Abstract
In songbirds, the seasonal growth of the song system is generally thought to be controlled by the spring increase in plasma levels of testosterone and/or related changes in singing activity. Here we report an extremely early seasonal growth (before February) of the song control nuclei HVC and RA in Corsican blue tits (Parus caeruleus) indicating that the vernal development of these nuclei occurs well before the vernal increase in plasma testosterone, testes size and song activity. The development of HVC and RA occurred simultaneously in two populations that are known to breed consistently one month apart as an adaptation to heterogeneous landscapes (predominance of broad-leaved deciduous versus evergreen oak trees). The unidentified environmental and/or physiological cues controlling the plasticity in the song system must therefore differ, at least in part, from those affecting other morphological and physiological traits controlling reproduction.
Keywords: Populations, Seasonal changes, Song system, HVC, Robust nucleus of the arcopallium, Parus caeruleus
In the brain of songbirds, the discrete nuclei that form the song control system undergo seasonal variations in structure that seem to mediate the annual changes in song quality and quantity [4,25]. During the spring, there is a marked increase in the volume of several song control nuclei such as HVC (formerly high vocal center) and the robust nucleus of the arcopallium (RA) [21]. The seasonal growth of these nuclei reflects changes in cell size and branching, variations in the volume of the neuropile, and in the case of HVC, incorporation of new neurons [2]. Plasma testosterone (T) is generally considered as the key factor controlling the seasonal growth of the song control nuclei HVC and RA (see review in [4]) but three types of observations indicate that this mechanism is not the only one involved: (a) High plasma levels of T are not always necessary to induce the growth of song control nuclei. In song sparrows (Melospiza melodia) for example, HVC and RA are already developed in early spring when plasma T levels are still at basal levels [21,26], (b) photoperiod, the most important cue regulating seasonal changes in reproductive physiology and behaviour, has been shown to increase the size of song nuclei in starlings (Sturnus vulgaris) [5] and Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) [21] independently of gonadal T (i.e. in castrated birds), and (c) social cues, such as those provided by a sexually receptive female, are also able to enhance the seasonal growth of song nuclei in white-crowned sparrows [27]. The respective roles of these different factors remain however unclear at present.
We compared here the seasonal changes in HVC and RA volumes in two Mediterranean blue tit (Parus caeruleus) populations that show considerable differences in the timing of reproduction [7,8,15]. These populations, situated at similar latitudes and altitudes, are adapted to heterogeneous landscapes differing by the prevalence of broad-leaved deciduous (Quercus humilis: Muro) versus evergreen (Q. ilex: Pirio) oak trees. In Muro, blue tits raise chicks early (in May) at the time when many caterpillars are available in this broad-leaved deciduous oak habitat. By contrast, in the other valley (Pirio), blue tits raise chicks in June (about one month later) when the highest density of caterpillars is available in evergreen oak. Experimental work with captive birds showed that the two populations do not have the same breeding responses in standardized outdoor conditions [7]. In addition, during a 12-years field study involving captures of 3000 adults on the nest at both sites, we only observed two reproducing males that had been marked in the other valley. Reproductive exchanges between these two populations are therefore assumed to be rare despite their proximity, which would favor adaptive population differentiation for timing of breeding [7,8]. A preliminary study of these two populations based on a small number of subjects (n=22), covering the period from February till April during a single year, failed to find any seasonal variation in the volumes of HVC and RA [10]. It was hypothesized that either Corsican blue tits do not display any seasonal variation in their song nuclei that maintain the same size all year round or that the seasonal growth of the song nuclei occur for the most part before the sampling period. The aims of the present study were thus (1) to determine whether seasonal changes occur in the song-control system of these populations, (2) to quantify the timing of the seasonal development (if present) in both populations, and (3) to relate any seasonal change observed in the song control system to the previously described phenology of other physiological and behavioural traits [10] that could be potentially related, such as plasma levels of testosterone, singing activity and egg laying. This study represents, to our knowledge, the first neurobiological analysis of the song control system carried out in the framework of long-term ecological studies of natural bird populations in which selective pressure leading to a specialized timing of reproduction have been clearly identified [7,15].
