Abstract
Background
Multiple risk factors possibly associated with lung cancer were examined as part of a large-scale residential radon case-control study conducted in Iowa between 1994 and 1997. We were particularly interested in stratifying risk factors by smoking status. Relatively little risk factor information is available for Midwestern rural women.
Methods
Four hundred thirteen female lung cancer cases and 614 controls aged 40-84, who were residents of their current home for at least 20 years, were included. Risk factors examined included cigarette smoking, passive smoking, occupation, chemical exposure, previous lung disease, family history of cancer, and urban residence. Multiple logistic regression analysis was conducted after adjusting for age, education, and cumulative radon exposure.
Results
As expected, active cigarette smoking was the major risk factor for lung cancer. While cessation of smoking was significantly associated with a reduced risk for lung cancer, the risk remained significantly elevated for 25 years. Among all cases, asbestos exposure was a significant risk. Among ex-smokers, pack-year history predominated as the major risk. Among never smokers, a family history of kidney or bladder cancer were significant risk factors (OR= 7.34, 95% CI = 1.91 - 28.18; and 5.02, 95% CI = 1.64-15.39, respectively), as was a history of previous lung disease (OR=2.28, 95% CI=1.24-4.18) and asbestos exposure. No statistically significant increase in lung cancer risk was found for occupation or urban residence.
Conclusions
Smoking prevention activities are urgently needed in rural areas of the United States. Relatives of individuals with smoking-related cancers are potentially at increased risk. Genetic risk factors should be more fully investigated in never smokers.
Keywords: Lung cancer, Case-control, Epidemiology, Risk factors, Family history, Tobacco
1. INTRODUCTION
Lung cancer is the leading cause of cancer mortality in men and women in the U.S. and is the second leading site of cancer incidence. Approximately 163,510 deaths, representing approximately 28.7 percent of all cancer deaths, are projected to occur in the United States in 2005. The five-year lung cancer relative survival rate, for all stages, and all races, was 15 percent in the 1995-2000 period. Mortality rates from lung cancer have been slowly decreasing for men since 1990 and began leveling off for white women between 1997 and 2000 [1].
The major risk factor for lung cancer is cigarette smoking, with a relative risk of 20 to 25 and an attributable risk of 85 to 90 percent [2,3]. Risk factors also contributing to this disease include occupational exposure to arsenic, asbestos, crystalline silica, environmental tobacco smoke, and radon progeny [4]. Non-occupational risk factors include environmental tobacco smoke, residential radon progeny, a diet low in fruit and vegetable intake, and lower educational level [4]. Most studies to date have focussed on risk factors for lung cancer in males, a group that has more exposure to tobacco products and occupational carcinogens. Males historically have spent less time at home than females. Little data exist on the magnitude of the various risk factors for lung cancer in females.
A previous publication focused on residential radon exposure and lung cancer in Iowa women [5]. Our goal in this manuscript is to focus on the remaining risk factors in the Iowa Radon Lung Cancer Study. Our overall interest is in risk factors for lung cancer in never smokers, particularly women. This study includes stratification by smoking status, adjustment for smoking and other risk factors, and a wide variety of risk factors. The scope and detail of the information collected in Iowa provided this opportunity.
2. MATERIALS AND METHODS
The inclusion criteria for cases were: 1) newly diagnosed with a microscopically confirmed primary invasive (not in situ) lung carcinoma, without any prior primary invasive lung carcinoma; 2) female Iowa resident at the time of diagnosis; 3) age 40-84 years; and 4) residence in their current home for at least 20 consecutive years. Iowa Cancer Registry (ICR) personnel identified cases, through a rapid-reporting mechanism, meeting eligibility criteria between May 1, 1993 and October 30, 1996. The ICR has been a member of the National Cancer Institutes' Surveillance, Epidemiology, and End Results (SEER) program since its inception in 1973. The consent of the case subject's physician was obtained 101 d prior to contacting the subjects. Histologic samples collected from the cases were independently reviewed by two experienced pathologists at the University of Iowa, College of Medicine to confirm primary site and histology [6].
Female controls met similar criteria, with the exception of not having lung cancer. Controls aged 40-64 were selected from drivers' license files of the state of Iowa, while female controls aged 65-84 were selected from records of the Health Care Financing Administration. Controls were group matched on age to the cases [5]. Controls were verified against the ICR to insure that they did not have lung cancer. They could, however, have other cancers.
Participants were mailed detailed questionnaires followed-up by face-to-face facilitation of the questionnaires as needed. Because of the rapid-reporting mechanism used by the Iowa Cancer Registry, a high percentage (69 percent) of self-reported information was collected from live cases. All controls were alive at the time of interview. The questionnaires collected information on numerous lung cancer risk factors, including active and passive smoking, occupation, chemical exposures on the job, family history of cancer, personal history of prior lung disease, and education. The response rate was 73 percent for the cases and 52 percent for the controls.
A brief follow-up questionnaire was sent to individuals who chose not to participate in the study. No differences were noted between the participating and the 224 non-participating controls who returned the questionnaire for the categories ever-worked, current worker, ever smoked, current smoker, and bedroom radon concentrations [5].
Active smoking information included packs per day and years smoked. The minimum criteria utilized to classify someone as a smoker was that the person would have had to smoke at least 100 cigarettes (5 packs) in their lifetime or smoked for a period of at least six months. Pack-years were utilized as the measure of measure of tobacco exposure. Ex-smokers were individuals who had quit smoking five or more years prior to either diagnosis of lung cancer or interview (for controls). Years since smoking cessation were utilized as the measure of risk.
Passive smoking information for exposure as an adult was collected for those individuals who either never smoked or were ex-smokers and included exposure on the job and at home (yes/no, anyone at home, any smoking in bedroom). Passive smoking information for exposure as a child was collected for all individuals and focused on exposures at home (yes/no).
Occupations were categorized as either exposed or not to asbestos or selected other workplace carcinogens (e.g., paint, silica, gasoline fumes, coal dust, metal fumes/dust, radiation, or dry cleaning agents). Occupations were classified as high risk if they included exposure to dusts, particulates, volatile organic compounds, or cooking fumes. These categories included bartender, metal worker, welder, cook, factory worker, machine operator, painter, gas station attendant, carpenter, waitress, and truck driver. High-risk industries included dry cleaning, restaurant, plastics manufacturing, ordnance plant, welding shop, construction, bar, café, foundry, battery factory, paint contractor, and trucking.
