faseb J. 2005;19:298–300. doi: 10.1096/fj.04-2549fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Camakaris J, Mercer JF. Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein, in cells from blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum Mol Genet. 1999;8:1069–1075. doi: 10.1093/hmg/8.6.1069. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leo JM, Devine AH, Brunjes PC. Focal denervation alters cellular phenotypes and survival in the developing rat olfactory bulb. J Comp Neurol. 2000;417:325–336. doi: 10.1002/(sici)1096-9861(20000214)417:3<325::aid-cne6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Jiang T, Wood J, Lawrence DS, Tsien RY. Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron. 1997;18:1025–1038. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- Leventer RJ, Kornberg AJ, Phelan EM, Kean MJ. Early magnetic resonance imaging findings in Menkes’ disease. J Child Neurol. 1997;12:222–224. doi: 10.1177/088307389701200314. [DOI] [PubMed] [Google Scholar]
- Liu PC, Chen YW, Centeno JA, Quezado M, Lem K, Kaler SG. Downregulation of myelination, energy, and translational genes in Menkes disease brain. Mol Genet Metab. 2005;85:291–300. doi: 10.1016/j.ymgme.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Mackay-Sima A, Chuahb MI. Neurotrophic factors in the primary olfactory pathway. Prog Neurobiol. 2000;62:527–559. doi: 10.1016/s0301-0082(00)00009-5. [DOI] [PubMed] [Google Scholar]
- Mercer J. Menkes syndrome and animal models. Am J Clin Nutr. 1998;67:1022S–1028S. doi: 10.1093/ajcn/67.5.1022S. [DOI] [PubMed] [Google Scholar]
- Mercer JF. The molecular basis of copper-transport diseases. Trends Mol Med. 2001;7:64–69. doi: 10.1016/s1471-4914(01)01920-7. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Llanos RM. Molecular and cellular aspects of copper transport in developing mammals. J Nutr. 2003;133:1481S–1484S. doi: 10.1093/jn/133.5.1481S. [DOI] [PubMed] [Google Scholar]
- Mercer JF, Barnes N, Stevenson J, Strausak D, Llanos RM. Copper-induced trafficking of the cU-ATPases: a key mechanism for copper homeostasis. Biometals. 2003;16:175–184. doi: 10.1023/a:1020719016675. [DOI] [PubMed] [Google Scholar]
- Mondola P, Annella T, Santillo M, Santangelo F. Evidence for secretion of cytosolic CuZn superoxide dismutase by Hep G2 cells and human fibroblasts. Int J Biochem Cell Biol. 1996;28:677–681. doi: 10.1016/1357-2725(96)00004-0. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- Morys J, Berdel B, Kowianski P, Dziewiatkowski J. The pattern of synaptophysin changes during the maturation of the amygdaloid body and hippocampal hilus in the rat. Folia Neuropathol. 1998;36:15–23. [PubMed] [Google Scholar]
- Nadi NS, Head R, Grillo M, Hempstead J, Grannot-Reisfeld N, Margolis FL. Chemical deafferentation of the olfactory bulb: plasticity of the levels of tyrosine hydroxylase, dopamine and norepinephrine. Brain Res. 1981;213:365–377. doi: 10.1016/0006-8993(81)90241-9. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Developmental changes in the expression of ATP7A during a critical period in postnatal neurodevelopment. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Norgate M, Lee E, Southon A, Farlow A, Batterham P, Camakaris J, Burke R. Essential roles in development and pigmentation for the Drosophila copper transporter DmATP7. Mol Biol Cell. 2006;17:475–484. doi: 10.1091/mbc.E05-06-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- Ohno M, Narita T, Abe J, Tsuzuki T, Yagi K, Takikita S, Takano T, Shimada M. Apoptosis in cerebrum of macular mutant mouse. Acta Neuropathol (Berl) 2002;103:356–362. doi: 10.1007/s00401-001-0473-9. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Bergmann M, Marqueze-Pouey B, Knaus P, Betz H, Grabs D, Reisert I, Gratzl M. Ontogeny of synaptophysin and synaptoporin in the central nervous system: differential expression in striatal neurons and their afferents during development. Brain Res Dev Brain Res. 1993;72:219–225. doi: 10.1016/0165-3806(93)90186-e. [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. Embo J. 1996;15:6084–6095. [PMC free article] [PubMed] [Google Scholar]
- Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci. 1971;9:347–377. doi: 10.1242/jcs.9.2.347. [DOI] [PubMed] [Google Scholar]
- Priest CA, Puche AC. GABAB receptor expression and function in olfactory receptor neuron axon growth. J Neurobiol. 2004;60:154–165. doi: 10.1002/neu.20011. [DOI] [PubMed] [Google Scholar]
- Qin Z, Itoh S, Jeney V, Ushio-Fukai M, Fukai T. Essential role for the Menkes ATPase in activation of extracellular superoxide dismutase: implication for vascular oxidative stress. Faseb J. 2006;20:334–336. doi: 10.1096/fj.05-4564fje. [DOI] [PubMed] [Google Scholar]