Blue tit populations from the broad-leaved deciduous (Muro) and evergreen site (Pirio) were studied using basic protocols established since 1976 (see [15] for details). Both sites are located at similar latitudes (Muro: 42°32′ north; Pirio: 42°22′ north) and altitudes (Muro: 280 m; Pirio: 200 m). Data were collected on both sites during the 2002 and 2003 breeding seasons and in December 2003. The birds were killed following the ethical guidelines of the CNRS, with certificate n° 34–96 provided by the French Ministère de l’Agriculture et de la Forêt.
In both study sites, males (n=64) were caught, at the end of February and March of 2002 and 2003, and in mid-December 2003. Another group was collected in the evergreen site (Pirio) at the end of April in both years, just before egg laying. The profile of testicular development in males and the average egg-laying dates (see also [7] for the stability of egg laying dates across years) were nearly identical during these two years and data collected during these two reproductive cycles were therefore analyzed together. These sampling dates were assumed to cover most of the seasonal development of the reproductive system in both populations. Males feeding chicks were never taken to avoid chick loss. Males were trapped in the morning (before 1.00 p.m.) with mist nets to which they were attracted by tape-recorded blue tit songs and by live blue tit decoys. A blood sample was taken with a heparinized syringe from the jugular vein as soon as possible (within 2 to 10 min) after capture and males were killed by decapitation. Brains were dissected out and immersed in a fixative solution made of buffered-phosphate saline containing 5% Acrolein during 4 hours: 1 hour first without agitation and 3 hours under agitation. Brains were cryoprotected in a 30% sucrose solution overnight and frozen on dry ice (−80 °C).
Brains were cut in 30 μm transverse frozen sections on a microtome. Every fifth section was mounted on gelatin-coated slides and Nissl-stained with toluidine-blue to allow identification and measure of the song control nuclei. The volumes of HVC (used as a name, formely the “High Vocal Center”) and RA (Robust nucleus of the Arcopallium, formerly Archistriatum; see [18]) were measured by computer-assisted morphometry (see [10]). Sections were digitized by a video camera connected to a microscope, the borders of the nuclei were delineated with the computer mouse on the screen and surfaces were calculated by the software NIH Image version 1.52 (Wayne Rasband, NIH, Bethesda MD, USA). The nucleus volumes were then reconstructed by multiplying the surfaces by the sampling interval. Due to various technical problems, volumes could not be accurately reconstructed in 5 subjects leaving a final total sample size of 59 subjects (see Fig. 1 for their distribution in the different study sites and periods). No left-right difference in HVC and RA volumes was found in analyses of variance testing the effect of side as a function of the time of the year (no main effect and no interaction; data not shown), so all nucleus volumes are reported here as a mean of both sides.
Fig.1.