Family history of any cancer was assessed in first-degree and other blood relatives. Previous lung disease ascertainment included doctor-diagnosed bronchitis, emphysema, asthma, tuberculosis, silicosis, asbestosis, and chronic obstructive pulmonary disease. Education was assessed by the number of years of schooling completed.
Urban residence was ascertained as either county of residence or as residence inside or outside of the city limit. The 99 Iowa counties were pre-categorized by population into counties containing the larger cities (n=4), large rural counties (n=15), and smaller rural counties (n=80).
Extensive radon monitoring was accomplished in the current home over a one-year period using up to five alpha track radon detectors on different living levels of the home. Outdoor radon concentrations were also obtained through a statewide year-long radon monitoring survey [7]. Retrospective assessment of cumulative radon exposure was estimated by linkage between historical occupancy patterns and multiple radon measurements [5].
All interviewers received rigorous training in interview techniques prior to initiation of the study. All interview and other study data were double entered into the database. Any discrepancies were noted and corrected. A ten percent random sample of these data was reviewed for quality control purposes. The study followed a detailed Quality Assurance/Quality Control Plan [8].
The University of Iowa's Human Subjects' Committee reviewed all procedures yearly to assure compliance with Human Subjects requirements. The investigation was performed after approval by this board and in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services. Written informed consent was obtained from each subject or guardian or surrogate respondent.
Simple descriptive analyses were performed utilizing a chi-square test for dichotomous variables. All relevant risk factors were included. Subsequently, an exploratory adjusted analysis was performed utilizing stepwise logistic regression. In the final models, the data were adjusted for age, education (some college), and radon exposure (WLM 20). The term WLM 20 refers to cumulative working level months for exposures occurring 5-19 years either prior to diagnosis (cases) or prior to interview (controls).
In order to enter the logistic model the p-value for the association had to be less than or equal to 0.10 (two-tail) based on the score chi-square statistic. Variables selected were retained if their p-value was less than or equal to 0.05 based on the Wald chi-square statistic. Logistic regression analyses were performed for all subjects combined, current smokers, ex-smokers, and never smokers. The analyses were repeated using the self-reported subjects (live cases and all controls) only. Further analysis focused on family history of cancer in first-degree relatives and the same focus combined with controls with cancer removed. A collinearity problem between childhood passive smoke exposure and smoking status (current, ex-, or never) was detected. As a result, the childhood passive smoke exposure variable was not included in the multivariable logistic regression models. The data were analyzed using SAS version 8.12 [9].
Results are presented for statistically significant and non-significant findings in the bivariate table (Table 1). In the multivariate analysis tables, statistically significant odds-ratios are presented. The risk analysis for prolonged radon exposure is presented elsewhere [5].
Table 1.
Unadjusted Odds-Ratios for Lung Cancer in Iowa Women (Total Population)
| Risk Factor |
Cases (%) [n=413] |
Controls (%) [n=614] |
OR | 95% CI |
|---|---|---|---|---|
| Ever Smoked | 357 (86.4) | 200 (32.6) | 13.20 | 9.50-18.33 |
| Current Smokersa | 253 (81.9) | 72 (14.8) | 25.98 | 17.72-38.09 |
| Family History of Cancer. |
||||
| Kidney | 21 (5.1) | 11 (1.8) | 2.95 | 1.41-6.20 |
| Bladder | 21 (5.1) | 16 (2.6) | 2.01 | 1.04-3.91 |
| Lung | 97 (23.7) | 94 (15.3) | 1.71 | 1.25-2.35 |
| Prostate | 55 (13.4) | 73 (11.9) | 1.15 | 0.79-1.67 |
| Colon | 72 (17.6) | 108 (17.6) | 1.00 | 0.72-1.38 |
| Breast | 87 (21.2) | 143 (23.3) | 0.89 | 0.65-1.20 |
| Any | 284 (68.8) | 437 (71.2) | 0.89 | 0.68-1.17 |
| Urban Residence | ||||
| Inside City limits | 340 (82.3) | 440 (71.7) | 1.84 | 1.35-2.51 |
| Large County vs. Small | 134 (51.0) | 144 (45.4) | 1.25 | 0.90-1.73 |
| Medium County vs. Small | 150 (53.8) | 297 (63.2) | 0.68 | 0.50-0.92 |
| Pre-existing Lung Disease 553 | ||||
| Bronchitis and emphysema | 100 (24.2) | 51 (8.3) | 3.53 | 2.45-5.08 |
| Any | 180 (45.6) | 163 (26.6) | 2.14 | 1.64-2.78 |
| Occupation | ||||
| High Risk | 30 (7.3) | 38 (6.2) | 1.19 | 0.72-1.95 |
| Asbestos Exposure | 10 (2.4) | 12 (2.0) | 1.24 | 0.53-2.91 |
| Any Chemical Exposure | 34 (8.2) | 38 (6.2) | 1.36 | 0.84-2.20 |
| At least some 561 | ||||
| College Education | 135 (32.7) | 268 (43.6) | 0.63 | 0.48-0.81 |
| Passive Smoke Exposure 564 | ||||
| Adult (yes/no in home)b | 60 (37.5) | 334 (61.6) | 0.37 | 0.26-0.54 |
| Adult (smoke bedroom)b | 11 (6.9) | 68 (12.6) | 0.51 | 0.27-1.00 |
| Childhood (0-15, yes/no) | 254 (61.5) | 383 (62.4) | 0.96 | 0.74-1.25 |
Compared to never smokers.
Never and Ex-Smokers only (n=160 for cases; n=542 for controls).
3. RESULTS
There were 413 cases and 614 controls included in the study. Of these 1,027 participants, 1,010 (98.3%) were white and not of Hispanic origin. Table 1 shows a number of the unadjusted odds-ratios (OR) for lung cancer. Not surprisingly, the largest category for cases is Ever Smoked (n=357), with an OR=13.20 (95% CI=9.80-18.33). For current smokers the odds-ratio is 25.98 (95% CI=17.72-38.09).