- Reed V, Boyd Y. Mutation analysis provides additional proof that mottled is the mouse homologue of Menkes’ disease. Hum Mol Genet. 1997;6:417–423. doi: 10.1093/hmg/6.3.417. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Snyder SH. Molecular messengers of olfaction. Trends Neurosci. 1992;15:508–513. doi: 10.1016/0166-2236(92)90104-g. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Payne R. A Tale of Two Senses. Neuron. 1995;15:11–16. doi: 10.1016/0896-6273(95)90058-6. [DOI] [PubMed] [Google Scholar]
- Roskams AJ, Bredt DS, Dawson TM, Ronnett GV. Nitric Oxide Mediates the Formation of Synaptic Connections in Developing and Regenerating Olfactory Receptor Neurons. Neuron. 1994;13:289–299. doi: 10.1016/0896-6273(94)90347-6. [DOI] [PubMed] [Google Scholar]
- Roskams AJI, Cai X, Ronnett GV. Expression of neuron-specific beta-III tubulin during olfactory neurogenesis in the embryonic and adult rat. Neuroscience. 1998;83:191–200. doi: 10.1016/s0306-4522(97)00344-8. [DOI] [PubMed] [Google Scholar]
- Rossi L, De Martino A, Marchese E, Piccirilli S, Rotilio G, Ciriolo MR. Neurodegeneration in the animal model of Menkes’ disease involves Bcl-2-linked apoptosis. Neuroscience. 2001;103:181–188. doi: 10.1016/s0306-4522(00)00562-5. [DOI] [PubMed] [Google Scholar]
- Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. Synaptic organization of the mammalian olfactory bulb. Physiol Rev. 1972;52:864–917. doi: 10.1152/physrev.1972.52.4.864. [DOI] [PubMed] [Google Scholar]
- Simpson PJ, Miller I, Moon C, Hanlon AL, Liebl DJ, Ronnett GV. Atrial natriuretic peptide type C induces a cell-cycle switch from proliferation to differentiation in brain-derived neurotrophic factor- or nerve growth factor-primed olfactory receptor neurons. J Neurosci. 2002;22:5536–5551. doi: 10.1523/JNEUROSCI.22-13-05536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PJ, Wang E, Moon C, Matarazzo V, Cohen DR, Liebl DJ, Ronnett GV. Neurotrophin-3 signaling maintains maturational homeostasis between neuronal populations in the olfactory epithelium. Mol Cell Neurosci. 2003;24:858–874. doi: 10.1016/j.mcn.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Smith RL, Baker H, Kolstad K, Spencer DD, Greer CA. Localization of tyrosine hydroxylase and olfactory marker protein immunoreactivities in the human and macaque olfactory bulb. Brain Res. 1991;548:140–148. doi: 10.1016/0006-8993(91)91115-h. [DOI] [PubMed] [Google Scholar]
- Stephenson SE, Dubach D, Lim CM, Mercer JF, La Fontaine S. A single PDZ domain protein interacts with the Menkes copper ATPase, ATP7A. A new protein implicated in copper homeostasis. J Biol Chem. 2005;280:33270–33279. doi: 10.1074/jbc.M505889200. [DOI] [PubMed] [Google Scholar]
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-amidating monooxygenase. Endocrinology. 2003;144:188–200. doi: 10.1210/en.2002-220716. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Interkinetic and migratory behavior of a cohort of neocortical neurons arising in the early embryonic murine cerebral wall. J Neurosci. 1996;16:5762–5776. doi: 10.1523/JNEUROSCI.16-18-05762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HB, Purcell AL, Greer CA. Glomerular formation in the developing rat olfactory bulb. J Comp Neurol. 1999;413:289–304. [PubMed] [Google Scholar]
- Trombley PQ, Horning MS, Blakemore LJ. Interactions between carnosine and zinc and copper: implications for neuromodulation and neuroprotection. Biochemistry (Mosc) 2000;65:807–816. [PubMed] [Google Scholar]
- Turner BJ, Atkin JD, Farg MA, Zang da W, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25:108–117. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagenen S, Rehder V. Regulation of neuronal growth cone filopodia by nitric oxide depends on soluble guanylyl cyclase. J Neurobiol. 2001;46:206–219. doi: 10.1002/1097-4695(20010215)46:3<206::aid-neu1003>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Greer CA, Margolis FL. B-50/GAP43 Gene Expression in the Rat Olfactory System During Postnatal Development and Aging. Eur J Neurosci. 1990a;2:397–407. doi: 10.1111/j.1460-9568.1990.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL. Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res. 1990b;26:31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]
- Waggoner DJ, Bartnikas TB, Gitlin JD. The role of copper in neurodegenerative disease. Neurobiol Dis. 1999;6:221–230. doi: 10.1006/nbdi.1999.0250. [DOI] [PubMed] [Google Scholar]
- Wang JK. Cu2+ induces Ca2+-dependent neurotransmitter release from brain catecholaminergic nerve terminals. Eur J Pharmacol. 1999;373:163–169. doi: 10.1016/s0014-2999(99)00275-7. [DOI] [PubMed] [Google Scholar]