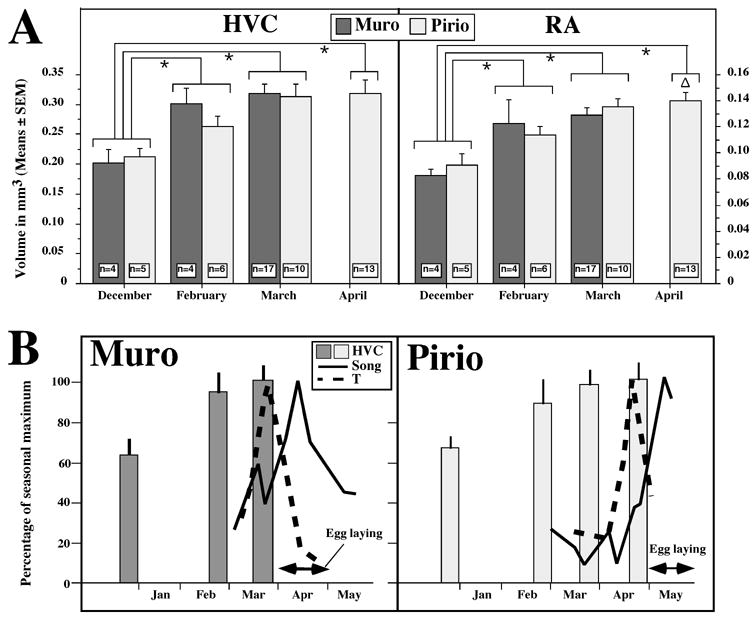
A. Seasonal changes in the volume of two song control nuclei, HVC and RA in Corsican male blue tits at the study sites of Muro and Pirio. Data are presented as mean ± SE. The number of independent data points at each date and site is indicated in the corresponding bar. Two-way ANOVA revealed significant difference between months but not study sites. Sampling periods were thus compared by one-way ANOVA followed by Fisher PLSD tests whose results are indicated by symbols above the bars. *: p<0.05 by comparison with December, Δ: p<0.05 by comparison with February. B. Comparison of the seasonal changes in Muro and Pirio of HVC volumes, singing rates (song; full lines), plasma T levels (T; dotted lines), all expressed as percentage of the annual maximum and of egg-laying periods based on the results of the present study and of previously published data [9].
Differences between study populations (Pirio vs. Muro) and sampling periods (months and/or years) were tested using one and two-way ANOVAs. Post hoc comparisons were carried out with Fisher Protected Least Significant Difference (PLSD) tests. Effects were considered significant for p≤0.05.
Analysis of HVC volumes by two-way ANOVA (months and location as factors) indicated a significant effect of the season (F3,52 = 7.861, P < 0.001), but not of the study site (F1,52 = 0.271, P = 0.605). The interaction between these two factors was also not significant (F2,52 = 0.383, P = 0.684; see Fig.1A). HVC volumes were thus similar in both populations at all periods of the year. Therefore, all data were pooled and re-analyzed by a one-way ANOVA (season as factor), which confirmed the very significant effect of the sampling period (F3,55 = 7.996, P < 0.001). Post hoc Fisher’s PLSD tests indicated that HVC volumes in December were significantly different from all other periods (February: P = 0.016, March: P < 0.001, April: P < 0.001; see symbols on Fig. 1A, left).
Similar results were observed in both years (2002 and 2003) and accordingly a two-way ANOVA of HVC sizes with year and months as factors revealed no significant effect of year (F1,44=1.275, p=0.2649) and no interaction of year with the other factor (F2,44=2.938, p=0.0634; analysis performed on data collected from February to April since December was sampled during a single year).
RA volume (Fig. 1A, right) was similarly affected by the season (F3,50 = 11.483, P < 0.001), but not by the study site (F1,50 = 0.064, P = 0.802) nor by the interaction between these two factors (F2,50 = 0.463, P = 0.632; Fig 1A). Like for HVC, these results were therefore reanalyzed after pooling the two study sites with a one-way ANOVA which confirmed the significance of the seasonal changes (F3,53 = 12.013, P < 0.001). Post hoc Fisher’s PLSD tests indicated that RA volume in December was significantly smaller than in February (P = 0.004), March (P < 0.001) or April (P < 0.001). In addition RA volumes measured in February were smaller than in April (P = 0.016).
No difference between years was also detected here in a two-way ANOVA analyzing RA volumes with year and months as factors for the months that were sampled in both years, i.e. from February to April (effect of year: F1,42=0.073, p=0.789; interaction of year with month: F2,42= 1,362, p=0.267).