The mean number of pack-years for cases is 37.70 (standard deviation ± 26.60) while the corresponding information for controls is 7.81 (standard deviation ± 16.32). The average years since quitting smoking for cases is 4.22 (standard deviation ± 6.86) while the corresponding number of years for controls is 12.45 (standard deviation ± 10.40).
A number of risk factors relate to family history of cancer. Of these, family histories 192 s of kidney cancer (OR=2.95, 95% CI=1.45-6.20), lung cancer (OR=1.71, 95% CI=1.25-2.35), and bladder cancer (OR=2.01, 95% CI=1.04-3.91) were statistically significant. In evaluating the risks among first-degree relatives only, we found unadjusted increased risks for kidney (n=14 cases), laryngeal (n=14 cases), and lung cancer (n=77 cases) (data not shown).
Urban residence was a significantly increased risk factor using inside city limits as the category (OR=1.84, 95% CI=1.35-2.51). There was a significant increase in risk for those with certain pre-existing lung diseases (OR=3.53, 95% CI=2.45-5.08) or for those with any lung disease (OR=2.14, 95% CI=1.64-2.78).
There was no significant increase or decrease in risk for those in high-risk occupations, those with any chemical exposure, those with asbestos exposure, or those with exposure to environmental tobacco smoke as a child. A significant inverse association was found for those with some college education (OR=0.63, 95% CI=0.48-0.81) and for those with adult passive smoke exposure at home (OR=0.37, 95% CI=0.26-0.54).
Table 2 shows the results of the logistic regression analysis for the total study population (413 cases and 614 controls) after adjustment for radon (WLM 20), education (some college), and age. For all subjects the largest risk factor is current smoker (OR=13.92, 95% CI=7.40- 26.18), followed by ex-smoker. For each single pack-year there was an increase in the risk (OR=1.03, 95% CI=1.01-1.04). The next highest odds-ratios were found for asbestos exposure (OR=3.39, 95% CI=1.18-9.75), family history of bladder cancer (OR=3.08, 95% CI=1.26-7.57), and family history of kidney cancer (OR=3.04, 95% CI=1.13-8.18). Years since quitting smoking (maximum of 25 years) is a significant protective variable (OR=0.92, 95% CI=0.88-0.96).
Table 2.
Adjusted Odds-Ratios for Lung Cancer in Iowa Women (Total Population)a
| Subject Category |
Original (Adjusted) #'s |
Risk Factor | OR | 95% CI |
|---|---|---|---|---|
| All Subjects | ||||
| Current Smoker | 13.92 | 7.40-26.18 | ||
| 413 (410) cases | Ex-Smoker | 13.47 | 5.17-35.12 | |
| 614 (613) controls |
Pack-year | 1.03 | 1.01-1.04 | |
| Quit Smoking | 0.92 | 0.88-0.96 | ||
| Asbestos Exposure | 3.39 | 1.18-9.75 | ||
| Family History Bladder Cancer |
3.08 | 1.26-7.57 | ||
| Family History Kidney Cancer |
3.04 | 1.13-8.18 | ||
| Current Smokers |
||||
| 253 (250) cases | Pack-year | 1.02 | 1.01-1.04 | |
| 72 (72) controls | Family History Lung Cancer |
2.43 | 1.12-5.28 | |
| Ex-Smokersb | ||||
| 104 (104) cases | Pack year | 1.03 | 1.01-1.05 | |
| 128 (128) controls |
Quit Smoking | 0.93 | 0.88-0.98 | |
| Never Smokersc |
||||
| 56 (56) cases | Family History Kidney Cancer |
7.34 | 1.91-28.18 | |
| 414 (413) controls |
Family History Bladder Cancer |
5.02 | 1.64-15.39 | |
| Any Lung Disease |
2.28 | 1.24-4.18 |
Adjusted for radon (WLM 20), education (some college), and age. Numbers in parentheses represent cases or controls with all relevant variables available for analysis.
Quit five or more years prior to either diagnosis of lung cancer or interview (for controls).
Smoked less than 100 cigarettes (5 packs) in their lifetime or less than six months.
Among current smokers each single pack-year is significantly elevated (OR=1.02, 95% CI=1.01-1.04). Family history of lung cancer is also significantly elevated (OR= 2.43, 95% CI=1.12-5.28).
For ex-smokers each single pack-year is significantly elevated (OR=1.03, 95% CI=1.01-1.05). Years since quitting smoking (maximum of 25 years) is also associated with a statistically significantly reduction in risk (OR=0.93, 95% CI=0.88-0.98).
Among never smokers, family history of kidney cancer shows a significant positive association (OR=7.34, 95% CI=1.91-28.18). Other significant variables are family history of bladder cancer (OR=5.02, 95% CI=1.64-15.39) and history of any 223 lung disease (OR=2.28, 95% CI=1.24-4.18). No effect is seen for any urban factor or passive smoking.
Table 3 presents the results of the logistic regression analysis for live cases (n=283) and controls (n=614; all controls in this study were alive at time of interview) after adjustment for cumulative radon exposure (WLM 20), education (some college), and age. As before, the major risk factor for all subjects is current smoker (OR=14.70, 95% CI=7.44-29.05), followed by ex-smoker. Family history of bladder, kidney, prostate, or lung cancer is also statistically significant (OR=3.39, 3.25, 1.88, and 1.60, respectively). A significantly reduced risk is seen for each year since quitting smoking (maximum of 25 years) (OR=0.92, 95% CI= 0.88-0.97).
Table 3.