This study demonstrates that, in blue tits, the vernal recrudescence of HVC and RA occurs at similar periods in Muro, a population that breeds early in spring, as in Pirio where birds breed approximately one month later [7,15]. This seasonal recrudescence occurred very early in the pre-breeding period, between December and February, when other reproductive parameters such as plasma T, song activity and testis volumes were still close to the basal level. As shown in our previous study [10], these endocrine traits only begin to increase later in the season (March or April) and reach their peak even later, between March and May during or just before egg-laying (see Fig. 1B). These findings raise two types of questions concerning the control of seasonal changes in song nuclei and the physiological mechanisms underlying the breeding asynchrony in these two Corsican blue tit populations.
Seasonal changes in song control nuclei
Current research has identified three semi-independent factors that could directly promote the growth of song nuclei: plasma T, photoperiod and singing activity, but their respective roles remain unclear at present. The originally postulated causal links were that daylength increases plasma T, which induces the growth of the control nuclei resulting in enhanced singing activity (see review in [4]). This view is however challenged by several observations. First, changes in the volume of song control nuclei in dark-eyed juncos (Juncos hyemalis) have been reported in the absence of detectable changes in plasma T [11,13]. In addition, in some species, an increasing photoperiod has by itself a stimulatory effect on the growth of HVC and RA in the absence of T. For example, the transfer from short days to long days induces growth of these song nuclei in castrated tree sparrows, Spizella arborea [6], Gambel’s white-crowned sparrows, Zonotrichia leucophrys gambelii [22] and European starlings, Sturnus vulgaris [5]. Furthermore in Juncos (Junco hyemalis), T-independent effects of photoperiod appear to explain completely seasonal changes in the volume of HVC [12].
Recent work also demonstrated that singing itself increases the expression of brain-derived neurotrophic factor (BDNF), which increases the size of HVC in canaries [1,16,17]. Accordingly, a suite of experiments in which starlings were prevented from singing by various means, ranging from social inhibition to central or peripheral lesions, indicated that the T-induced development of HVC is significantly reduced in the absence of singing [3]. The originally postulated causal links may thus not be the only ones. It is also conceivable that the increase in plasma T leads to a recrudescence of the singing activity, which then causes the enlargement of HVC and other song control nuclei (see [3] for a detailed discussion).
In the present study on blue tits, the annual re-growth of HVC and RA in males preceded by at least one month (possibly two) the vernal increase of most physiological parameters controlling reproductive activity, including plasma T and singing activity (see Fig. 1B). Although it is conceivable that a moderate increase in plasma T could occur during the winter and stimulate HVC and RA growth, this is improbable based on the available literature on songbird field endocrinology [29]. It is thus unlikely that these factors alone are able to explain the early recrudescence of HVC and RA size. Most if not all the increase in HVC and RA size indeed occurs BEFORE there is any change in plasma T or singing activity (i.e. before February; see [10] and Fig. 1B; no plasma samples were assayed in January but in a few samples collected at the end of December, both plasma T and singing activity were low; unpubl. data). Furthermore, though the photoperiod begins to increase soon after the winter solstice, it only reaches approximately 11 hours of light per day by the end of February when song control nuclei were fully regrown. Based on a large amount of literature in a variety of avian species, it is unlikely that this photoperiod is long enough to be stimulatory, at least for the pituitary-gonadal axis. The minimal photoperiodic requirements to directly stimulate the growth of the song control nuclei and to activate the endocrine axis may however be different. It cannot be thus completely ruled out that such a relatively short photoperiod could have direct effects by itself on the growth of song control nuclei although this is not supported at this time by any experimental evidence.
Even if such an early growth of HVC and RA was previously reported in great tits Parus major [20] and song sparrows Melospiza melodia morphna [21,26], the nature of the stimuli that triggers song nuclei growth still remains elusive. A few options are however available. The growth could for example reflect an endogenous circannual rhythm (see [14]). In addition, the recrudescence of HVC and RA could be steroid-dependent but controlled by steroids that would not be of gonadal origin (and were thus not assayed here). A growing body of evidence indicates that aspects of the reproductive physiology can be activated outside of the breeding season by neurosteroids, i.e. steroids produced in the brain either from cholesterol of from adrenal androgens. The best evidence for such a control mechanism concerns at this point the activation of aggressive behavior during the winter and development of HVC in song sparrows (Melospiza melodia morphna), which seem to depend on estrogens produced centrally by aromatization of androgens that would be either produced entirely in the brain or synthesized in the adrenal gland [23]. A similar control by neurosteroids might potentially explain the early growth of song nuclei observed here. Alternatively, there is scattered information in the literature suggesting that social and sexual interactions can modulate the production of sex steroids and possibly the size of song control nuclei. Since blue tits are often seen on their reproduction territories all year round, it is possible that the social interactions between males or interactions between males and females were responsible for the early increases in HVC and RA size reported here. Additional experimental work will be needed to identify the mechanism involved.
The full development of HVC was already observed by the end of February while RA size still continued to grow later in the season, resulting is a significantly larger volume in April than in February. This difference in the growth rate of HVC and RA might reflect a differential sensitivity of these two nuclei to T [25] but given that most of the growth occurred well before the vernal increase in plasma T, this explanation seems unlikely. Alternatively, this finding is reminiscent of results from earlier studies suggesting that the growth of RA is triggered at least in part by afferent inputs from HVC: in white-crowned sparrows, lesions of HVC decrease the response of RA to increasing long days and plasma T [9] and in canaries, systemic treatment with T for 11 days increases HVC but not RA volume [19]. It is therefore logical that the regrowth of RA in the spring follows HVC growth and the different patterns observed here may reflect this phenomenon.
Breeding asynchrony between blue tit populations in Corsica
The seasonal recrudescence of the song system occurred here simultaneously in two blue tits populations that show otherwise marked asynchrony in breeding physiology (see Fig. 1B and [10]). A similar phenomenon has also been reported in song sparrows [26]. This suggests that the (unidentified) environmental cues controlling the song system may differ from those affecting other morphological and physiological traits later in the season. We speculated earlier that the breeding asynchrony observed around the egg-laying period between these two blue tit populations, is probably the result of differential supplementary cues, such as food availability or the behavior of females nearing ovulation. In this case, even if both male populations seem to initiate their testicular development at the same time (suggesting a similar reaction to photoperiod), the later phase of development would then slow down in Pirio presumably due to the lack of proper responses from the females. The present data are fully consistent with this interpretation in that the earlier events in the male reproductive system development (in this case the song nuclei and the gonadal recrudescence) occurred simultaneously in both populations. Further studies will help to understand the mechanisms controlling this early development.
These data thus demonstrate an early seasonal recrudescence of HVC and RA in two distinct populations of Mediterranean blue tits occurring before the vernal peaks in singing activity and plasma T levels. This early growth thus appear to be independent of gonadal steroids but could still be controlled by steroids of adrenal origin (e.g., dehydroepiandrosterone) or by steroids directly produced in the brain. Although important differences in the reproductive phenology of these two populations were previously reported, no difference could be found in the timing of the seasonal growth of the song system. The current results suggest that (a) the early seasonal growth of the song system is independent of high levels of plasma T and singing activity and (b) the action of environmental cues on the seasonal male development may differ for the song system and the other physiological and morphological parameters.