Adjusted Odds-Ratios for Lung Cancer in Iowa Women (Live Cases and Controls Only)a
| Subject Category |
Original (Adjusted) #'s |
Risk Factor | OR | 95% CI |
|---|---|---|---|---|
| All Subjects | ||||
| Current Smoker | 14.70 | 7.44-29.05 | ||
| 283 (282) cases | Ex-Smoker | 14.40 | 5.01-41.39 | |
| 614 (613) controls |
Pack-year | 1.02 | 1.01-1.04 | |
| Quit Smoking | 0.92 | 0.88-0.97 | ||
| Family History Bladder Cancer |
3.39 | 1.25-9.18 | ||
| Family History Kidney Cancer |
3.25 | 1.12-9.42 | ||
| Family History Prostate Cancer |
1.88 | 1.10-3.22 | ||
| Family History Lung Cancer |
1.60 | 1.02-2.52 | ||
| Current Smokers |
||||
| Pack-year | 1.02 | 1.00-1.04 | ||
| 166 (165) cases | Family History Colon Cancer |
3.00 | 1.14-7.89 | |
| 72 (72) controls | Family History Lung Cancer |
2.43 | 1.08-5.45 | |
| Ex-Smokersb | ||||
| 80 (80) cases | Pack-year | 1.03 | 1.01-1.05 | |
| 128 (128) controls |
Quit Smoking | 0.93 | 0.88-0.97 | |
| Never Smokersc |
||||
| 37 (37) cases | Family History Kidney Cancer |
10.96 | 2.76-43.58 | |
| 414 (413) controls |
Family History Bladder Cancer |
6.05 | 1.72-21.31 |
Adjusted for radon (WLM 20), education (some college), and age. Numbers in parentheses represent cases or controls with all relevant variables available for analysis.
Quit five or more years prior to either diagnosis of lung cancer or interview (for controls).
Smoked less than 100 cigarettes (5 packs) in their lifetime or less than six months.
Among current smokers each single pack-year is marginally significantly elevated (OR=1.02, 95% CI=1.00-1.04). Family history of both colon and lung cancer are significantly elevated (OR=3.00 and 2.43, respectively).
For ex-smokers each single pack-year is significantly elevated (OR=1.03, 95% CI=1.01-1.05). Years since quitting smoking (maximum of 25 years) is also associated with a statistically significantly reduction in risk (OR=0.93, 95% CI=0.88-0.97).
Among never smokers, family history of kidney and bladder cancer shows a significant positive association (OR=10.96, 95% CI=2.76-43.58, and OR=6.05, 95% CI=1.72-21.31, respectively). No significant effect is seen for urban factor, passive smoking, or previous lung disease.
Table 4 restricts the family history portion of Table 3 to those reporting on first-degree relatives only. The focus is on the percentage of first-degree relatives reporting these cancers. The odds-ratios decrease for all the family history variables. Asbestos exposure emerges as a significant risk factor for lung cancer in never smokers (OR=4.38, 95% CI=1.10-17.45).
Table 4.
Adjusted Odds-Ratios for Lung Cancer in Iowa Women (Live Cases and Controls Only)a
| Subject Category |
Original (Adjusted) #'s |
Risk Factor | OR | 95% CI |
|---|---|---|---|---|
| All Subjects | ||||
| Current Smoker | 14.99 | 7.66-29.34 | ||
| 283 (282) cases | Ex-Smoker | 16.68 | 5.91-47.06 | |
| 614 (613) controls |
Pack-year | 1.02 | 1.01-1.03 | |
| Quit Smoking | 0.92 | 0.87-0.96 | ||
| % 1st. Degree Relatives with Kidney Cancer |
1.09 | 1.01-1.19 | ||
| % 1st. Degree Relatives with Lung Cancer |
1.04 | 1.01-1.07 | ||
| Current Smokers |
||||
| Pack-year | 1.02 | 1.00-1.04 | ||
| 166 (165) cases | % 1st. Degree Relatives with Colon Cancer |
1.13 | 1.01-1.28 | |
| 72 (72) controls | % 1st. Degree Relatives with Lung Cancer |
1.15 | 1.04-1.28 | |
| Ex-Smokersb | ||||
| 80 (80) cases | Pack-year | 1.03 | 1.01-1.05 | |
| 128 (128) controls |
Quit Smoking | 0.93 | 0.88-0.97 | |
| Never Smokersc |
||||
| 37 (37) cases | % 1st. Degree Relatives with Kidney Cancer |
1.25 | 1.10-1.43 | |
| 414 (413) controls |
Asbestos Exposure |
4.38 | 1.10-17.45 |
Adjusted for radon (WLM 20), education (some college), and age. Family history restricted to those reporting first-degree relatives with cancer. Numbers in parentheses represent cases or controls with all relevant variables available for analysis.
Quit five or more years prior to either diagnosis of lung cancer or interview (for controls).
Smoked less than 100 cigarettes (5 packs) in their lifetime or less than six months.
As can be seen in Table 5, when controls are removed who have other types of cancer (n=90), results are similar to Table 4 with the following exceptions: 1) family history of any cancer replaces the percentage of first-degree relatives reporting lung cancer and 2) the percentage of first-degree relatives reporting colon cancer drops out as a significant predictor.
Table 5.
Adjusted Odds-Ratios for Lung Cancer in Iowa Women (Live Cases and Controls Only)a
| Original (Adjusted) #'s |
Risk Factor | OR | 95% CI | |
|---|---|---|---|---|
| All Subjects | ||||
| Current Smoker | 17.65 | 8.78-35.47 | ||
| 283 (282) cases | Ex-Smoker | 18.37 | 6.22-54.27 | |
| 614 (523) controls |
Pack-year | 1.02 | 1.01-1.03 | |
| Quit Smoking | 0.92 | 0.87-0.96 | ||
| % 1st. Degree Relatives with Kidney Cancer |
1.10 | 1.00-1.20 | ||
| Family History Any Cancerb |
1.59 | 1.03-2.45 | ||
| Current Smokers |
||||
| 166 (165) cases | Pack-year | 1.02 | 1.00-1.04 | |
| 72 (61) controls | % 1st. Degree Relatives with Lung Cancer |
1.14 | 1.03-1.26 | |
| Ex-Smokersc | ||||
| 80 (80) cases | Pack-year | 1.03 | 1.01-1.05 | |
| 128 (103) controls |
Quit Smoking | 0.93 | 0.88-0.98 | |
| Never Smokersc |
||||
| 37 (37) cases | % 1st. Degree Relatives with Kidney Cancer |
1.34 | 1.13-1.58 | |
| 414 (359) controls |
Asbestos Exposure |
5.17 | 1.20-22.36 |
Adjusted for radon (WLM 20), education (some college), and age. Family history restricted to those reporting first-degree relatives with cancer. Controls with other cancers removed. Numbers in parentheses represent cases or controls with all relevant variables available for analysis.
Information on first-degree relatives was unavailable for family history of any cancer.
Quit five or more years prior to either diagnosis of lung cancer or interview (for controls).
Smoked less than 100 cigarettes (5 packs) in their lifetime or less than six months.
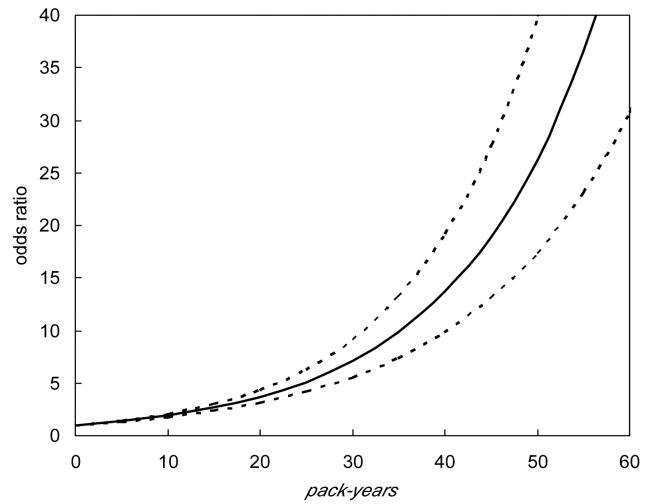
Figures 1 and 2 are based upon a model that adjusts for radon (WLM 20), education (some college), age, pack-years, and years since quitting smoking [maximum of 25 years]. Figure 1 illustrates the strong increase in lung cancer risk as the number of pack-years increases. Figure 2 illustrates the decrease in the lung cancer odds-ratio among ex-smokers as the time since quitting increases. As can be seen, the risk drops to about 14 percent of the risk among ever smokers about 25 years after quitting smoking. Women who have quit smoking recently have about seven times the risk of developing lung cancer as women who have quit smoking for 25 years.
Fig. 1.
Lung Cancer Risk Associated with Cigarette Use. Estimates of the lung cancer odds ratio associated with cigarette pack- year exposure. The dashed lines represent 95 percent pointwise confidence intervals. This figure is based on a model adjusting for radon (WLM 20), education (some college), and years since quitting smoking (maximum of 25 years). Comparison is made to never smokers.
Fig. 2.
Lung Cancer Risk Subsequent to Smoking Cessation. Estimates of the lung cancer odds ratio associated with years since smoking cessation (maximum of 25 years) (smkquit) among current and ex-smokers. The dashed lines represent 95 percent pointwise confidence intervals. This figure is based on a model adjusting for radon (WLM 20), education (some college), age, and pack-years. Comparison is made to current smokers.
4. DISCUSSION
As might be expected, the major risk factor for lung cancer emerging from this study is active cigarette smoking. Quitting smoking reduced the risk of lung cancer; with the risk dropping continuously up to 25 years. The decrease agrees qualitatively with other predictions of reduction in lung cancer risk after quitting smoking [10]. However, the odds-ratio remained significantly elevated above that for never smokers even after that lapse of time. The odds-ratio comparing those who quit for 25 years to those who never quit was 0.14 (95% CI=0.04-0.58) (Figure 2). However, this seven-fold protective effect did not overcome the thirteen-fold increased risk of lung cancer found by comparing ever-smokers to never smokers (Table 1). A population-based prospective cohort study of 41, 836 Iowa women aged 55 to 69 years has also been conducted [11]. In that study, The Iowa Women's Health Study, former smokers had an elevated risk of lung cancer compared to never smokers after 30 years of follow-up (RR=6.6, 95% CI=5.0-8.7).
Among never smokers no significant effect of urban factor, passive smoking, or occupation (including high risk) was detected. However, an increased risk was seen for exposure to asbestos (either direct or indirect). It is likely that the sample sizes for the never and ex-smoking categories in this study were too small to fully examine risk factors in these groups.
Familial risk factors for lung cancer were observed (Table 3) despite the fact that the control group included individuals with cancer (including breast [n=38], uterine [n=7], and colon cancers [n=9]). Those controls with cancer would also be expected to show some familial clustering of cancer due to genetic and lifestyle characteristics. Despite the inclusion of such individuals, there still is a reported excess of cancer in relatives of cases, compared to relatives of controls. When analysis was first restricted to first-degree relatives with cancer (Table 4) and then to first-degree relatives with cancer but with controls with cancer eliminated from the analysis (Table 5), the familial tendency for cancer remains. However, the use of a family history variable (yes/no) in this case-control study is not as definitive as would be a family-based cohort study that allowed for the adjustment of smoking habits for each relative.
A number of studies have evaluated the influence of family history of cancer on subsequent lung cancer [12-18]. Family history of cancer, particularly lung cancer in younger first-degree relatives has been shown in some studies to be a significant risk factor. Some studies have found an increase in risk with a reported familial history of reproductive cancer [12], others with reported family history of any cancer [13,15], while others have found an increased lung cancer risk in those with a reported excess of familial lung cancer [14,16]. Two studies [17,18] found a significant increase in risk with reported family history of lung cancer only for younger patients (e.g., only for patients aged less than 40 or 45). Odds ratios tend to be in the 1.5-3.0 range. A recent study in Detroit, Michigan [19] found an increased risk for early- onset lung cancer among those with a first-degree relative with lung cancer after adjusting for race, age, sex, and pack-years of smoking (OR=1.71, 95% CI=1.16-2.52). With regard to the genetic component of such familial risk, a susceptibility locus for lung cancer has recently been identified on chromosome six [20].
The associations with familial risk for some tobacco-related cancers noted in this study, as well as others, suggests that programs designed to reduce lung cancer risks should include an educational component addressing the significance of family history of cancer. Smoking intervention programs that provide information on the implications of genetic susceptibility to lung cancer may be more effective at reaching individuals on a personal level, thereby affecting lifestyle behaviors that reduce smoking-related cancer risks.