Acknowledgments
Supported by grants from the NINDS (NS 35467), the Belgian FRFC (2.4555.01) and the French Community of Belgium (ARC 99/04-241), to J.B. and from the Belgian FNRS (Crédits de mission) to S.P.C. M.M.L. received financial support from the European Commission and the CNRS. S.P.C. is a FRIA Grant recipient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. JNeurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- 3.Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal Plasticity in the song control system. Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2002;1016:1–25. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- 4.Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- 5.Bentley GE, Van't Hof TJ, Ball GF. Seasonal neuroplasticity in the songbird telencephalon: A role for melatonin. Proc Natl Acad Sci USA. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard DJ, Wilson FE, Ball GF. Testis-dependent and -independent effects of photoperiod on volumes of song control nuclei in American tree sparrows (Spizella arborea) Brain Res. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- 7.Blondel J, Dias PC, Perret P, Maistre M, Lambrechts MM. Selection-based biodiversity at a small spatial scale in a low-dispersing insular bird. Science. 1999;285:1399–1402. doi: 10.1126/science.285.5432.1399. [DOI] [PubMed] [Google Scholar]
- 8.Blondel J, Perret P, Dias PC, Lambrechts MM. Is phenotypic variation of blue tits (Parus caeruleus) in mediterranean mainland and insular landscapes adaptive? Genet Sel Evol. 2001;33:S121–S139. [Google Scholar]
- 9.Brenowitz EA, Lent K. Afferent input is necessary for seasonal growth and maintenance of adult avian song control circuits. J Neurosci. 2001;21:2320–2329. doi: 10.1523/JNEUROSCI.21-07-02320.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caro SP, Balthazart J, Thomas DW, Lacroix A, Chastel O, Lambrechts MM. Endocrine correlates of the breeding asynchrony between two corsican populations of blue tits (Parus caeruleus) Gen Comp Endocrinol. 2005;140:52–60. doi: 10.1016/j.ygcen.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Deviche P, Gulledge CC. Vocal control region sizes of an adult female songbird change seasonally in the absence of detectable circulating testosterone concentrations. J Neurobiol. 2000;42:202–211. doi: 10.1002/(sici)1097-4695(20000205)42:2<202::aid-neu4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Dloniak SM, Deviche P. Effects of testosterone and photoperiodic condition on song production and vocal control region volumes in adult male dark-eyed juncos (Junco hyemalis) Horm Behav. 2001;39:95–105. doi: 10.1006/hbeh.2000.1621. [DOI] [PubMed] [Google Scholar]
- 13.Gulledge CC, Deviche P. Photoperiod and testosterone independently affect vocal control region volumes in adolescent male songbirds. JNeurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- 14.Gwinner E. Circannual rhythms. Berlin: Springer Verlag; 1986. [Google Scholar]
- 15.Lambrechts MM, Blondel J, Hurtrez-Boussès S, Maistre M, Perret P. Adaptive inter-population differences in blue tit life-history traits on Corsica. Evolutionary Ecology. 1997;11:599–612. [Google Scholar]
- 16.Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survical. Proc Natl Acad Sci USA. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 18.Reiner AD, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gütürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005 doi: 10.1016/j.yhbeh.2004.12.004. In press. [DOI] [PubMed] [Google Scholar]
- 20.Silver DW, Silverin B, Ball GF. Seasonal changes and sex differences in the song control system of free-living great tits. Soc Neurosci Abtracts, Abst. 2003;200.10 [Google Scholar]
- 21.Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32:426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Soma KK, Wissman AM, Brenowitz EA, Wingfield JC. Dehydroepiandrosterone (DHEA) increases territorial song and the size of an associated brain region in a male songbird. Horm Behav. 2002;41:203–212. doi: 10.1006/hbeh.2001.1750. [DOI] [PubMed] [Google Scholar]
- 24.Stamps JA, Buechner M. The Territorial Defense Hypothesis and the Ecology of Insular Vertebrates. Quarterly Review of Biology. 1985;60:155–181. doi: 10.1086/414314. [DOI] [PubMed] [Google Scholar]
- 25.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 26.Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinol. 2001;122:1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- 27.Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wingfield JC, Jacobs J, Hillgarth N. Ecological constraints and the evolution of hormone-behavior interrelationships. Ann NY Acad Sci. 1997;807:22–41. doi: 10.1111/j.1749-6632.1997.tb51911.x. [DOI] [PubMed] [Google Scholar]
- 29.Wingfield JC, Silverin B. Ecophysiological studies of hormone-behavior relations in birds. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. San Diego, CA: Academic Press; 2002. pp. 587–647. [Google Scholar]