The urban factor, which has shown up in several publications as a risk factor for lung cancer in Iowa [21-23], drops out as a risk factor after suitable adjustment. Our previous ecologic study showed urban residence to be a risk factor at the group level of detail, but there was evidently considerable confounding from smoking and radon in the earlier study [22]. There generally is more smoking in urban areas of Iowa.
Occupation was not found to be a risk factor for lung cancer in this study; however, exposure to asbestos did present a risk (Tables 4 and 5). There were a relatively small number of women who worked in high-risk occupations (n=68). While occupational causes of lung cancer among men have been studied extensively for years, relatively little is known about the effect of occupational exposures on lung cancer in women [4,24-27]. Studies in males have documented lung cancer related exposures such as underground uranium mining (radon progeny), asbestos, arsenic, beryllium, hexavalent chromium, crystalline silica, and polycyclic aromatic hydrocarbons. It is possible that lung cancer risk among never smokers in these studies is quite small. It has been estimated that 57 percent of female cases can be attributed to occupation [26,28]. A study of non-smoking women in Missouri [26] found an excess risk among women who worked with asbestos, pesticides, or in dry-cleaning facilities. After adjusting for age, race, and pack-years of smoking, there were no significant occupational or industrial risks for lung cancer in women in Detroit, Michigan [25]. In Illinois, significantly increased risks were found for non-smoking white women employed in eating and drinking places; occupation as a registered nurse was of borderline significance [27]. A more detailed discussion of occupational risks for lung cancer in non-smokers may be found elsewhere [4].
Pre-existing lung disease was not a risk factor for lung cancer in this study. However, it has been found to be a risk factor for lung cancer in other studies [29,30]. In Missouri the risk was low, generally with an OR < 1.5 [29]. Among lifetime non-smokers, the highest risk for previous lung disease was for asthma (OR=2.7, 95% CI=1.4-5.4). However, the literature overall is inconclusive on asthma. Most studies of tuberculosis and pneumonia show positive associations. In another study in five metropolitan areas, statistically significant increases on the order of 1.6 were seen for any lung disease, asthma, and chronic bronchitis, while marginally significant increases in risk were seen for pneumonia (OR=1.37) and for emphysema (OR=2.61) [30]. In both these studies the number of nonsmokers was larger than that found herein. In an overview of the causes of lung cancer in nonsmokers significantly elevated odds-ratios in the range of 1.4-2.9 were found for a number of previous non-malignant lung diseases, including emphysema and pneumonia [31].
No increased risk was noted for passive smoking in this study, but information on passive smoke exposure as an adult was only based on the smaller subset sample of ex and never smokers. Although this approach is consistent with that used by a number of other studies, the decision not to collect passive smoke exposure in current smokers affected our ability to detect this hazard in the overall model (which included current smokers). In addition, pack-years or packs per day were unreliable measures of exposure to second-hand smoke because the respondent frequently didn't know how many packs per day were smoked in their presence. A number of respondents reported exposure in the home for a full 24 hours per day, an extremely unlikely scenario. Thus, we were unable to determine time-response relationships with any confidence. Unfortunately, this study did not have available to it objective measurements of recent tobacco smoke exposure, such as urinary cotinine measurements.
Passive smoking by a spouse or in childhood has been evaluated in over 30 studies. Results have been mixed as to whether or not it is a risk factor for lung cancer in nonsmokers. Some studies report a positive association with spousal exposure [32-34], while others do not [35,36]. Some studies have found a positive association with childhood exposure [34,35], while others have not [32,33]. The study in Missouri found no increase in risk for those exposed in childhood [33]. For those exposed at home there was a non-significantly increased risk of 1.3. Exposure of more than 40 pack-years duration increased the risk among non-smokers by about 30 percent. In a case series conducted in Olmsted County, Minnesota, 57 percent of never smoking female lung cancer cases reported environmental tobacco smoke exposure in childhood [37]. This compares with 61.5 percent in this study. However, there were no controls utilized in the Minnesota study. The authors suggest that lung cancer, smoking, and environmental tobacco smoke exposure aggregate in families. A German study found a significantly increased risk for exposure to environmental tobacco smoke at work [38]. However, a non-significant increase in risk was found for spousal exposure. A report by the U.S. Environmental Protection Agency indicates the result of a pooled analysis to be a non-significantly elevated risk of 1.19 for exposure to spousal smoke [39]. A meta-analysis of 37 studies (4,626 cases and 477,924 controls) of non-smoking women exposed to spousal tobacco smoke at home found a pooled relative risk of 1.24 (95% CI=1.13-1.36). Only seven of the original studies found a significantly elevated risk [40]. Additional discussion of studies may be found in our previous summary [4].
This study was population-based and had excellent retrospective radon exposure assessment, a relatively large sample size (413 cases and 614 controls), independent pathologic review, and good quality assurance and quality control. Rapid-reporting was utilized (which allowed for a high percentage of direct interviews with cases), questionnaire information was double entered, a 10 percent random sample of questionnaire data was compared to the computerized database, relevant risk factors for lung cancer were included, and comprehensive statistical analyses were performed. A relatively uniform socio-economic status was assured by requiring 20 years residency in the current home, by utilizing the relatively homogeneous population of Iowa, and by adjusting for education at various points in the analysis.
Limitations of the study include a response rate of 52 percent in the controls. The need for scientific staff to visit homes, place radon detectors for a year, and later retrieve them, may have contributed to the low response rate. In addition, the 20-year residency restriction may have also contributed to this problem [5]. The limited sample size for some of the analyses and the potential for reduced information quality from proxy interviews are also noteworthy. We used both stratified and adjusted analyses to partially counter some of these problems. The absence of reliable time of exposure information for passive smoking has previously been mentioned. Thus, one may question the validity of the second-hand smoking data. If the sample size of never smokers increased this would allow for improved evaluation of histologic subtypes. The family history of cancer variable needs to be interpreted cautiously because of the potential bias from recall. The results of this study are best generalizable to midwestern women who spent at least 20 years in their current home. Additional information on the strengths and weaknesses of the study are reported elsewhere [5].
Given the fact that there is no known threshold for cigarette smoking and lung cancer, perhaps the definition of “ever smoker” used in research studies should be revisited. We used the standard United States guideline herein, requiring the smoking of either 100 or more cigarettes over a lifetime or the smoking of any amount of cigarettes over at least a six-month period. Therefore, some of our never smokers (cases or controls) could have smoked, but at a low level. Unfortunately, more detailed smoking information was not obtained for this group.
Given the findings in never smokers, including the family history of smoking related cancers, the etiology of lung cancer in never smoking women should be a high priority area for future research. A more in-depth family history approach should be utilized (41). Sample sizes of never smokers will need to be dramatically increased over the numbers utilized herein in order to more fully evaluate risk factors. While all risk factors need to be addressed, key areas of potential interest should include genomic instability, DNA adducts, and deficiencies in DNA repair mechanisms. The genetic factors that underlie familial susceptibility to tobacco-related cancers need to be explored in more detail. A detailed genetic analysis and an historical family profile would be helpful. Lung cancer education and prevention efforts should also include information on the potential link between lung cancer and both asbestos exposure and a family history of cancer, particularly lung and other tobacco-related cancers.
In conclusion, continued and urgent efforts are needed in Iowa and other rural states to reduce smoking rates. Measures that could be used to reduce tobacco usage include additional taxation, eliminating smoking in all work and public places, and intensive community and school-based educational programs.
ACKNOWLEDGEMENTS
This publication was made possible by grant numbers R01 ES05653 and P30 ES05605 from the National Institute of Environmental Health Sciences, NIH. Dr. Mayo's effort was supported by grant 5R24 CA095835 from the National Cancer Institute, NIH. The authors acknowledge the invaluable assistance of the individuals and their families who participated in this study and the Iowa Radon Lung Cancer scientific staff. We also thank Drs. Charles Lynch and Brian Smith for reviewing an earlier version of this manuscript.
Footnotes
Sources of Support: This publication was made possible by grant numbers R01 ES05653 and P30 ES05605 from the National Institute of Environmental Health Sciences, NIH. Computer system support was partially provided by the Center for Health Effects of Environmental Contamination at the University of Iowa. Dr. Mayo's effort was supported by grant 5R24 CA095835 from the National Cancer Institute, NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Blot WJ, Fraumeni JF., Jr. Cancers of the lung and pleura. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and Prevention. 2nd ed. Oxford University Press; New York, NY: 1996. [Google Scholar]
- 3.Baron JA, Rohan TE. Tobacco. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and Prevention. 2nd ed. Oxford University Press; New York, NY: 1996. [Google Scholar]
- 4.Neuberger JS, Field RW. Occupation and Lung Cancer in Non-Smokers. Reviews Environ Health. 2003;18(4):251–267. doi: 10.1515/reveh.2003.18.4.251. [DOI] [PubMed] [Google Scholar]
- 5.Field RW, Steck DJ, Smith BJ, Brus CP, Fisher EL, Neuberger JS, et al. Residential radon gas exposure and lung cancer: The Iowa radon lung cancer study. Am J Epidemiol. 2000;151(11):1091–1102. doi: 10.1093/oxfordjournals.aje.a010153. [DOI] [PubMed] [Google Scholar]
- 6.Field RW, Smith BJ, Platz CE, Robinson RA, Neuberger JS, Brus CP, et al. Lung cancer histologic type in Surveillance, Epidemiology, and End Results registry versus independent review. J Natl Cancer Inst. 2004;96(14):1105–1107. doi: 10.1093/jnci/djh189. [DOI] [PubMed] [Google Scholar]
- 7.Steck DJ, Field RW, Lynch CF. Exposure to atmospheric radon. Environ Health Perspect. 1999;107(2):123–127. doi: 10.1289/ehp.99107123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field RW, Lynch CF, Steck DJ, Fisher EF. Dosimetry quality assurance: the Iowa residential radon lung cancer study. Radiat Prot Dosimetry. 1998;78(4):295–303. [Google Scholar]
- 9.SAS Institute Inc . SAS/STAT User's Guide, Version 8. SAS Institute Inc; Cary, NC: 1999. [Google Scholar]
- 10.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation and lung cancer in the UK since 1950: Combination of national statistics with two case-control studies. Br Med J. 2000;321(7257):323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebbert JO, Yang P, Vachon CM, Vierkant RA, Cerhan JR, Folsom AR, et al. Lung cancer risk reduction after smoking cessation: Observations from a prospective cohort of women. J Clin Oncol. 2003;21(5):921–926. doi: 10.1200/JCO.2003.05.085. [DOI] [PubMed] [Google Scholar]
- 12.Sellers TA, Potter JD, Folsom AR. Association of incident lung cancer with family history of female reproductive cancers: The Iowa women's health study. Genet Epidemiol. 1991;8:199–208. doi: 10.1002/gepi.1370080306. [DOI] [PubMed] [Google Scholar]
- 13.Mayne ST, Buenconsejo J, Janerich DT. Familial cancer history and lung cancer risk in United States nonsmoking men and women. Cancer Epidemiol Biomarkers Prev. 1999;8:1065–1069. [PubMed] [Google Scholar]
- 14.Ooi WL, Elston RC, Chen VW, Bailey-Wilson JE, Rothschild H. Increased familial risk for lung cancer. J Natl Cancer Inst. 1986;76(2):217–222. [PubMed] [Google Scholar]
- 15.Brownson RC, Alavanja MCR, Caporaso N, Berger E, Chang JC. Family history of cancer and risk of lung cancer in lifetime non-smokers and long-term ex-smokers. Int J Epidemiol. 1997;26:256–263. doi: 10.1093/ije/26.2.256. [DOI] [PubMed] [Google Scholar]
- 16.Wu AH, Fontham ETH, Reynolds P, Greenberg RS, Buffler P, Liff J, et al. Family history of cancer and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1996;143:535–542. doi: 10.1093/oxfordjournals.aje.a008783. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AG, Yang P, Swanson GM. Familial risk of lung cancer among nonsmokers and their relatives. Am J Epidemiol. 1996;144:554–562. doi: 10.1093/oxfordjournals.aje.a008965. [DOI] [PubMed] [Google Scholar]
- 18.Kreuzer M, Kreienbrock L, Gerken M, Heinrich J, Bruske-Hohlfeld I, Muller K-M, et al. Risk factors for lung cancer in young adults. Am J Epidemiol. 1998;147:1028–1037. doi: 10.1093/oxfordjournals.aje.a009396. [DOI] [PubMed] [Google Scholar]
- 19.Coté ML, Kardia SLR, Wenzlaff AS, Ruckdeschel JC, Schwartz AG. Risk of lung cancer among white and black relatives of individuals with early-onset lung cancer. JAMA. 2005;293:3036–3042. doi: 10.1001/jama.293.24.3036. [DOI] [PubMed] [Google Scholar]
- 20.Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, et al. A major lung cancer susceptibility locus maps to chromosome 6q23-25. Am J Hum Genet. 2004;75:460–474. doi: 10.1086/423857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg MR. The United States Experience, 1950-1975. Oxford University Press; New York: 1983. Urbanization and cancer mortality. [Google Scholar]
- 22.Neuberger JS, Lynch CF, Kross BC, Field RW, Woolson RF. Residential Radon Exposure and Lung Cancer: Evidence of an Urban Factor in Iowa. Health Phys. 1994;66(3):263–269. doi: 10.1097/00004032-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lynch CF, Oppliger B, Burmeister L, Van Hoesen C, Olson D. Excess of smoking related cancers in urban relative to rural areas of Iowa. Abstract; 16th Annual Meeting of the American Society of Preventive Oncology; Bethesda, MD.. 1992. [Google Scholar]
- 24.Samet JM, editor. Epidemiology of lung cancer. Marcel Dekker; New York: 1994. [Google Scholar]
- 25.Swanson GM, Burns PB. Cancer incidence among women in the workplace: a study of the association between occupation and industry and 11 cancer sites. J Occup Environ Med. 1995;37(3):282–287. doi: 10.1097/00043764-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Brownson RC, Alavanja MCR, Chang JC. Occupational risk factors for lung cancer among nonsmoking women: a case-control study in Missouri (United States) Cancer Causes Control. 1993;4(5):449–454. doi: 10.1007/BF00050864. [DOI] [PubMed] [Google Scholar]
- 27.Keller JE, Howe HL. Risk factors for lung cancer among non-smoking Illinois residents. Environ Research. 1993;60(1):1–11. doi: 10.1006/enrs.1993.1001. [DOI] [PubMed] [Google Scholar]
- 28.Alavanja MCR, Brownson RC, Benichou J, Swanson C, Boice JD., Jr. Attributable risk of lung cancer in lifetime nonsmokers and long-term ex-smokers (Missouri, United States) Cancer Causes Control. 1995;6:209–216. doi: 10.1007/BF00051792. [DOI] [PubMed] [Google Scholar]
- 29.Alavanja MCR, Brownson RC, Boice JD, Jr., Hock E. Preexisting lung disease and lung cancer among nonsmoking women. Am J Epidemiol. 1992;136:623–632. doi: 10.1093/oxfordjournals.aje.a116542. [DOI] [PubMed] [Google Scholar]
- 30.Wu AH, Fontham ETH, Reynolds P, Greenberg RS, Buffler P, Liff J, et al. Previous lung disease and risk of lung cancer among lifetime nonsmoking women in the United States. Am J Epidemiol. 1995;141:1023–1032. doi: 10.1093/oxfordjournals.aje.a117366. [DOI] [PubMed] [Google Scholar]
- 31.Brownson RC, Alavanja MCR, Caporaso N, Simoes EJ, Chang JC. Epidemiology and prevention of lung cancer in nonsmokers. Epidemiol Rev. 1998;20(2):218–236. doi: 10.1093/oxfordjournals.epirev.a017982. [DOI] [PubMed] [Google Scholar]
- 32.Fontham ETH, Correa P, Reynolds P, Wu-Williams A, Buffler PA, Greenberg RS, et al. Environmental tobacco smoke and lung cancer in non-smoking women: A multicenter study. JAMA. 1994;271:1752–1759. [PubMed] [Google Scholar]
- 33.Brownson RC, Alavanja MCR, Hock ET, Loy TS. Passive smoking and lung cancer in nonsmoking women. Am J Public Health. 1992;82:1525–1530. doi: 10.2105/ajph.82.11.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockwell HG, Goldman AL, Lyman GH, Noss CI, Armstrong AW, Pinkham PA, et al. Environmental tobacco smoke and lung cancer risk in non-smoking women. J Natl Cancer Inst. 1992;84:1417–1422. doi: 10.1093/jnci/84.18.1417. [DOI] [PubMed] [Google Scholar]
- 35.Janerich DT, Thompson WD, Varela LR, Greenwald P, Chorost S, Tucci C, et al. Lung cancer and exposure to tobacco in the household. N Engl J Med. 1990;323:632–636. doi: 10.1056/NEJM199009063231003. [DOI] [PubMed] [Google Scholar]
- 36.Kabat GC, Stellman SD, Wynder EL. Relation between exposure to environmental tobacco smoke and lung cancer in lifetime non-smokers. Am J Epidemiol. 1995;142:141–148. doi: 10.1093/oxfordjournals.aje.a117612. [DOI] [PubMed] [Google Scholar]
- 37.de Andrade M, Ebbert JO, Wampfler JA, Miller DL, Marks RS, Croghan GA, et al. Environmental tobacco smoke exposure in women with lung cancer. Lung Cancer. 2004;43:127–134. doi: 10.1016/j.lungcan.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Kreuzer M, Heinrich J, Kreienbrock L, Rosario AS, Gerken M, Wichmann HE. Risk factors for lung cancer among nonsmoking women. Int J Cancer. 2002;100(6):706–713. doi: 10.1002/ijc.10549. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Environmental Protection Agency . Respiratory health effects of passive smoking: Lung cancer and other disorders. US EPA, Office of Research and Development RD-689; Washington, DC: Dec, 1992. (EPA/600/6-90/006F) [Google Scholar]
- 40.Hacksaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–988. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoury MJ, Beaty TH, Cohen BH. Epidemiologic approaches to familial aggregation. In: Khoury MJ, Beaty TH, Cohen BH, editors. Fundam Genet Epidemiol. Oxford University Press; New York: 1993. pp. 170–176. [Google Scholar]